

Aust J Crop Sci. 18(09):591-597 (2024) ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.09.p153
Efficient and rapid in vitro micropropagation of MD2 and H4 pineapple varieties
Oumar SILUE*1, Samuel Kouadio Oi KOUADIO2, Koffi Honoré KOUAME3, Emmanuel ZRAN1, Mah TOURE1, Modeste Kan KOUASSI4
1Central Biotechnology Laboratory, National Agronomic Research Center (CNRA), Abidjan, 01 BP 1740 Abidjan 01, Côte d’Ivoire
2Biological Sciences Department, Peleforo Gon Coulibaly University, B.P. 1328 Korhogo, Côte d’Ivoire
3Sciences and Technology Training and Research Unit, Alassane Ouattara University, 01 BP V 18 Bouaké 01, Côte d’Ivoire
4Laboratoire de Biotechnologie, Agriculture et Valorisation de Ressources Naturelles, UPR de Physiologie et Pathologie Végétales, Université Félix Houphouët-Boigny d’Abidjan, 08 BP 1085 Abidjan 08, Côte d’Ivoire
*Corresponding author: silueoumarnatogoma@gmail.com
ORCID: https://orcid.org/0009-0009-2695-6127
Abstract: Pineapple plays an important role in international trade and supports of more than a million households in Côte d’Ivoire. However, its yield is subject to pedoclimatic problems and erosion of genetic potential of plant material. This study aims to establish an effective in vitro yield protocol for MD2 and H4 pineapple varieties. Therefore, the effects of 6-benzylaminopurine (BAP) combined with different concentrations of 2-isopentenyladenine (2-iP), with variable light intensity and activated charcoal on leaf shoot proliferation and elongation were tested. Analysis of variance revealed that Murashige and Skoog medium (MSB5) supplemented with 2 mg/L BAP combined with 0.25 mg/L 2-iP recorded the highest average number of microshoots (13.50 microshoots) in MD2 variety, while 2 mg/L of BAP combined with 0.5 mg/L of 2-iP recorded the best average number of shoots (15.20 microshoots) in H4 variety. Maximum microshoot elongation (7.81 cm and 7.03 cm for MD2 and H4, respectively) was obtained with 2 mg/L BAP combined with 0.25 mg/L of 2-iP. Light intensity of 1500 lux produced 14.06 and 14.86 microshoots in MD2 and H4, respectively. In contrast, maximum microshoot elongation (4.40 cm and 4.13 cm in MD2 and H4, respectively) was recorded at 2000 lux light intensity. A 2 g/L of activated charcoal inhibited microshoot proliferation. However, it induced an elongation of 7.50 cm in MD2 and 7.07 cm in H4. Microshoots were rooted on MS medium supplemented with 100 mg/L myo-inositol, 30 g/L sucrose, 200 mg/L glutamine, 1 mg/L naphthalene acetic acid (NAA), 2 mg/L indole-3-butyric acid (IBA), 2 g/L activated charcoal, all solidified with 6g/L agar. In conclusion, the combination of 2 mg/L BAP and 2-iP (0.25 - 0.5 mg/L) is recommended for better in vitro shoot proliferation in MD2 and H4 varieties. Light intensities of 1500 - 2000 lux are ideal for better shoot proliferation and elongation. Finally, the addition of 2 g/L activated charcoal to the medium improves shoot elongation and rooting in MD2 and H4 varieties.
Keywords: Bromeliaceae, in vitro multiplication, light intensity, microshoots, plant hormones.
Abbreviations: AC_Activated charcoal, BAP_6-benzylaminopurine, FAO-Food and Agriculture, GDP_Gross Domestic Product, H4_Hydrid 4, IBA_Indole-3-butyric acid, MD2_Del Monte Extra Sweet, MSB5_Murashige and Skoog medium including vitamin B5, NAA_Naphtalene Acetic Acid, TDZ_Thidiazuron,2-iP_2-isopentenyladenine.
Introduction
Pineapple is the second most traded tropical fruit in the world. Indeed, with 29.361 million tons in 2022, pineapple contributes 20% of the world’s tropical fruit production (FAO, 2023). In Côte d’Ivoire, pineapple plays a strategic role in the economy of Eastern Comoé River including Grand-Bassam, Bonoua, Adiaké, Aboisso, which provide 70 to 80% of national production. It contributes 0.6% to national GDP and 1.6% to agricultural GDP (Kouadio, 2018). The significant income generated by the marketing of pineapples, mainly on the international market, supports more than one million people. As a result, pineapple cultivation contributes to food security and maintaining a balance that is essential for the development of rural and urban populations (FAO, 2020).
