

Aust J Crop Sci. 18(09):539-546 (2024)
ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.09.p65
Quantitative eco-anatomical analysis reveals distinctive stem traits of six Cannabis sativa cultivars in Rif Mountains (Northern Morocco)
Ismail El Bakali1*, Soufian Chakkour1, Samir El Bakali2,3, Aboubakr Boutahar1, Mohamed Kadiri1, Abderrahmane Merzouki1
1Laboratory of Applied Botany, Department of Biology, Faculty of Sciences of Tetuan, Abdelmalek Essaâdi University, Mhannech II. 93002, Tetuan, Morocco
2Laboratory of Biology, Ecology, and Health, FS, Abdelamlek Essaadi University, Mhannech II. 93002, Tetuan, Morocco
3Marine Ecotoxicology Team, Department of chemistry, Faculty of Sciences of Tetuan, Abdelmalek Essaâdi University, Mhannech II. 93002, Tetuan, Morocco
Abstract
Cannabis sativa stands out as an important crop with significant socioeconomic importance in the Rif Mountains of North Morocco. Recently the number of new cultivars developed in this region has gradually increased for different purposes. In this current study, we delve into the eco-anatomical characteristics of stems across six of the most prevalent Cannabis sativa cultivars in Morocco: ‘Avocat’, ‘Beldiya’, ‘Critical Plus’, ‘Industriel’, ‘Khardala’, and ‘Mexicana’. The investigation encompasses the measurement and analysis of six key eco-anatomical traits: plant height, stem diameter, vessel size, vessel density, number of vessels joined in radial files, and coefficient of hydraulic conductivity. Our aim is to elucidate the variations among these cultivars and underscore their agricultural and ecological relevance. Through anatomical examinations of stem cross-sections, our findings reveal notable disparities and distinctive features among the cultivars. The ‘Khardala’ cultivar exhibits the highest values across most measured eco-anatomical traits, while ‘Beldiya’ and ‘Mexicana’ show similar trait values, particularly with low stem diameter and vessel density. In contrast, ‘Industriel’ stands out with the lowest vessel size and highest vessel density. ‘Critical Plus’ and ‘Avocat’ closely resemble ‘Khardala’ in vessel size and stem diameter. Regarding hydraulic conductivity, ‘Khardala’ ranks highest, followed by ‘Avocat’ and ‘Critical Plus’, while ‘Industriel’ registers the lowest. Our findings underscore increased water demand and uptake potential in ‘Khardala’, ‘Critical Plus’, and ‘Avocat’, contrasting with ‘Industriel’, ‘Beldiya’, and ‘Mexicana’. Anatomical diversity among Cannabis cultivars in our study likely stems from genetic lineage and ecological adaptation.
Keywords: Hemp, functional traits, cultivars discrimination, northern Morocco, eco-anatomical analysis, stem anatomy.
Abbreviations: ANOVA_analysis of variance, C. sativa_Cannabis sativa, CI_confidence interval, DM-stem section diameter, DVS_Stem vessel density, NVS_number of vessels joined in radial files, SVS_stem vessel size, THC_Tetrahydrocannabinol.
Introduction
Cannabis sativa L. (C. Sativa), commonly referred to as hemp or ‘Kif’ in the Moroccan local dialect, is a dioecious herbaceous annual plant belonging to the Cannabaceae family and originating from Central and South-East Asia (Miller, 1970; Wu et al., 2003; Jiang et al., 2006; Stevens et al., 2016; Bonini et al., 2018). Its hollow aerial stem are reinforced in the radical direction by nodes at distances of ~5 cm. With an outer diameter of ~4 cm it grows to a height of ~3 m. Hamp is one of the earliest known cultivated plants since agricultural farming started around 10,000 years ago (Schultes et al., 1974; Li, 1974). It is an emblematic example of a multi-purpose crop plant with diverse agricultural and industrial applications ranging from the production of paper, wood, and fiber, to potential use in the medicinal and pharmaceutical industries (Hanus, 2009; Whiting et al., 2013; Savo et al., 2013; Tang et al., 2016; Bonini et al., 2018; El Ghacham et al., 2023). The first-ever report to reveal the prospects of C. sativa L. as a medicinal plant was already published in 1843 and described the use of plant extracts to treat patients suffering from tetanus, hydrophobia, and cholera (O’Shaughnessy, 1843).
