

Aust J Crop Sci. 18(09):528-534 (2024)
ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.09.p4107
Acaricidal and anticholinesterase activities of ethanol extracts from low-quality green and roasted Coffea arabica beans
Antonia Isadora Fernandes1, Maria das Graças Cardoso1*, Luís Roberto Batista2, Marcus Vinicius Prado Alves1, Vanuzia Rodrigues Fernandes Ferreira1, Cassia Duarte Oliveira2, Isaac Filipe Moreira Konig1, Rafael Neodini Remedio3, David Lee Nelson4, Pâmela Aparecida dos Santos1
1Chemistry Department, Federal University of Lavras (UFLA), Lavras, 37200-900, MG, Brazil
2Food Sciences Department, Federal University of Lavras (UFLA), Lavras, 37200-900, MG, Brazil
3Veterinary Medicine Department, Federal University of Lavras (UFLA), Lavras, 37200-900, MG, Brazil
4Postgraduate Program in Biofuels, Federal University of The Jequitinhonha and Mucuri Valleys, Diamantina, 39100-000, MG, Brazil
Abstract
The acaricidal and enzymatic inhibition potentials of extracts from lower quality green (GC) and roasted (RC) coffee beans were analyzed. Coffee extracts were obtained using the solid-liquid reflux technique, in a 1:5 ratio. The extracts were characterized by HPLC and the compounds were identified by comparison with the retention times of the standards used. Acaricidal activity was assessed by the Adult Immersion Test (AIT) and acetylcholinesterase inhibition was determined by monitoring the formation of 5-thio-2-nitrobenzoate. For every 100 g of plant material, yields equal to 3.44% and 13.34% were obtained for green and roasted coffees, respectively. Caffeic and chlorogenic acids were present in concentrations equal to 43.75 mg g-1 and 15.1 mg g-1 for green coffee, respectively, whereas concentrations equivalent to 4.5 mg g-1 and 0.019 mg g-1 were observed for roasted coffee. An efficient acaricidal activity was observed with all the doses of the extracts analyzed when compared with the water and Tween control groups, with a high mortality for all the individuals and a reduction in oviposition. Enzyme inhibition was observed at all the concentrations tested, with mean inhibitory concentrations equal to 0.2980 mg mL-1 for green coffee extracts and 0.1991 mg mL-1 for roasted coffee. Thus, green and roasted coffee extracts from lower quality beans can be used to develop new organic products.
Keywords: Rhipicephalus microplus. Phenols. Secundary metabolites. Plant extracts. Bioactive compounds.
Introduction
Coffee is one of the most widely traded and consumed products. In production terms, Brazil stands out as the world's largest coffee producer, with an estimated harvest of 55.7 million bags of Arabica coffee and 17 million bags of Conilon coffee for the biennium 2022-2023. Research by Pereira et al. (2020) and Montenegro et al. (2021) showed that the biochemical and bioactive composition of coffee beans is related to human health benefits. For example, caffeine can reduce the risk of Parkinson's and Alzheimer's diseases, Type 2 diabetes, and certain types of cancer. Trigonelline, an alkaloid present in coffee, has antimicrobial, anticancer, and antihyperglycemic effects. In addition, coffee is a source of chlorogenic acids, which have antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, hepatoprotective, antimicrobial and anti-hypertensive properties.
The concentration of compounds in coffee that are related to health benefits can be influenced by factors such as roasting, grinding and fermentation. The roasting process is one of the main steps that modify the concentration of bioactive compounds because chemical reactions occur, such as dehydration, Maillard reactions and Strecker degradation, which are responsible for the characteristic aroma and color of coffee (Montenegro et al., 2021).
Because coffee is widely consumed by the Brazilian population and widely exported, the generation of solid waste and effluents during the coffee production chain, from harvesting to preparation of the beverage, is directly proportional to the production of the product suitable for consumption. These by-products are a worrying obstacle because they are often improperly discarded and result in strong environmental impacts (Vegro and Carvalho, 1994; Silva et al., 2022).
Unlike the food industry, several manners of utlizing the residues have already been studied, such as the skins, pulp and sludge that are applied in the production of biofuels, biofertilizers and animal feed, whereas the water used in the process is treated by aerobic digestion and reused for irrigation. Furthermore, it is known that the application of non-defective green beans as food additives and sources of enzymes, fertilizers, preservatives and antioxidants already exists, as well as the use of roasted beans for the formulation of cosmetics, pharmaceuticals and food. However, new alternatives for the use of defective coffee beans are still limited and scarce (Esquivel and Jimenéz, 2012; Marto et al., 2016; Durán et al., 2017). The objectives of this work were to analyze the acaricidal potential of extracts of green and roasted coffee beans of inferior quality, in addition to evaluating the potential for inhibiting the acetylcholinesterase.