However, Ivorian pineapple production has been declining for more than a decade, despite the efforts made by producers. Thus, in 2006, Côte d’Ivoire exported 94,000 tons of fresh pineapple, but by 2022, less than 23,834 tons were exported (FAO, 2023). One reason for this situation is the lack of financial and technical support for pineapple producers, which has led many to abandon pineapple cultivation in favor of other crops such as oil palm and rubber trees. Additionally, falling income and the proliferation of natural
Table 1. Effect of different concentrations of 2-iP in combination with BAP on in vitro shoots multiplication and elongation in two ananas varieties (MD2 and H4).
| Hormonal combination | Varieties | ||||
|---|---|---|---|---|---|
| MD2 | H4 | ||||
| Mean number of shoots | Mean length of shoots (cm) | Mean number of shoots | Mean length of shoots (cm) | ||
| C1 | 10.80 ± 0.24d | 7.06 ± 0.11b | 11.85 ± 0.27d | 6.75 ± 0.18a | |
| C2 | 13.50 ± 0.31a | 7.81 ± 0.15a | 14.40 ± 0.28b | 7.03 ± 0.14a | |
| C3 | 12.75 ± 0.20b | 6.76 ± 0.12b | 15.20 ± 0.21a | 6.13 ± 0.13b | |
| C4 | 11.60 ± 0.23c | 5.50 ± 0.10c | 11.50 ± 0.25d | 5.02 ± 0.13c | |
| p | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | |
Mean values within a column followed by the same letters are not significantly different at p ≤ 0.05 by Fisher LSD test ± standard error. C1 (2 mg/L BAP + 0 mg/L 2-iP); C2 (2 mg/L BAP + 0.25 mg/L 2-iP); C3 (2 mg/L BAP + 0.5 mg/L 2-iP); C4 (2 mg/L BAP + 1 mg/L 2-iP).
enemies of the crop, such as diseases and pests, are contributing factors. Finally, the aging and erosion of genetic potential also play a role. Considering the socio-economic importance of pineapple in Côte d'Ivoire, it is necessary to revalue in its varietal dimension to reduce poverty among vulnerable groups. Therefore, pineapple vegetative multiplication by suckers (Harahap et al., 2015) has been the safest method of multiplication to preserve the fruit quality of selected varieties. However, this traditional multiplication method, which is very slow, remains of very limited scope to meet the needs of an ever-increasing demand for planting material to expand existing pineapple plantations or create new ones (Chotangui et al., 2019).
In contrast, in vitro micropropagation appears to be the most promising means of producing pineapple plantlets to fill the planting material deficit (Kouadio et al., 2017). This technique has the advantage of producing a significant quantity of healthy and rejuvenated planting material in a relatively short time and in a reduced space, unlike conventional vegetative propagation methods. However, the production of planting material through in vitro techniques production of planting material is dependent on several factors.
This study aims to establish an efficient protocol to produce MD2 and H4 varieties plants using micropropagation. Factors such as hormonal combination, light intensity and inorganic adjuvants can influence plant production. Thus, several authors have recommended 6-benzylaminopurine (BAP) as the most effective growth regulator for pineapple shoot production (Kouadio et al., 2017). Several studies have reported that a concentration of 2 mg/L of BAP is optimal for pineapple shoot proliferation (Atawia et al., 2016; Sani et al., 2019).
In contrast, Thidiazuron (TDZ) at 2 mg/L was recommended by some authors for pineapple shoot proliferation (Manal et al., 2021). In addition, Manal et al. (2021) observed that high light intensity of 4000 lux was beneficial for shoot proliferation but inhibited shoot elongation in pineapple. The role of Activated charcoal (AC) in banana micropropagation has already been reported (Ahmed et al., 2014, Gehan et al., 2015; Emiru and Gerema, 2021). The search for an efficient protocol for producing pineapple planting material would make it possible to provide highly productive material capable of revitalizing pineapple cultivation in Côte d’Ivoire.
Results
Effect of combining two cytokinins on in vitro shoot multiplication and elongation in pineapple
Analysis of variance revealed a significant difference for the concentration factor (p ≤ 0.05) (Table 1). The results showed that the addition of 2-iP to the medium containing BAP resulted in an increase in the number of shoots compared to the control containing BAP alone (C1) (Fig 1). Thus, the combination C2 (2 mg/L BAP + 0.25 mg/L 2-iP) recorded the
highest average number (13.25 shoots) and average length (7.81 cm) of shoots in MD2 variety. In contrast, in H4 variety, C3 combination (2 mg/L BAP + 0.5 mg/L 2-iP) induced the highest average number of shoots (15.20 shoots), while C2 combination (2 mg/L BAP + 0.25 mg/L 2-iP) recorded the highest average length (7.03 cm). Therefore, the results suggest that increasing the concentration of 2-iP in the presence of BAP inhibited shoot elongation in both varieties compared to the control. Similarly, the addition of high concentrations of 2-iP (1 mg/L) to the medium, in the presence of BAP, also inhibited shoot induction in both varieties (Table 1).