The cultivation of cannabis in Morocco, particularly in the Rif mountains, has been a longstanding practice dating back to a period between the 7 th and the 15 th century, yet its precise origins remain obscure (Chouvy, 2008). It is believed that cannabis was initially cultivated for the production of both kif (chopped cannabis herb smoked in pipes) and hashish (resin-based drug) (Chouvy & Afsahi, 2014). Despite cannabis cultivation being illegal since the country's independence, Morocco continues to hold the title of the world's largest producer of cannabis resin, as reported by the United Nations (UNODC, 2022).
The Cannabis cultivation sector has witnessed significant development in recent centuries. Initially centered around the cultivation of unique cultivars called ‘Beldiya’, which are native varieties well-suited to the challenging conditions of the Rif mountains and cultivated through generations of farmer expertise, the sector has now shifted towards the adoption of modern farming techniques and the introduction of new hybrid high yielding varieties (Chouvy & Macfarlane, 2018; Clarke, 1998). The introduction of these new cultivars is primarily aimed at boosting hashish production due to their higher resin yields and potency (Chouvy & Afsahi, 2014; El Bakali et al., 2022). However, the origins and morpho-anatomical characteristics of these new cultivars remain largely unknown, and their response to environmental and farming conditions has led to alterations in crop cycles and resource requirements. Nonetheless, this transition poses a threat to local cannabis through genetic contamination, depletion, and pollution of the region’s already scarce water resources (Bachir et al., 2022).
Hydric stress is a defining characteristic of the Mediterranean climate, distinguished by more pronounced warming and drying trends compared to other climatic ecoregions. These trends are driven by higher temperatures and shifts in precipitation patterns (Choat et al., 2018; Cramer et al., 2018). In Morocco, these features are particularly evident, with the escalating frequency and severity of drought periods over the last two decades. This is largely attributed to global climate change and water scarcity. Additionally, the increasing replacement of resilient local seed varieties by newly introduced seed varieties, especially cannabis in the Rif mountains, worsens the situation. This has led to heightened competition for water resources among farmers who are utilizing various water catchment methods to meet the demands of these new cultivars (CARE, 2023).
Face of this situation, detailed knowledge of plant anatomical traits and their variation among closely related taxa is key to understanding their evolution and function (Du et al.2020; Schweingruber et al., 2014; Carlquist, 1969). Eco-anatomical plant traits refer to the structural features of plants that are closely linked to their ecological functions and adaptations to the environment (Zanne et al., 2010; Poorter & Bongers, 2006; Wright et al., 2004). These traits provide insights into the evolutionary strategies that plants utilize to thrive in specific ecological conditions, such as climate, soil type, or nutrient availability (Gleason & Westoby, 2007; Zanne & Falster, 2010). Generally, variations in plant anatomy are expected to result in variations in physiological functions. As a result, the unique morphological structure and physiological functions of plants enable them to tolerate changes in environmental conditions to different degrees (Schweingruber et al., 2014). Stem anatomical traits have captured the attention of scientists due to their correlation with various crucial plant functions, including growth rate, water storage, mechanical strength, efficiency, and safety of hydraulic transport, as well as resistance to herbivory (Jacobsen et al., 2007; Sperry et al., 2008; Chave et al., 2009; Maguire & Kobe, 2015; Furze et al., 2019; Puntieri & González, 2023).
One of the most extensively researched anatomical stem traits revolves around vessel size and density, which are crucial factors in determining a plant's hydraulic efficiency (Pandey, 2021). Vessels serve as the primary conduits in angiosperms, and the flow rates per vessel are positively correlated with the cross-sectional area of their lumens (Tyree & Zimmermann, 2002). Nevertheless, the significance of vessel diameter in explaining the efficiency of water transport is well-documented (Rosell et al., 2017; Guo et al., 2017; Rodriguez-Zaccaro et al., 2019, Lens et al., 2022).