Results and Discussion
Determination of moisture and yield of extracts
The results obtained for the extraction yield of green and roasted beans are shown in Table 1.
Leite et al. (2021) reported that the reagents used in the extraction process should be defined with a view to using the extract in industrial processes. Gualberto et al. (2021) showed that the composition of the extracts can be enhanced by extraction with solvents of different polarities to extract compounds with different chemical structures.
Characterization of extracts
The compounds present in the green and roasted coffee extracts were identified by comparison with the retention times of the standards and their respective concentrations (mg g-1). They are listed in Table 2.
In both extracts, caffeic acid was the compound found in the highest concentration, corresponding to 43.75 mg g-1 and 4.5 mg g-1 in green and roasted coffee extracts, respectively. A larger quantity of chlorogenic acid (CGA) was identified in the green coffee extract (15.1 mg g-1). A smaller concentration of other detected compounds was observed when the roasted coffee extract was compared to the green coffee extract. These results can be explained by the formation and degradation of phenolic compounds during the roasting process (Elias et al, 2022).
The trigonelline content in the green coffee extract was considerably greater than that found in roasted coffee extracts. Bressani and collaborators (2021) state that the expressive decrease in the trigonelline content between the green coffee and roasted coffee extracts can be explained by its degradation during the roasting process because it has a significant thermal instability. According to the authors, the degradation of this compound is related to the formation of pyrazines, furans, pyrroles and alkyl-pyridines, which are responsible for the sensory characteristics of aroma and taste.
Chlorogenic acids are defined as a general term used to describe a variety of phenolic compounds found in plant species, including coffee. This group includes esters of trans-cinnamic acids, such as caffeic, ferulic, p-coumaric and quinic acids. These acids have satisfactory antioxidant and anticancer activities. They are effective in combating degenerative and cardiovascular diseases, protecting plants against abiotic stresses and pathogen attacks, in addition to contributing to the bitterness and astringency of the beverage (Monteiro and Farah, 2012; Ayelign and Sabally, 2013).
According to studies by Jeszka-Skowron, Zgoła-Grześkowiak and Grześkowiak (2015), compounds belonging to the chlorogenic acid group are responsible for the quality of coffee and its flavor and can vary because of genetic factors, such as the species in question, and environmental factors related to agricultural practices, maturation of beans, climate and soil. Previously, Duarte, Pereira and Farah (2010) reported that, in addition to these factors, post-harvest management can also influence the hydrophilic components of the beans, such as sugars and other compounds quantified in this study.
Similar results for chlorogenic acid concentrations in ethanolic coffee extracts were obtained by Kiattisin, Nantarat and Leelapornpisid (2016), where the authors detected the presence of chlorogenic acid only in samples of green beans. Significantly different levels of chlorogenic acid between samples of green and roasted coffee were also found by Kim et al. (2018), who observed values between 59.04 μg g-1 and 15.37 μg g-1, respectively. For Stefanello et al. (2019), variables such as high temperatures, type of roasting and the speed of air flow in the roaster can all be crucial for reducing the concentration of chlorogenic acid in coffee samples.
According to Farah et al. (2005), the high temperature in the roasting process promotes the cleavage of carbon-carbon bonds in CGA, results in the isomerization and degradation of some compounds in roasted coffee. In addition, hydrolysis and dehydration of compounds can occur, which explained the difference in the concentrations of compounds present in the extracts.
In this study, glucose, fructose and sucrose were only detected in green coffee extracts, whereas the same sugars were not identified in roasted coffee extracts. Caporaso et al. (2018) explained that the difference between sugar levels in coffee samples can be related to the species and the post-harvest processing steps. Studies by Bertuzzi et al. (2020) show that these sugars, when subjected to high temperatures, can be converted to degradation products. Therefore, the absence of these sugars in the roasted coffee extracts can be justified by the roasting process to which the beans were submitted. Bondam et al. (2022) explain that the phenolic compounds present in coffee extracts can vary significantly according to the extraction method because the modification of the applied method and the solvent used in the extractions can direct the origin of quantitative products.
Acaricide activity
The acaricidal activity data obtained by the Immersion Test in Adults using green and roasted coffee extracts are presented in Tables 2 and 3, respectively. A dose-dependent behavior was observed for the individual mortality, with higher individual mortalities at higher concentrations. The application of the roasted coffee extract, in addition to the mortality of individuals within the five days of treatment, considerably inhibited oviposition at all concentrations, whereas the egg laying decreased in the presence of green coffee extract at concentrations equal to 125 and 62.5 mg mL-1. The controls used in the test, water and Tween 0.01%, did not cause death or inhibition of egg laying during the evaluation period. The lethal concentration was not obtained for 50% of the individuals because dead individuals were observed at all the concentrations used during the evaluation period, but it is possible to infer that the LC50 of both extracts is lower than 7.8 mg mL-1.