Effect of light intensity on in vitro shoot multiplication and elongation
It appears from the analysis of the results (Table 2) that light intensity significantly influenced the average number of shoots induced in both varieties (p ≤ 0.05). The highest average number of shoots was observed at a light intensity of 1500 lux for both pineapple varieties (14.06 and 14.86 shoots in MD2 and H4, respectively), while light intensities above 1500 lux inhibited shoot multiplication in both varieties (Fig 2). In contrast, the analysis of variance did not reveal any significant difference between the light intensities tested in terms of shoot elongation, (p ≤ 0.48 in MD2 and p ≤ 0.32 in H4). However, the results show that shoot elongation increased with increasing light intensities. The maximum elongation was recorded with a light intensity of 2000 lux, in both varieties (4.40 cm and 4.13 cm in MD2 and H4, respectively).
Effect of activated charcoal on in vitro multiplication and elongation of leafy shoots
Analysis of variance of shoot multiplication and elongation parameters in two pineapple varieties (MD2 and H4) (Table 3) showed that the different concentrations of activated charcoal recorded a significant effect (p ≤ 0.05) on shoot multiplication and elongation in both varieties.
The results indicated that the addition of activated charcoal to the culture medium inhibited the proliferation of shoots, as indicated by the average number of shoots. The medium without activated charcoal; therefore, recorded the large number of shoots in MD2 (13.55 shoots) and H4 (13.75 shoots) varieties. An increase in the concentration of activated charcoal resulted in a decrease in the number of shoots (Fig 3 and Fig 4). In contrast, the results indicated that shoot elongation increased in both varieties as the activated carbon content of the culture medium rose (Fig 3 and Fig 4). The highest average shoot length was recorded with 2 mg/L activated charcoal in both varieties (7.50 cm and 7.07 cm in MD2 and H4, respectively).
Table 2. Effect of light intensity on shoot number and length in two ananas varieties (MD2 and H4).
| Light intensity (Lux) | Varieties | ||||
|---|---|---|---|---|---|
| MD2 | H4 | ||||
| Mean number of shoots | Mean length of shoots (cm) | Mean number of shoots | Mean length of shoots (cm) | ||
| 1000 | 11.13 ± 0.19c | 3.73 ± 0.22a | 12.60 ± 0.28c | 3.73 ± 0.22a | |
| 1500 | 14.06 ± 0.34a | 4.06 ± 0.44a | 14.86 ± 0.19a | 3.86 ± 0.13a | |
| 2000 | 12.20 ± 0.20b | 4.40 ± 0.42a | 13.46 ± 0.29b | 4.13 ± 0.19a | |
| p | ≤ 0.05 | 0.48 | ≤ 0.05 | 0.32 | |
Mean values within a column followed by the same letters are not significantly different at p ≤ 0.05 by Fisher LSD test ± standard error.
Table 3. Effect of Activated charcoal on shoot number and length in two ananas varieties (MD2 and H4).
Activated charcoal concentrations (g/L) |
Varieties | ||||
|---|---|---|---|---|---|
| MD2 | H4 | ||||
| Mean number of shoots | Mean length of shoots (cm) | Mean number of shoots | Mean length of shoots (cm) | ||
| 0 | 13.55 ± 0.34a | 4.25 ± 0.09d | 13.75 ± 0.25a | 4.05 ± 0.07d | |
| 0.5 | 12.25 ± 0.30b | 5.76 ± 0.07c | 12.95 ± 0.30b | 5.25 ± 0.05c | |
| 1 | 10.85 ± 0.36c | 6.70 ± 0.12b | 11.90 ± 0.26c | 6.15 ± 0.12b | |
| 2 | 10.05 ± 0.29c | 7.50 ± 0.14a | 10.60 ± 0.29d | 7.07 ± 0.13a | |
| p | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | ≤ 0.05 | |
Mean values within a column followed by the same letters are not significantly different at p ≤ 0.05 by Fisher LSD test ± standard error.

Fig. 1. In vitro multiplication and elongation of pineapple shoots under the effect of BAP and 2-iP combination in MD2 (A) and H4 (B) varieties. C1 (2 mg/L BAP + 0 mg/L 2-iP); C2 (2 mg/L BAP + 0.25 mg/L 2-iP); C3 (2 mg/L BAP + 0.5 mg/L 2-iP); C4 (2 mg/L BAP + 1 mg/L 2-iP).