In this study, we investigate the stem eco-anatomical traits of six C. sativa cultivars from the Rif Mountains, which have not previously undergone anatomical analysis. Our main objective is to compare the eco-anatomical traits of stems among these cultivars and delve into their possible correlation with ecological factors, particularly focusing on water uptake and conductivity.
Results
Eco-anatomical trait variations among C. sativa cultivars
As summarized in Table 2, significant variations in mean height were observed among the six cannabis cultivars. Utilizing ANOVA and Tukey's test allowed for the descrimination of these cultivars into four distinct groups. Among them, ‘Industriel’ and ‘Khardala’ exhibited the tallest stature, with no significant difference observed between them but with noticeable disparities when compared to the remaining cultivars. Conversely, ‘Beldiya’ and ‘Mexicana’ displayed the lowest height measurements, demonstrating significant deviations from the other cultivars. Additionally, ‘Avocat’ and ‘Critical Plus’ showcased intermediate height values and were found to be significantly different from each other as well as from the remaining cultivars.
The mean stem diameter results revealed variations among the cultivars. Specifically, the cultivar ‘Khardala’ exhibited the largest DM (5.7 ± 3.1 cm) in comparison to the other cultivars. A significant difference was observed only between ‘Khardala’ with the highest DM and ‘Beldiya’ with the lowest DM (2.8 ± 1.2 cm). No significant differences were found among the other cultivars (Table 2).
The DVS results showed significant differences, particularly between ‘Industriel’ and 'Beldiya,' with the former recording the highest density at 120.6 ± 11.5 vessels/mm², and the latter having the lowest at 79.3 ± 8.8 vessels/mm². However, among the remaining cultivars, there were no substantial variations in vessel density, indicating no significant difference compared to the two aforementioned cultivars.
The cultivar ‘Avocat’ had the highest mean NVS, at 2.9 ± 0.43. The cultivars ‘Industriel’, ‘Mexicana’, ‘Beldiya’ and ‘Khardala’ followed closely behind with respective averages of 2.7 ± 0.34, 2.7 ± 0.28, 2.7 ± 0.26 and 2.6 ± 0.25. In contrast, the cultivar ‘‘Critical Plus’ recorded the lowest value, with an average of 2.5 ± 0.23. Nevertheless, the ANOVA with Tukey's post hoc test indicated significant differences between the cultivars ‘Khardala’ and ‘Industriel’, while no significant differences were observed between the other cultivars or between them and the two aforementioned cultivars.
The SVS results indicated significant differences among the cultivars that were studied. The cultivar ‘Khardala’ had the highest mean SVS value (1847.5 ± 226.6 µm2), while the cultivar ‘Industriel’ had the lowest mean value (923.2 ± 107.6). The remaining cultivars, namely ‘Avocat’, ‘Critical Plus’, ‘Mexicana’, and ‘Beldiya’, had intermediate positions with mean values of 1551.4 ± 197.2, 1403.4 ± 159.7, 1304.6 ± 129.1, and 1147.1 ± 131.5 respectively.
The results of CD showed no significant differences. The highest average values were observed in the cultivars ‘Critical Plus’, ‘Avocat’, and ‘Beldiya’, with averages of 12553.1 ± 4103, 4230.5 ± 1846, and 3884.8 ± 1359, respectively. On the other hand, the lowest CD value was recorded in the cultivar, with an average of 1034.3 ± 275 (Table 2).
Correlations among stem eco-anatomical traits
Pearson's correlation tests among stem eco-anatomical traits revealed several statistically significant relationships at p-values of < 0.05, < 0.01, and < 0.001 (Table 3). Plant height demonstrated positive correlations with most examined traits, except NVS and SVS. Significant positive correlations were found between SVS and CD, CD and DM, as well as SVS and DM. Conversely, significant negative correlations were observed between SVS and DVS, CD and DVS, and DVS and DM (Table 3).