Bravo-Ramos et al. (2021) analyzed extracts of Carica papaya leaf, Moringa oleifera root, and Randia aculeata seeds and bark at concentrations equal to 100, 50, 25, 12.5, 6.25, 3.13, and 1.56 mg mL- 1 against R. microplus. The authors observed a mortality between 5-55% using C. papaya leaf extracts, 7.5-65.6% for M. oleifera extracts, 17.5-85.5% with extracts of R. aculeata seeds and 15-75% using R. aculeata bark extracts, in addition to inhibition of egg hatching at the highest concentrations analyzed.
Shanmuganath et al. (2021) tested extracts of A. conyzoides for resistant species of R. microplus and noted the positive influence of the extracts on changes in the tegument of the tested individuals, in addition to the decrease in the activity of acid and alkaline phosphatase, enzymes responsible for the transport and digestion of food. In the same year, Oliveira et al. (2021) observed the action of Acmella oleracea (jambu) extracts on oocytes I and II of female R. sanguineus and, consequently, interference with the genetic material, which compromised their cellular functions.
Acetylcholinesterase inhibitory activity
The enzymatic activity as a function of the concentrations of green and roasted coffee extracts analyzed in this study is presented in Figure 1, whereas the average inhibitory concentration of the extracts (IC50), as well as that of carvacrol, the standard used for comparison can be seen in Table 4. There is a dose-dependent effect of the extracts on the enzymatic activity, that is, the activity of the AChE enzyme decreases as the concentration of extracts and standard increases. The greatest potential for AChE enzymatic inhibition by the extracts was observed at the highest concentration (1 mg mL-1), where 100% inhibition was observed with the roasted coffee extract, and 87% inhibition of the enzymatic activity was observed with the green coffee extract.
It is known that plant substances have a complex nature, thanks to their chemical diversity and that the complex nature of extracts. When linked to possible antagonistic or synergistic interactions, this fact can explain their actions (Zengin et al., 2020). A similar effect was confirmed by Gomes et al. (2022), who showed that the Ammocharis coranica extracts have a greater potential for enzymatic inhibition than fractions of the same extracts. Furthermore, bioactive compounds, when compared to other extracts, may differ quantitatively because of several factors, such as the portion of the plant utilized, the environment in which it was collected and the season of the year, which can strongly influence the active metabolites.
The extraction process is also crucial. Parameters such as the method, solvent and temperature utilized interfere with the efficiency of extraction and the amount of plant biomolecules obtained (Şenol et al., 2018; Zengin et al., 2020).
Akomolafe et al. (2017) and Zengin et al. (2020) mentioned that coffee has a high enzyme inhibitory activity, proven by the action of isolated compounds, such as caffeine and chlorogenic and caffeic acids. The inhibitory activity of caffeic acid, when used with caffeine, is lower than that of pure caffeine, but with a greater inhibitory effect when compared to caffeic acid alone. Thus, caffeic acid is a weaker inhibitor than caffeine. The authors mention that, in addition to the bioactive compounds being analyzed, factors such as the enzyme and the method used can directly influence the result.
Similar to the results obtained in this study, Nemzer, Kalita and Abshiru (2021) analyzed coffee extracts with distinct components of caffeine and chlorogenic acid for fins of AChE controls. In their studies, they concluded that the inhibitory action of extracts with higher levels of caffeine and chlorogenic acid is dependent on the avoided dose, while extracts with reduced levels of these substances did not show inhibitory activity of the enzyme under certain conditions. These findings are consistent with those found in the present study, in which a lower degree of inhibition of the AChE enzyme was observed with the green coffee extracts containing higher levels of caffeine and chlorogenic and caffeic acids than was observed with the roasted coffee extract, which contains lower concentrations of these bioactives. High concentrations of these compounds probably act with an antagonistic effect and decrease the inhibitory activity against enzymes such as the AChE enzyme. These results are consistent with the effect found by Oboh and collaborators (2013), who analyzed the inhibition of AChE by caffeine and chlorogenic acid, separately and combined. When analyzed together, the enzymatic activity was lower in the presence of equal and equivalent amounts of these substances. According to the authors, the action of biocompounds is related to the chemical structure of the bioactives, which can positively or negatively interfere with the inhibitory or neuroprotective potential.