Discussion
The main objective of this study was to establish an efficient protocol for in vitro production of MD2 and H4 pineapple variety plants using a combination of two cytokinins (BAP and 2-iP), light intensity and activated charcoal concentration. The results indicated that BAP alone was effective for in vitro induction of multiple shoots in both varieties. However, the addition of low concentrations of 2-iP to BAP improved the number of induced shoots in both varieties. Thus, the addition of 0.25 mg/L or 0.5 mg/L of 2-iP to a medium containing 2 mg/L of BAP made it possible to induce 13.50 and 15.20 shoots in MD2 and H4 varieties, respectively. However, the combination of 2 mg/L BAP with 0.25 mg/L 2-iP induced the best shoot elongation in both varieties (7.81 cm and 7.03 cm in MD2 and H4, respectively). The positive effect of the synergy between two cytokinins may be explained by the fact that the combined supply of BAP and 2-iP, at different concentrations, improved the suboptimal level of endogenous cytokinins, resulting in an optimal balance capable of reducing apical dominance and stimulating the formation of axillary and adventitious shoots in both MD2 and H4 varieties (Van Staden et al., 2008). Contrary to our work, some authors have reported that fusing BAP alone in the shoot multiplication medium enabled to obtain better results in certain pineapple varieties. For example, Sani et al. (2019) were able to produce 120 to
130 shoots using a concentration of 2 mg/L BAP, while Maqsood et al. (2023) and José et al. (2023) produced 16.7 and 8 shoots, respectively. However, increasingly higher concentrations such as 3 mg/L of BAP (Zuraida et al., 2018) and 5 mg/L of BAP (Adeoye et al., 2020) allowed to induce a maximum of 94 and 7.80 shoots, respectively. This suggests not only the physiological ability of BAP to induce shoot budding but also a differential response of pineapple explants to BAP concentration. Our study found that using a fortified medium of 2 mg/L of BAP in combination with 0.25 mg/L of 2-iP resulted in a maximum elongation of 7.81 cm and 7.03 cm in MD2 and H4 pineapple varieties, respectively. These results suggest that the combination of two cytokinins was essential to stimulate cell division and elongation. Our results corroborate with Atawia et al. (2016), who also observed long pineapple shoots after infusing 0.25 mg/L BAP in the culture medium. Furthermore, several authors have reported the synergistic effect of combining BAP with 2-iP on other plant species. Thus, in Ziziphus Jujuba Mill the combinations of 4 mg/L BAP + 0.5 mg/L 2iP and 0.5 mg/L BAP + 0.5 mg/L 2iP induced 4.6 shoots and an optimal length of 2.57 cm, respectively (Hemaid and Ghada, 2013). Similarly, in Chrysanthemum morifolium Ramat, the addition of 0.5 mg/L 2iP to the culture medium in the presence of 2 mg/L BAP induced an elongation of 1.88 cm (Rinaldi et al., 2016).
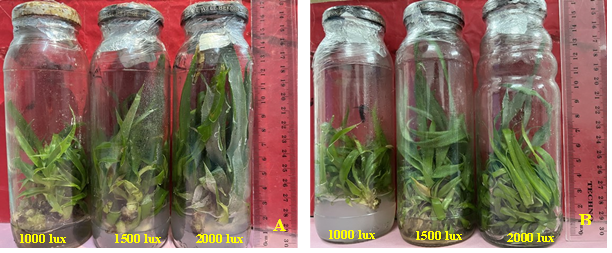
Fig. 2. In vitro multiplication and elongation of pineapple shoots under the effect of different light intensities in MD2 (A) and H4 (B) varieties.

Fig. 3. In vitro multiplication and elongation of pineapple leafy shoots under the effect of different concentrations of activated charcoal in MD2 varieties.

Fig. 4. In vitro multiplication and elongation of pineapple leafy shoots under the effect of different concentrations of activated charcoal in H4 varieties
Furthermore, in Caralluma adscendens, Aruna et al. (2012) found that MS medium supplemented with 2 mg/L BA and 0.5 mg/L 2-iP resulted in an average of 5.16 shoots per explant, with an average shoot length of 2.98 cm.