Cluster analysis
The results of the cluster analysis revealed the distinction of six cannabis cultivars (Figure 2 ) into four distinct clusters, determined by the similarity of their morpho-anatomical traits. Specifically, Cluster 1 comprised the ‘Khardala’ cultivar, Cluster 2 showcased the ‘Industriel’ cultivar, Cluster 3 encompassed both the ‘Critical Plus’ and ‘Avocat’ cultivars, and Cluster 4 comprised the ‘Beldiya’ and ‘Mexicana’ cultivars (Figure 2).
Discussion
The examination of eco-anatomical traits is imperative for a comprehensive understanding of cultivar and variety identification, as well as for evaluating their correlation with resource demands and agricultural conditions. Previous research indicates that the vascular anatomy plays a crucial role in the acclimation potential of plants (Levanic et al., 2011; Kim et al., 2014; Tietjen et al., 2017; Mirwais et al., 2019).
Despite being cultivated under identical plantation conditions, the morpho-anatomical traits of the sampled plants exhibited significant variations. The ‘Khardala’ cultivar distinguishes itself by exhibiting the highest values across nearly all measured eco-anatomical traits. Specifically, it showcases a substantial height, DM, elevated DVS, and a large SVS. These characteristics collectively contribute to its notably high CD. Both the ‘Avocat’ and ‘Critical Plus’ cultivars demonstrate trait values similar to ‘Khardala’ in terms of SVS and stem DM, may indicating a comparable high water water uptake and transport. In contrast, the ‘Beldiya’ cultivar exhibits noteworthy distinctions from other cultivars, particularly in DM and DVS. It records the lowest values for the majority of the measured traits and has a smaller CD value, suggesting better adaptation to water deficiency and scarcity. The ‘Mexicana’ cultivar demonstrates traits closely aligned with ‘Beldiya’, particularly in DVS. Furthermore, the ‘Industriel’ cultivar exhibits significant differences compared to other cultivars, specifically in SVS with the lowest value and DVS with the highest value. This results in the CD, indicating mreduced efficiency in water transport.
These findings highlight the increased water demand and uptake potential observed in ‘Khardala’, ‘Critical plus’, and ‘Avocat’, contrasted with the opposite trend seen in ‘Beldiya’ and ‘Mexicana’. This discrepancy may be attributed to the genetic characteristics of the first three cultivars, which exhibit heightened irrigation demands and are typically cultivated using intensive and modern farming methods. Conversely, the ‘Beldiya’ cultivar appears better adapted to abiotic factors and is characterized by a relatively short life cycle (Chouvy & Macfarlane, 2018).
In addition to the critical role of stem vessel diameter in sap uptake and conductivity, it may also contribute to increased susceptibility to drought stress in plants (Baas & Schweingruber, 1987; Lens et al., 2007; Schweingruber et al., 2014; Puntieri & González, 2023). Levanic et al. (2011) suggested that under drought conditions, plants with larger vessel diameters may exhibit greater vulnerability to drought stress compared to those with smaller vessel diameters.
The correlation results reveal a significant relationship between the Hydraulic Conductivity Coefficient (CD) and both Vessel Surface (SVS) and Vessel Density (DVS). Specifically, a positive correlation exists between CD and SVS, while a negative correlation is observed between CD and DVS. This suggests that as SVS increases, CD also increases, whereas as DVS decreases, CD increases. Conversely, an increase in DVS corresponds to an increase in CD, while SVS decreases. Therefore, this implies that C. sativa's hydraulic conductivity is more closely associated with SVS than DVS. In line with this, several studies have emphasized that the vascular conductivity of plants is influenced by their anatomical characteristics and vessel structures. It has been noted that vascular conductivity tends to rise with larger vessel sizes and diameters (Choat et al., 2005; Petit et al., 2008; Tyree & Zimmermann, 2002; Olson & Rosell, 2013; Jensen et al., 2016; Skelton et al., 2018; Soriano et al., 2020; De Moraes et al., 2022). The negative correlation observed in our results between SVS and DVS underscores the plant's inclination to establish an equilibrium between SVS and DVS. Similarly, Kassout et al. (2021) highlighted this negative correlation in vascular plants experiencing low hydric deficits, emphasizing their propensity to maintain a balance between SVS and DVS. This adaptive strategy enables the plants to sustain a relatively consistent sap conductance and water supply. Therefore, our results may be explained by the fact that cannabis cultivars exhibit genetic variability in how they respond to their original ecological conditions. This genetic variability allows them to show distinct anatomical responses, even when they are grown under identical farming conditions. Several studies indicate that genetic plasticity tends to decrease in cultivated plants when compared to their wild counterparts (Sanad et al., 2016; Zhang et al., 2017; Hauvermale et al., 2020).