According to Pohanka and Dobes (2013), caffeine is a compound with an inhibitory capacity of a non-competitive character, but with low potential when compared to other substances already applied for this purpose and that possess lower toxicity and for which high doses can be more easily administered. Research by Nemzer, Kalita and Abshiru (2021) point to caffeine IC50 values equal to 90 μg mL-1, whereas known anticholinesterase inhibitors, such as physostigmine, present almost complete inhibition with doses equivalent to 5 μg mL-1.
Similar to the present study, Erdem et al. (2016) evaluated extracts obtained from commercial coffee for inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. The researchers did not observe inhibition of AChE at low concentrations (up to 100 μg mL-1), but they observed a 30.8% inhibition of the BChE enzyme, which lead to the assumption that these compounds require higher concentrations to inhibition against the AChE enzyme. Their results are consistent with those found in the present work.
Studies by Pavlica and Gebhardt (2009) and by Metwally et al. (2020) showed that chlorogenic acid protects neuronal cells by cleaning free radicals and inhibiting their formation when analyzed in intact cells, and It also acts in the regulation of molecular markers and cytoprotective signaling. Taram et al. (2016) studied the action of chlorogenic acid and its metabolites in reducing neuronal damage and observed that this occurs by protecting against stressors such as nitric oxide and glutamate, as well as by reducing lipid peroxidation.
Materials and Methods
Samples
The green and roasted coffee beans (Coffea arabica L.) of inferior quality were purchased from the Department of Agronomy of the Federal University of Lavras (UFLA). The beans were produced in the south of Minas Gerais during the 2019/2020 harvest.
The treatment of green beans (raw) consisted only of grinding them in an industrial blender. The coffee beans were roasted in the Agricultural Product Processing Laboratory of Agricultural Engineering at UFLA in a roaster with a capacity of 5 kg. A priori, the roasting temperature and pressure were maintained at 200 ºC and 10 mbar, respectively, and then they were maintained at 220 ºC and 5 mbar for 9.5 minutes at the sound of the signal that indicates the expansion of the bean. Finally, the reasted beans were ground. Both samples were stored at room temperature in polyethylene bags with a capacity of 1 kg for further analysis.
Plant extracts
The coffee extracts were prepared by the simple solid-liquid reflux technique using ethanol as the solvent. The ground beans were refluxed for 4 hours at 78 °C with a liquid/solid ratio of 5:1 for effective extraction of constituents. The extracts were filtered and the solvent was removed on a rotary evaporator (Rotavapor Buchi R-144) over a two-hour period. The extracts were left for 24 hours in a fume hood, transferred to hermetically sealed flasks and stored in a freezer at -10 °C. The moisture content of coffee beans was determined according to Pimentel et al. (2006), and the extraction yield (%R) was calculated on a moisture-free basis according to Equation 1.
$$\% R = \frac{100\ \bullet \ mass\ of\ extract}{\frac{sample\ mass - (sample\ mass\ x\ moisture\ content)\ }{5}}\ \ (1)$$
Characterization of the extracts
The characterization of the extracts was achieved by HPLC on a Shimadzu UHPLC chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with two LC-20AT high pressure quaternary pumps, a DGU-20A5 degasser, a SIL automatic injector -20A, a CBM-20A controller, a CTO-20AC oven, an SPDM-20A UV-Vis detector, a RID-10A differential refractive index detector, and an FRC-10A fraction collector. A VP-ODS-C18 Shim-pack column (250 mm x 4.6 mm) and Shim-pack pre-column (10 mm x 4.6 mm) were used.
The Mobile Phase (A) was composed of 2% acetic acid in water and the Mobile Phase (B) consisted of a mixture of methanol, water and acetic acid in the proportions of 70:28:2, respectively. The total analysis time was 65 min at 40 ºC, with a flow rate of 1 mL min-1 and a wavelength of 280 nm. The phenol standards used were gallic acid, catechin, chlorogenic acid, caffeic acid, vanillin, ferulic acid, m-coumaric acid, o-coumaric acid and resveratrol, all obtained from Sigma-Aldrich (Saint Louis, Missouri, USA). Stock solutions were prepared in HPLC grade 70% methanol.
The extracts (0.5 g) were dissolved in 20 mL of 70% methanol and submitted to an ultrasonic bath for 20 minutes. The solution was centrifuged (4000G for 7 minutes), and the supernatant was filtered through a 0.45 µm nylon membrane (Millipore). The compounds present in the extract were identified by comparison of their retention times with the retention times of the standards.