To determine the optimal light intensity for in vitro shoot multiplication, the cultures were exposed to different light intensities. Indeed, light intensity influences shoot regeneration during micropropagation in various plants (Meziani et al., 2015; Farhadi et al. 2017). Furthermore, light influences the biological effectiveness of growth regulators added to the culture medium, as well as the endogenous hormonal balance in tissues (Ding et al., 2011). Our results revealed that the light intensity of 1500 lux induced the
highest average number of shoots in both varieties MD2 (14.06 shoots) and H4 (14.86 shoots). These results suggest that this light intensity is optimal for increasing the availability of nutrients, such as sucrose, and consequently the multiplication and elongation of shoots. According to Hosseini et al. (2013) low light intensity reduces the availability of sucrose for explants, resulting in a significant reduction in shoot induction. They also suggest that endogenous auxins may be activated at these light intensities, working together with cytokinins to induce cell division. In fact, plant hormones influence changes in plant physiology and morphogenesis that are caused by light intensity or quality (Kissoudis et al., 2017). However, it is
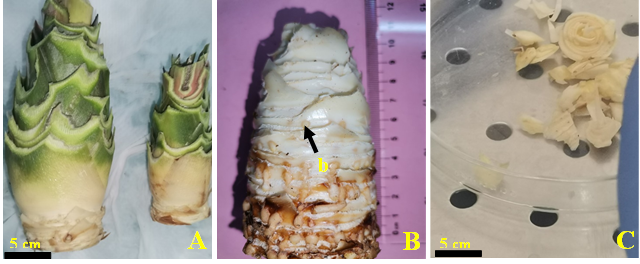
Fig. 5. Preparation of pineapple explant for in vitro shoot initiation. (A) Trimmed pineapple sucker; (B) Trimmed sucker exposing buds; (C) Isolated buds; (b) buds.
important to note that the optimal light intensity for pineapple shoot multiplication may vary depending on the variety. For example, Manal et al. (2021) reported that high light intensities were optimal for pineapple shoot multiplication. Therefore, it is possible that each variety requires a different light intensity to produce multiple shoots. In contrast, Manal et al. (2021) found that low light intensity (500 lux) results in maximum shoot elongation, while our study required a high light intensity of 2000 lux to achieve long shoots. This difference could be due to varietal effects. However, our results are consistent with previous studies on Phoenix dactylifera L. (Meziani et al., 2015) and Ipomea batatas (Ibrahim et al., 2016), which also found that high intensities (2000-3000 lux) stimulate shoot elongation.
Concerning the effect of activated charcoal on in vitro shoot multiplication and elongation of MD2 and H4 pineapple varieties, our results revealed that the addition of activated charcoal to the culture medium can improve shoot elongation while reducing proliferation in both varieties. In contrast, the medium without activated carbon induced the highest number of shoots. This result suggests that shoot proliferation in pineapple was inhibited by the addition of activated charcoal to the culture medium. This agrees with the findings of Fernando et al. (2010), who demonstrated that incorporating activated charcoal into the regeneration medium, inhibits shoot growth and development. The authors suggest that activated charcoal adsorbs significant quantities of growth regulators from the medium; thus, limiting the available quantity below the threshold necessary to stimulate shoot proliferation in both pineapple varieties. This adsorption role of activated charcoal was also reported by Praveen et al. (2009). The authors suggest that the addition of activated charcoal to the culture medium can fix certain compounds, such as phenolic compounds, which reduces oxidation of the medium, browning, and death of the explants. Our study confirms this role of fixing compounds, including growth regulators, as the inhibition of shoot proliferation was induced by the addition of activated charcoal to the culture medium. Our results agree with those of Sanputawong et al., (2015) in Cardamine lyrata and Priyanka et al., (2015) in Aloe vera L., who reported that media without activated charcoal favored shoot proliferation. However, we also found that supplementing the medium with 2 g/L of activated charcoal resulted in maximum shoot elongation in both pineapple varieties. This could be explained by the fact that activated charcoal adsorbs excess growth regulators and toxic phenolic compounds released by stressed explants; thus, favoring cell growth and differentiation. Certain authors have confirmed the positive effect of activated charcoal on shoot elongation. For example, in Aloe vera L., a lower concentration of activated charcoal (0.5 g/L) than ours had a positive effect on
shoot elongation (Nayanakantha et al., 2010; Priyanka et al., 2015). In contrast, a concentration like ours allowed shoot elongation in Elaeis guineensis (Periasamy et al., 2011), in sugar cane (Mittal et al., 2016).
Materials and Methods
Plant material
The plant material used during this study consisted of suckers of MD2 and H4 pineapple varieties obtained from the pineapple collection at the station of the National Center for Agronomic Research (CNRA) in Anguédédou (Côte d’Ivoire).
Preparation and disinfection of explants
Pineapple suckers were trimmed (Fig 5A) to expose the buds (Fig 5B). They were then carefully washed with soapy water. The suckers were then immersed in a sodium hypochlorite solution with 3.8% active chlorine for 20 min after rinsing with tap water. Under a laminar flow hood, the buds were gently isolated (Fig 5C) and soaked in 70% ethanol for 5 min. After three rinses with sterile distilled water, the buds were immersed in a 2.5% calcium hypochlorite solution for 15 min.