Different cultivars of cannabis can be distinguished based on the anatomical characteristics of their stems. The indigenous cannabis variety known as ‘Beldiya’ exhibits the smallest plant height and DM values, indicating potential adaptation to the local climate and environmental conditions. This adaptation is reflected in its stem anatomy, which features a lower SVS, reduced DVS, and decreased CD. Similarly, the ‘Mexicana’ cultivar shares significant morpho-anatomical traits with ‘Beldiya’, suggesting a possible genetic relationship between the two cultivars. It is plausible that ‘Mexicana’ is a hybrid cultivar originating from ‘Beldiya’. The ‘Critical Plus’ and ‘Avocat’ cultivars demonstrated considerable similarity, displaying intermediate morpho-anatomical trait values distinct from other varieties. They exhibited relatively high water uptake and conductivity compared to other cultivars, although not as pronounced as the ‘Khardala’ cultivar, which displayed notably larger SVS and CD. These observations suggest a high resource demand for these introduced cultivars, emphasizing the need for specific agricultural practices and attention to ensure their survival in the climatic conditions of the Rif Mountains. The ‘Industriel’ cultivar exhibited unique characteristics in comparison to other varieties, featuring a small DM and considerable height. This resulted in a high DVS but relatively small SVS, ultimately contributing to the cultivar's lowest coefficient of CD. These traits are likely attributed to genetic selection, specifically targeted for fiber density enhancement.
Materıals and methods
Plant material and growing conditions
This study centered on six of the most commonly cultivated Cannabis sativa L. cultivars in the Rif Mountains, namely: ‘Beldiya’, a Moroccan cultivar well-suited to the environmental conditions of the Rif Mountains; ‘Avocat’, ‘Critical Plus’, ‘Khardala’, and ‘Mexicana’, introduced as high-yielding cultivars renowned for their elevated THC levels; and ‘Industriel’, an introduced cultivar from Spain primarily utilized for fiber production. The cultivars are named based on local designations. All these cultivar seeds were sourced from farmers in Bab Taza locality in the Chefchaouen province, northwest Morocco. Detailed information for the six cultivars is presented in Table 1.
The cultivar seeds were sown in black plastic pots, each measuring 30 cm in height and 25 cm in diameter. These pots were filled with 10 liters of commercial peat substrate and placed in an greenhouse at the Faculty of Sciences of Tétouan (located at 35°33'38"N 5°21'46"W, 10 meters above sea level) in April 2018. Throughout the growing season in the greenhouse, day and night temperatures were maintained between 20–45°C and 10–30°C, respectively, with a relative humidity ranging from 60% to 80% and a natural photoperiod. The seedlings were irrigated every two days, three times a day, with each watering session lasting for two minutes.
Morphological and anatomical observations
After maturation, we randomly selected 15 female plants from each cultivar for measurements. Initially, the plants were dried, and their leaves were removed. The stems were transported in plastic bags to the laboratory for measurements and cuts.
Haut du formulaireFor each stem, three 2 cm sections were obtained from internodes using razor blades. These sections were taken from the lower (between nodes 1-2), middle (nodes 4-5), and upper parts (nodes 8-9) of the stem, starting from the ground level and moving towards the top of the plant. The sections were then soaked in water for one day. Stem cross sections of 8μm were obtained with a sliding microtome (G.S.L.1, Microm International GmbH, Walldorf, Germany). The sections treated with sodium hypochlorite diluted to 10% before staining with safranin and fast green. Subsequently, they progressively dehydrated by passing through successive alcohol baths at 40, 50, and 100°. Finally, the sections mounted on slides with synthetic Canadian balsam for observation and measurements. Sections were observed under a light microscope (Olympus BX43) and images were captured using a microscope attached camera (Toupcam U3CMOS).