The trigonelline and caffeine contents were measured using the method proposed by Santiago et al. (2020). Chromatographic runs were performed using a Shimadzu high-performance liquid chromatograph equipped with a high-pressure quaternary pump (LC-20AT), a degasser (DGU-20A5), an interface (CBM-20A), an automatic injector (SIL-20A- HT) and a UV-Vis detector (SDP-20A). A Supelcosil LC-C18 column (4.6x250 mm, 5 µm) and a Supelcosil C18 pre-column (4.6x12.5 mm, 5 µm) were used, whereas the mobile phase was isocratic, and the flow rate was 1 mL min-1. Solvent A was composed of ultrapure water and glacial acetic acid (99:1 v/v) and Solvent B was composed of methanol, water and acetic acid (85:14:1 v/v). The sample volume was 20 µL, the compounds of the samples were identified by comparison of their retention times with the retention times of the standards, and they were quantified by external calibration at a wavelength of 272 nm. For the preparation of the analytical curve of trigonelline and caffeine standards, stock solutions of each compound were prepared at concentrations equal to 1 mg mL-1. Dilutions of these solutions to concentrations between 0.01 and 0.7 mg mL-1 were used to prepare the standard curves.
The identification and quantification of sugars (glucose, fructose and sucrose) in the extracts were accomplished according to the method of Lopes et al. (2020) using the same equipment and the same stationary phase mentioned above. The mobile phase, consisted of sulfuric acid 0.005 M, and the flow rate was 0.5 mL min-1. The sugars were identified from the comparison of their retention times with the retention times of the standards and they were quantified by external calibration.
Acaricide activity
Engorged Rhipicephalus microplus females were manually collected from naturally infested cattle in the region of Lavras, MG, Brazil that had not received any type of acaricide treatment in the thirty days prior to the experiment. The ticks were taken to the Parasitic Biology Laboratory (BIOPAR) of the Department of Veterinary Medicine at UFLA, Minas Gerais, Brazil, washed in running water, dried on absorbent paper and divided into groups of ten individuals, according to their weight, for the experiment (Reis et al., 2021). The tick test was performed using the Adult Immersion Test according to a modification of the method of Drummond et al. (1973).
The selected ticks were weighed and divided into groups of ten individuals of homogeneous weight. In control groups, ticks were exposed to distilled water (CI) and to 20% to 0.01% Tween (CII). In the treatment groups, ticks were treated with concentrations of green and roasted coffee extracts, equivalent to 7.8 (TI); 15.6 (T2); 31.25 (T3); 62.5 (T4) and 125 (T5) mg mL-1 diluted in 0.01% Tween solution. Ticks from each group were immersed for five minutes in beakers containing 10 mL of each solution described above, both for Control and Treatment Groups. The ticks were dried with a paper towel, placed in Petri dishes under ambient conditions, and monitored for seven days. Ticks that did not respond to CO2 and stimulation of the underside were considered to be dead.
Acetylcholinesterase inhibitory activity
The test of acetylcholinesterase inhibition was performed according to the method of Ellman et al. (1961). For the activation of the enzyme, 2970-µL aliquots of Tris-HCl buffer (composed of HCl, NaCl and MgCl2.6H2O) and 254 µL of acetylcholinesterase enzyme solution (0.04 U mL-1) were added to a test tube and incubated at 37 ºC for five minutes. Then, 25 µL of extract samples at concentrations of 0.25, 0.5, 1.0, 10, 50 and 100 mg mL-1; 100 µL of Ellman's Reagent and 80 µL of substrate were added, and the mixture was incubated again at 37 ºC for fifteen minutes. The absorbance of the samples was measured in a spectrophotometer at a wavelength of 412 nm (Shimadzu UV-1601PC).
For the purpose of comparison, Negative and Positive Controls were performed for each sample, where the samples were replaced by ethanol and equivalent dilutions of carvacrol, respectively. To compensate for spontaneous hydrolysis of acetylthiocholine, non-enzymatic controls were performed for each sample, where the enzyme was replaced by Tris-HCl buffer. The tests were performed in three repetitions, and the percentage of enzymatic activity was calculated according to Equation 2.
| $$A(\%) = \ \left( \frac{A_{T} - A_{C}}{A_{O}} \right)\ .\ 100$$ | (2) |
|---|
where A is the percentage absorbance; AT is the absorbance of the treatment containing the extract/positive control; AC is the absorbance of the non-enzymatic control; and AO is the absorbance of the negative control.
Statistical analysis
Data from the yield and AChE inhibitory activity were analyzed using the SISVAR® software to assess whether significant differences existed between treatments, with means compared by the Tukey test and the Scott-Knott test at 5% significance (Ferreira, 2011).
Conclusions
The acaricide activity obtained with the use of the extracts at concentrations between 7.81 and 125 mg mL-1 was satisfactory, with gradual mortality during the following days of the experiment. The decrease in oviposition of individuals treated with roasted coffee extracts was noticeable at the aforementioned concentrations, whereas egg laying decreased only at 125 and 62.5 mg mL-1 with the green coffee extracts.
Conflict of interest
The authors declare that no conflict of interests exists.