Preparation of media
All media were prepared from MSB5 basal medium (Murashige and Skoog, 1962), fortified with 30 g/L sucrose and solidified with 6 g/L agar. The pH of the media was adjusted to 5.8 and the media were autoclaved at 121 °C for 20 min under a pressure of 1 bar.
In vitro initiation of shoots
After 10 min of drying on previously sterilized blotting paper, the disinfected buds were inoculated into test tubes containing 15 mL of initiation medium. This medium consists of MSB5 basal medium (Murashige and Skoog, 1962); supplemented with 100 mg/ L myoinositol, 200 mg/L glutamine, 1 mg/L kinetin, 40 mg/L gibberellic acid (GA3) and 2 g/L activated charcoal. Cultures were incubated for two months under 2000 lux light intensity provided by white fluorescent lamps.
Assessment of the effect of combining two cytokinins on in vitro shoot multiplication and elongation
To assess the effect of cytokinins, the initiated shoots were transferred to MSB5 medium supplemented with 2 mg/L of BAP (6-benzylaminopurine) combined at different concentrations (0; 0.25; 0.5 and 1 mg/L) of 2-iP (2-Isopentenyladenine). Four hormonal combinations were tested as follows:
Combination 1 or C1: 2 mg/L BAP + 0 mg/L 2-iP
Combination 2 or C2: 2 mg/L BAP + 0.25 mg/L 2-iP
Combination 3 or C3: 2 mg/L BAP + 0.5 mg/L 2-iP
Combination 4 or C4: 2 mg/L BAP + 1 mg/L 2-iP
The cultures were maintained for eight weeks under the same conditions as previously mentioned.
Assessment of the effect of light intensity on in vitro shoot multiplication and elongation
After two-month culture period, the shoots were transplanted into jars containing 50 mL of the previously selected best multiplication medium. The cultures were then incubated in culture chambers under varying light intensities (1000; 1500 and 2000 Lux).
Assessment of the effect of activated charcoal on in vitro leafy shoot multiplication and elongation
After two months of culture on multiplication medium, the shoots were transplanted onto a selected medium. Different concentrations (0, 0.5, 1, and 2 g/L) of activated charcoal were added to evaluate its effect on in vitro shoot multiplication and elongation.
In vitro shoot rooting
The shoots were individually seeded on MSB5 medium containing 100 mg/L myoinositol, 30 g/L sucrose, 200 mg/L glutamine, 1 mg/L NAA, 2 mg/L IBA, 2 g/L activated charcoal and solidified with 6 g/L agar.
Culture conditions
The test tubes and jars containing the explants were tightly closed and then sealed with stretch film. The cultures were randomly arranged and incubated in a culture room at a temperature of 25 ± 2 °C under a photoperiod of 16/8 hours (16 h of light and 8 h of darkness).
Acclimatization of in vitro regenerated plantlets
Well-rooted plantlets were gently removed from the agar medium. The roots were carefully washed with sterile distilled water to remove agar medium residues. Subsequently, the plantlets were transplanted into perforated polyethylene bags, previously filled with sterile sand. The bags containing the vitroplants were placed under a tunnel at room temperature which oscillate between 30 and 40 °C, for two months.
Statistical analysis
All cultures were placed in the light following a completely randomized design. Each treatment consisted of 30 jars, with one explant per jar, and was repeated three times. After 60 days of cultivation, the parameters (number and length of shoots) were using STASTICA 7.1 software was used for data analysis. A one-way Analysis of Variance (ANOVA) was performed and the means were compared using Fisher's LSD test at 5% threshold.
Conclusion
The study results highlight a protocol for producing microshoots of MD2 and H4 varieties. The combination of 2 mg/L of BAP and 2-iP (0.25 – 0.5 mg/L) induced better in vitro shoot proliferation in MD2 and H4 varieties. Light intensities of 1500 – 2000 lux are ideal for better shoot multiplication and elongation. Finally, the addition of 2 g/L of activated charcoal to the culture medium improves shoot elongation and rooting in MD2 and H4 varieties. The search for an efficient protocol to produce pineapple planting material would allow producers to provide highly productive material capable of revitalizing pineapple cultivation in Côte d’Ivoire
Conflict of interest
The authors declare that they have no conflict of interest regarding this paper.