Vessel traits were assessed in the stem sections of C. sativa cultivars using optical magnification at 100×, 200×, and 400× through digital image analysis software (Toupview 3.7). A total of six morpho-anatomical traits were measured. Alongside plant height and stem section diameter (DM), three anatomical parameters linked to sap conduction were evaluated for each stem section: vessel density (DVS), defined as the number of vessels per square millimeter of cross-sectional area; number of vessels joined in radial files (NVS); and the vessel size (SVS) for each individual vessel in µm² (also referred to as vessel surface area) (Figure 1).
To evaluate the vascular conductivity of each cultivar, we determine the Hydraulic Conductivity Coefficient (CD) using the formula:
$$CD = \frac{{(\frac{SVS}{\pi})}^{2}}{\begin{matrix} DVS \\ \\ \end{matrix}}$$
Where: SVS represents the vessel surface area, π is the ratio of a circle's circumference to its diameter (≈ 3.14), and DVS corresponds to vessel density (Zimmermann, 1983; Carlquist, 1988).
Statistical analysis
Descriptive statistics were employed to analyze the trait data. The mean value of 30 replicates for DVS and 50 replicates for SVS and NVS, as well as the confidence interval (CI), were calculated for all measured traits based on the three diameter section values. We used analysis of variance (ANOVA) and Tukey's post hoc tests for pairwise comparisons to test for differences. Morover, we employed Pearson's correlation test to assess relationships among measured eco-anatomical traits. A classical hierarchical cluster analysis was conducted utilizing Bray-Curtis disimilarity distance to delineate assemblages according to the eco-anatomical trait values of cultivars. The PAST 4 statistical software package (Hammer et al., 2001) was used to perform all the statistical analysis.
Conclusion
This study contributes new insights into the anatomical characteristics of stems across six C. sativa cultivars in Morocco. Variations in stem anatomy were observed among the five species investigated, particularly in terms of plant height, vessel size, vessel density, and the coefficient of vascular conductivity. The considerable anatomical diversity observed among Cannabis cultivars within our study material can be attributed to both genetic descent and ecological adaptation. Our findings aid in identifying cultivars suitable for cultivation in the Rif Mountains while minimizing water resource risks, highlighting the ‘Beldiya’ and ‘Mexicana’ cultivars as better adapted to the study area's abiotic factors. In contrast, introduced high-yield cultivars display anatomical traits linked to increased water demand, potentially compromising their resilience to the Moroccan climate and weather patterns. Hence, there is a pressing need for increased attention and political backing to regulate the introduction of new cultivars while safeguarding and preserving local varieties. This endeavor aims to conserve resources and prevent seed contamination effectively.
Acknowledgments
We thank all members of the applied botany team for their valuable feedback and suggestions. The authors are grateful to the reviewers for their useful suggestions and comments.
Statements and Declarations
Funding: No funding was received for conducting this study.
Competing Interests: The authors declare that they have no conflict of interest.
Ethics approval: Not applicable.
Availability of data and material: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions: Ismail El Bakali: Conceptualization, Data collection, Methodology, Investigation, Writing - original draft. Soufian Chakkour: Writing – review & editing, Statistical analysis, Visualization. Samir El Bakali: Data collection, Writing - original draft. Aboubakr Boutahar: Investigation, Writing - original draft. El Hassan Sakar: Software, Writing – original draft. Mohamed Kadiri: Identified the botanical taxa, Methodology, Writing - original draft. Abderrahmane Merzouki: Conceptualization, Methodology, Supervision, Writing - original draft.
References
Baas P, Schweingruber FH (1987) Ecological trends in the wood anatomy of trees, shrubs and climbers from Europe. IAWA J. 8:245–74.