Acknowledements
This work was supported by the Brazilian Coffee Research and Development Consortium, the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG — Project CAG/APQ 02390/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq— Project CNPQ 311183/2022-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance code 001) for the scholarships. The authors are thankful for the scholarships and financial support and thank the Central of Analysis and Chemical Prospecting of the Federal University of Lavras for supplying the equipment for chromatographic analyses.
References
Akomolafe SF (2017) Effect of caffeine, caffeic acid and their various combinations on enzymes of cholinergic, monoaminergic and purinergic systems critical to neurodegeneration in rat brain — In vitro. Neurotoxicology. 62: 6-13.
Ayelign A, Sabally K (2013) Determination of Chlorogenic Acids (CGA) in Coffee Beans using HPLC. Am J Res Commun. 1: 78-91.
Bertuzzi T, Martinelli E, Mulazzi A, Rastelli S. (2020) Acrylamide determination during an industrial roasting process of coffee and the influence of asparagine and low molecular weight sugars. Food Chem. 303: 125372.
Bondam AF, da Silveira DD, dos Santos JP, Hoffmann JF (2022) Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci Technol. 123:172-186.
Bravo-Ramos JL, Flores-Primo A, Paniagua-Vega D, Sánchez-Otero MG, Cruz-Romero A, Romero-Salas D (2021) Acaricidal activity of the hexanic and hydroethanolic extracts of three medicinal plants against southern cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp Appl Acarol. 85:113-129.
Bressani APP, Batista NN, Ferreira G, Martinez SJ, Simão JBP, Dias DR, Schwan, R F (2021) Characterization of bioactive, chemical, and sensory compounds from fermented coffees with different yeasts species. Food Res Int. 150: 110755.
Caporaso N, Whitworth MB, Grebby S, Fisk ID (2018) Non-destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging. Food Res Int. 106:193-203.
Drummond REA, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973)
Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Econ Entomol. 66:130-133.
Duarte GS, Pereira AA, Farah A (2010) Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chem. 118:851-855.
Durán CAA, Tsukui A, Santos FKF, Martinez ST, Bizzo HR, Rezende CM (2017) Café: Aspectos Gerais e seu Aproveitamento para além da Bebida. Rev Virtual Quim. 9:107-134.
Elias AMT, Silva MED, Neto VFM, Santos WWV, Oliveira RL, Porto TS, Silva SP (2022) Utilização do planejamento fatorial para avaliação do impacto do processo de
torrefação no perfil físico-químico de blends de café. Res. Soc. Dev. 11:1-12.
Ellman GL, Courtney KD, Andres Jr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88-95.
Erdem SA, Senol FS, Budakoglu E, Orhan IE, Sener B (2016) Exploring in vitro neurobiological effects and high-pressure liquid chromatography-assisted quantitation of chlorogenic acid in 18 Turkish coffee brands. J Food Drug Anal. 24:112-120.
Esquivel P, Jiménez VM (2012) Functional properties of coffee and coffee by-products. Food Res Int. 46:488-495.
Farah A, Paulis T, Trudo LC, Martins PR (2005) Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee. J Agric. 53:1505-1513.
Ferreira DF (2011) SISVAR: a computer statistical analysis system. Ciênc. Agrotec. 35:1039-1042.
Gomes JVD, Tosta CL, Neto AC, Fagg CG, Silva CAG, Gomes-Copeland KKP, Magalhães PO, Fonseca-Bazzo YM, Jamal CM, Silveira D (2022) Chemical profile and biological activity of Crinum americanum L. (Amaryllidaceae). S Afr J Bot. 146:25-35.
Gualberto NC, Oliveira CS, Nogueira JP, Jesus MS, Araújo HCS, Rajan M, Neta MTSL, Narain N (2021) Bioactive compounds and antioxidant activities in the agro-industrial residues of acerola (Malpighia emarginata L.), guava (Psidium guajava L.), genipap (Genipa americana L.) and umbu (Spondias tuberosa L.) fruits assisted by ultrasonic or shaker extraction. Food Re Int. 147:1-13.
Jeszka-Skowron M, Zgoła-Grześkowiak A; Grześkowiak T (2015) Analytical methods applied for the characterization and the determination of bioactive compounds in coffee. Eur Food Res Tec. 240:19-31.
Kiattisin K, Nantarat T, Leelapornpisid P (2016) Evaluation of antioxidant and anti-tyrosinase activities as well as stability of green and roasted coffee bean extracts from Coffea arabica and Coffea canephora grown in Thailand. J Pharmacogn Phytotherapy. 8:182-192.
Kim W, Kim SY, Kim DO, Kim BY, Baik MY (2018) Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chem. 240:594-600.