Statement of contributions
OS, EZ, MT conceived and designed the study. OS and OKSK wrote the manuscript. HKK and OKSK provided guidance on the whole study and MKK improved the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
The authors are grateful to the National Center for Agronomic Research of Côte d'Ivoire for providing the technical platform for this research. The authors are also grateful to the Central Biotechnology Laboratory
References
Adeoye BA, Lawyer EF, Hassan KO, Ilesanmi AO, Richard-Olebe TC, Oyedeji TT, Aderemi TA, Ajongbolo FB, Adedeji AA (2020) Optimization of Plant Growth Regulator (PGR) on in vitro propagation of pineapple (Ananas comosus (L.) var. Smooth Cayenne). Int J Recent Res in Life Sci. 7(1): 13-20.
Ahmed S, Sharma A, Bhushan B, Wali VK, Bakshi P, Singh AK (2014) Studies on hardening and acclimitization of micropropagated plantlets of banana cv. Grand Naine. Bioscan. 9(3): 965-967
Aruna V, Kiranmai C, Karuppusamy S, Pullaiah T (2012) Influence of aseptic seedling explants on in vitro shoot multiplication of Caralluma adscendens var. attenuata Wight. Afr J Plant Sci. 6(11): 290-294.
Atawia AR, EL-Latif FM, EL-Gioushy SF, Sherif SS, Kotb OM (2016) Studies on Micropropagation of Pineapple (Ananas comosus L.). Middle East J Agric Res. 05(02): 224-232.
Chotangui AH, Kenhoung C, Mandou MS, Kouam EB (2019) Effect of different substrates on the mass production of vivo plantlets of smooth cayenne cultivar of pineapple (Ananas comosus) in the western highlands of Cameroon. Int J Curr Res Biosci Plant Biol. 6 (11): 1-8. Doi: https://doi.org/10.20546/ijcrbp.2019.611.001
Ding Z, Galván-Ampudia CS, Demarsy E, Langowski L, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, Firmil J (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol. 13 (4) : 447–452.
Emiru CG, Gerema A (2021) Control of browning in plant tissue culture: A review. J Sci Innov Res. 10(4): 89-93
FAO. (2020). Perspectives à moyen terme : perspectives concernant la production et le commerce mondiaux des bananes et des fruits tropicaux 2019-2028. Rome : Food and Agriculture Organization of the United Nations. http://www.fao.org/3/ca7568fr/ca7568fr.pdf
FAO (2023). https://www.fao.org/faostat/fr/?data/QC/visualize#data/QCL/visualize. Acces on 18/07/2024
Farhadi N, Panahandeh J, Azar AM, Salte SA (2017) Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sc. Hortic. 218 : 80–86.
Fernando SC, Santha ES, Hewarathna DJA (2010) Activated coconut shell charcoal as a component of tissue culture media of Cocos nucifera L. J Natn Sci Foundation Sri Lanka 38(3): 181-185.
Gehan S, Fatima AR, El Sharbasy S (2015) The effect of some antioxidants on blackening and growth of in vitro culture of banana (Musa spp.cv. Grand naine). Egypt J Genet Cytol. 44: 47-59
Harahap F, Suriani C, Poerwanto R, Siallagan J (2015) Sterilization of pineapple explant from Sipahutar, North Sumatera, Indonesia (Ananas comosus L.) and in vitro growth induction. Asian J Microbiol Biotech Env Sc. 17: 469–478.
Hemaid IS, Ghada AEl-MH (2013) In Vitro clonal propagation and molecular characterization of Jujube (Ziziphus Jujuba Mill.). Life Sci J. 10 (2) : 573-582.
Hosseini R, Moradnejad M, Nezami-Alanagh E, Ashrafi S, Ghane-Golmohammadi F (2013) Somatic embryogenesis and bulblet production in Narcissus papyraceus cv. Shirazi: effect of plant growth regulators, light intensity, sucrose concentration, methyl jasmonate and anti-gibberellins. Iranian J Genet Plant breed. 2 (1) : 27-34.
Ibrahim AI, Emara HA, Nower AA, Abodiab AY (2016) In vitro cultivation of potato plants. Int J Curr Microbiol App Sci. 5(12) : 858-868.
José RTR, Carlos ALG, María del CSG, Federico AGM, Nancy RL, Nancy SB (2023) Direct organogenesis in landrace pineapple induced by 6-benzylaminopurine. Rev Mexicana cienc agricole 14(6): 3159.
Kissoudis C, Seifi A, Yan Z, Islam ATM, Van der Schoot H, Van de Wiel CCM, Visser RGF, Van der Linden CG, Bai Y (2017) Ethylene and abscisic acid signaling pathways differentially influence tomato resistance to combined powdery mildew and salt stress. Front Plant Sci. 9 (7): 1-16.
Kouadio OKS, Yapo ESS, Kouassi K, Silue O, Koffi E, Kouakou TH (2017) Improved callogenesis and somatic embryogenesis using amino acids and plant growth regulators combination in pineapple [Ananas comosus (L.) Merr. (Bromeliaceae)]. European J Biotechnol Biosci. 5 (5): 06-16.
Kouadio OKS (2018). Effet de la composition du milieu de culture sur la régénération in vitro de l’ananas [Ananas comosus (L.) var. Cayenne lisse] par embryogenèse somatique indirecte et impact du stress salin sur les paramètres morphophysiologiques des vitroplants régénérés. Thèse de Doctorat de l’Université Nangui Abrogoua-Côte d’Ivoire, soutenue publiquement le 13 Octobre 2018, pp 238
Manal EAEA, Reda EE, Abo El-F, Mohamed NSS, Tamer M, Abd E (2021) Effect of micropropagation conditions on adventitious buds formation and the circadian expression of the ACO013229.1 gene in Ananas comosus. Egyptian J Desert Res. 71(2): 191-208.
Maqsood AL, Mushtaque AJ, Najamuddin S, Adel A, Abul‑S, Muneer AQ, Gholamreza A (2023) Optimizing in vitro nutrient and ex vitro soil mediums‑driven responses for multiplication, rooting, and acclimatization of pineapple. Sci Rep. 13:1275.
Meziani R, Jaiti F, Mazri MA, Anjarne M, Chitt MA, El Fadile J, Alem C (2015) Effects of plant growth regulators and light intensity on the micropropagation of date palm (Phoenix dactylifera L.) cv. Mejhoul. J Crop Sci Biotechnol. 18 (5): 325-331.
Mittal P, Devi R, Gosal SS (2016) Effect of genotypes and activated charcoal on gigh frequency in vitro plant regeneration in sugarcane. Indian J Biotechnol. 15: 261-265.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tabacoo tissue culture. Physiol Plant. 15: 473-497
Nayanakantha NMC, Singh BR, Kumar A (2010) Improved culture medium for micropropagation of Aloe vera L. Tropical Agricult Res Ext. 13(4): 87-93.
Periasamy S, Uma RS, Sreeramanan S, Maheran A, Aziz NR, Saikat G (2011) Effect of plant growth regulators and activated charcoal on in vitro growth and development of oil palm (Elaeis guineensis Jacq. var. Dura) zygotic embryo. A J Biotechnol. 10(52):10600-10606.
Praveen N, Naik PM, Manohar SH, Nayeem A, Murthy HN (2009) In vitro regeneration of brahmi shoots using semisolid and liquid cultures and quantitative analysis of Bacoside A. Acta Physiol Plant. 31(4): 723-728.
Priyanka D, Alok KS (2015) To study the effect of activated charcoal, ascorbic acid and light duration on invitro micropropagation of Aloe vera L. Int J Innov Res Sci Engin Technol. 4(5) : 3131-3138.
Rinaldi S, Feranita H, Muhammad R, Muhammad DR, Raham SK, Arjunayanti A, Trisnawaty AR (2016) Performance of NAA, 2iP, BAP and TDZ on callus multiplication, shoots initiation and growth for efficient plant regeneration system in chrysanthemum (Chrysanthemum morifolium Ramat.). Int J Agric Syst. 4 (1): 52 – 61.
Sanputawong S, Raknim T, Benchsri S (2015) Influence of different type of culture media and activated charcoal on callus induction and shoot multiplication of Cardamine lyrata. J Agricult Technol. 11(8):1697-1704.
Sani LA, Usman IS, Nasir AU, Abdulmalik MM (2019) Micropropagation of pineapple (Ananas comosus L. var. Smooth cayenne) in temporary immersion bioreactor system (TIPS). Bayero J Pure App Sci. 12(2): 207 - 209.
Souza FVD, Souza AS, Santos-Serejo JA, Souza EH, Junghans TG, Silva MJ (2013) Micropropagação do abacaxizeiro e outras bromeliáceas. In T. G. Junghans & A. S. Souza (Eds.), Aspectos práticos da micropropagação de plantas (2a. ed., p. 177-205). Brasília, DF: Embrapa.
Van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: Cytokinins, their analogues and antagonists. In E. F. George, M. Hall & G. J. De Kleck, Plant propagation by tissue culture, 1: 205-226. Dordrecht, Netherlands: Spring
Zuraida ABR, Hartinee A, Ayu N (2018) “Rapid micropropagation of MD2 pineapple (Ananas comosus L.) using the Temporary Immersion System (TIS) and liquid-shake culture (LSC)”, Asian J Sci Technol. 09(10):8833-8836.