Leite PIP, Barreto SMAG, Freitas PR, Araújo ACJ, Pauo CLR, Almeida RS, Assis CF, Padilha CEA, Ferrari M, Junior FCS (2021) Extraction of bioactive compounds from buriti (Mauritia flexuosa L.) fruit by eco-friendly solvents: Chemical and functional characterization. Sustain Chem Pharm. 22: 100489.
Lopes ACA, Andrade RP, Oliveira LCC, Lima LMZ, Santiago WD, Resende MLV, Cardoso MG, Duarte WF (2020) Production and characterization of a new distillate obtained from fermentation of wet processing coffee by-products. J Food Sci Technol. 57:4481-4491.
Marto J, Gouveia LF, Chiari BG, Paiva A, Isaque V, Pinto P, Simões P, Almeida AJ, Ribeiro HM (2016) The green generation of sunscreens: Using coffee industrial sub-products. Ind Crops Prod. 90:93-100.
Metwally DM, Alajmi RA, El-Khadragy MF, Yehia HM, Al-Megrin WA, Akabawy AMA, Amin HK, Moneim AEA (2020) Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. Journal of Functional Foods. 75:1-9.
Monteiro MC, Farah A (2012) Chlorogenic acids in Brazilian Coffea arabica cultivars from various consecutive crops. Food Chem. 134:611-614.
Montenegro J, Santos LS, Souza RGG, Lima LGB, Mattos DS, Viana BAPB, Bastos ACSF, Muzi L, Conte-Junior CA, Gimba HRP, Freitas-Silva O, Teodoro AJ (2021) Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE). Food Research International. 140:1-8.
Nemzer B, Kalita D, Abshiru N (2021) Quantification of major bioactive constituents, antioxidant activity, and enzyme inhibitory effects of whole coffee cherries (Coffea arabica) and their extracts. Molecules. 26: 4306.
Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha EA (2013) Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem Res. 38:413-419.
Oliveira PR, Monteiro OS, Rocha CQ, Costa-Junior LM, Câmara MBP, Pereira TCS, Maia JGS (2021) Exposure of Rhipicephalus sanguineus sensu lato Latreille, 1806 (Acari: Ixodidae) to hexane extract of Acmella oleracea (Jambu): semi-engorged and engorged ticks. Ticks Tick Borne Dis. v. 12:1-12.
Pavlica S, Gebhardt R (2009) Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic Res. 39:1377-1390.
Pereira GVM, Neto DPC, Junior AIM, Prado FG, Pagnoncelli MGB, Graça SK, Soccol CR (2020) Chapter Three - Chemical composition and health properties of coffee and coffee by-products. Adv Food Nutr Res. 91:65-96.
Pimentel FA, Cardoso MG, Salgado APSP, Aguiar PM, Silva VF, Morais AR, Nelson DL (2006) A convenient method for the determination of moisture in aromatic plants. Quim Nova. 29:373-375.
Pohanka M, Dobes P (2013) Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int J Mol Sci. 14:9873-9882.
Reis AC, Konig IFM, Rezende DACS, Gonçalves RRP, Lunguinho AS, Ribeiro JCS, Cardoso MG, Remédio RN (2021) Cytotoxic effects of Satureja montana L. essential oil on oocytes of engorged Rhipicephalus microplus female ticks (Acari: Ixodidae). MRT. 84:1375-1388.
Santiago WD, Teixeira AR, Santiago JA, Lopes ACA (2020) Development and validation of chromatographic methods to quantify organic compounds in green coffee (Coffea arabica) beans. Aust J Crop Sci. 14:1275-1282.
Şenol Fs, Şekeroğlu N, Gezici S, Kilic E, Orhan I. E (2018) Neuroprotective potential of the fruit (acorn) from Quercus coccifera L. Turk J Agric For. 42:82-87.
Shanmuganath C, Kumar S, Singh R, Sharma AK, Saminathan M, Saini M, Chigure G, Fular A, Kumar R, Julieta S, Upadhaya D, Kumar B, Srivastava S, Ghosh S (2021) Development of an efficient antitick natural formulation for the control of acaricide-resistant ticks on livestock. Ticks Tick Borne Dis. 12:1-10.
Silva MF, Pettinato M, Casazza AA, Maciel MIS, Perego P (2022) Design and evaluation of non-conventional extraction for bioactive compounds recovery from spent coffee (Coffea arabica L.) grounds. Chem Eng Res Des. 177:418-430.
Stefanello N, Spanevello RS, Passamonti S, Porciúncula L, Bonan CD, Olabiyi AA, Rocha JBT, Assmann CE, Morsch VM, Schetinger MRS (2019) Coffee, caffeine, chlorogenic acid, and the purinergic system. FCT. 123:298-313.
Taram F, Winter AN, Linseman DA (2016) Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res.1648:69-80.
Vegro CLR, Carvalho FC (1994) Disponibilidade e utilização de resíduos gerados no processamento agroindustrial do café. Instituto da Economia Agrícola, São Paulo – SP, 1994.
Zengin G, Sinan KI, Mahomoodally MF, Ângelo S, Mustafá AM, Vittori S, Maggi F, Caprioli G (2020) Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods. 9:1-17.
Table 1. Yield of ethanolic extracts of green coffee (GC) and roasted coffee (RC).
| Extract | Yield (g) |
|---|---|
| Roasted coffee (RC) | 13.34 ± 1.48a |
| Green coffee (GC) | 3.44 ± 0.81b |
| *GC (%) | 14.09 |
*GC (%) – Coefficient of Variation.
Table 2. Compounds present in the extracts identified by HPLC.
| Concentration (mg g –1) | ||
|---|---|---|
| Compound | GC | RC |
| Gallic acid | ND | 0.001 |
| Catechin | ND | 0.012 |
| Chlorogenic Acid | 15.1 | 0.019 |
| Caffeic acid | 43.75 | 4.50 |
| Vanillin | 0..2 | 0.008 |
| Ferulic acid | 0.22 | ND |
| m-Coumaric acid | ND | 0.007 |
| o-Coumaric acid | 0.53 | ND |
| Resveratrol | 0.25 | ND |
| Trigonelline | 9.95 | 0.34 |
| Caffeine | 73.86 | 6.09 |
| Glucose | 0.155 | ND |
| Fructose | 1.5 | ND |
| Sucrose | 4.71 | ND |
ND: Not detected.
Table 3. Mortality of green coffee extracts on Rhipicephalus microplus at different concentrations.
| Mortality (GC) | ||||
|---|---|---|---|---|
| Concentration (mg mL-1) | 24 h | 48 h | 72 h | 96 h |
| 125 | 6 | 10 | 10 | 10 |
| 62.5 | 2 | 8 | 8 | 10 |
| 31.25 | 2 | 4 | 8 | 10 |
| 15.6 | 2 | 4 | 6 | 10 |
| 7.8 | 0 | 2 | 4 | 10 |
Table 4. Mortality of roasted coffee extracts on Rhipicephalus microplus at different concentrations.
| Mortality (RC) | |||||
|---|---|---|---|---|---|
| Concentration (mg mL-1) | 24 h | 48 h | 72 h | 96 h | 120 h |
| 125 | 10 | 10 | 10 | 10 | 10 |
| 62.5 | 4 | 8 | 10 | 10 | 10 |
| 31.25 | 0 | 4 | 4 | 10 | 10 |
| 15.6 | 2 | 2 | 4 | 10 | 10 |
| 7.8 | 0 | 6 | 6 | 8 | 10 |
Table 5. Inhibition of AChE enzymatic activities observed with carvacrol and green and roasted coffee extracts.
| Sample | IC50 |
|---|---|
| Carvacrol | 0.0158±0.001a |
| Roasted coffee | 0.1991±0.010b |
| Green coffee | 0.2980±0.045c |
Means followed by the same lowercase letters in the rows and uppercase letters in the columns do not differ from one another by the Scott-Knott Test at the 5% probability level.
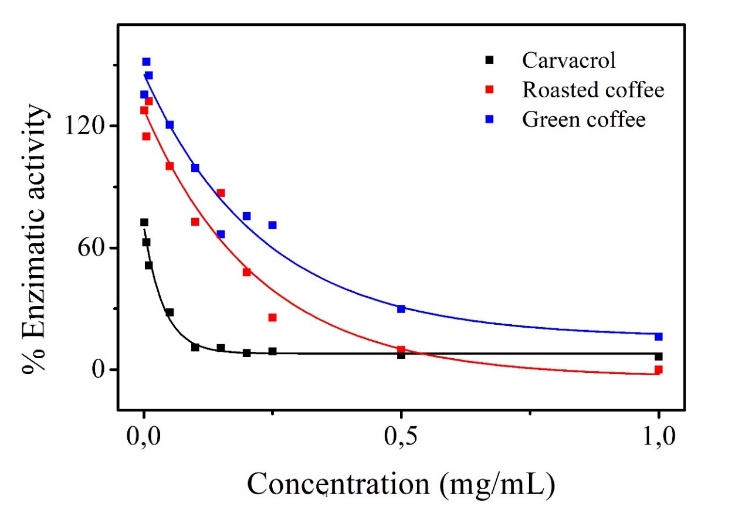
Figure 1. Acetylcholinesterase activity as a function of the concentrations of green and roasted coffee extracts and of the standard.