Bachir F, Eddouks M, Arahou M, Fekhaoui M (2022) Origin, Early History, Cultivation, and Characteristics of the Traditional Varieties of Moroccan Cannabis sativa L. Cannabis Cannabinoid Res. 7(5): 603-615.
Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, & Mastinu A (2018) Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 227: 300-315. https://doi.org/10.1016/j.jep.2018.09.004.
CARE by Ayham Taha (2023) Morocco: Drought Assessment Report Brief.
Carlquist S (1969) Wood anatomy of Lobelioideae (Campanulaceae). Biotropica. 1:47–72.
Carlquist S (1988) Comparative Wood Anatomy. Systematic, Ecological and Evolutionary Aspects of Dicotyledons Wood. Springer, Heidelberg.
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett. 12(4): 351-366.
Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature. 558: 531–539. https://doi.org/10.1038/s41586-018-0240-x.
Chouvy PA (2008) Production de cannabis et de haschich au Maroc: contexte et enjeux. L’Espace Politique. Revue en ligne de géographie politique et de géopolitique, (4).
Chouvy PA, Afsahi K (2014) Hashish revival in Morocco. Int J drug policy. 25(3): 416-423.
Chouvy PA, Macfarlane J (2018) Agricultural innovations in Morocco’s cannabis industry. Int J drug policy. 58: 85-91.
Clarke RC (1998) Part III: hashish cultures. In:Hashish!. Red Eye Press: Los Angeles, CA: 175.
Cramer W, Guiot J, Fader M, Garrabou J, Gattuso JP, Iglesias A, Lange MA, Lionello P, Llasat MC, Paz S, Peñuelas J, Snoussi M, Toreti A, Tsimplis MN, Xoplaki E (2018). Climate change and interconnected risks to sustainable development in the Mediterranean. Nat Clim Chang. 8(11): 972-980.
De Moraes DHM, Mesquita M, Graciano-Ribeiro D, De Araújo D S, Battisti R, Flores RA, De Melo HC, Casaroli D (2022) The effect of xylem vessel diameter on potential hydraulic conductivity in different rice stem longitudinal positions. Flora. 295: 152147. https://doi.org/10.1016/j.flora.2022.152147.
Du Q, Jiao X, Song X, Zhang J, Bai P, Ding J, Li J (2020) The response of water dynamics to long-term high vapor pressure deficit is mediated by anatomical adaptations in plants. Front Plant Sci. 11: 758. https://doi.org/10.3389/fpls.2020.00758.
El Bakali I, Sakar EH, Boutahar A, Kadiri M, Merzouki A (2022) A comparative phytochemical profiling of essential oils isolated from three hemp (Cannabis sativa L.) cultivars grown in central-northern Morocco. Biocatal Agric Biotechnol. 102327. https://doi.org/10.1016/j.bcab.2022.102327
El Ghacham S, El Bakali I, Zarouki MA, Aoulad El Hadj Ali Y, Ismaili R, El Ayadi A, Souhail B, Tamegart, L Azzouz A (2023) Wound healing efficacy of Cannabis sativa L. essential oil in a mouse incisional wound model: A possible link with stress and anxiety. S Afr J Bot. 163:488-496. https://doi.org/10.1016/j.sajb.2023.11.005
Furze ME, Huggett BA, Aubrecht DM, Stolz CD, Carbone MS, Richardson AD (2019). Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 221: 1466-1477. doi: 10.1111/nph.15462.
Gleason SM, & Westoby M (2007) J. W. Shipley revisited: a meta-analysis of plant responses to competition. New Phytol. 176(3): 528-545.
Guo SP, Chi Y, Guo GC (2017) Recent achievements on middle and far-infrared second-order nonlinear optical materials. Coord Chem Rev. 335: 44-57.
Hammer M (2001). The superefficient company. Harv Bus Rev. 79(8): 82-93.
Hanus LO (2009) Pharmacological and therapeutic secrets of plant and brain (endo) cannabinoids. Med Res Rev. 29: 213–271. https://doi.org/10.1002/med.20135.
Hauvermale AL, Sanad MNME (2020) Phenological plasticity of wild and cultivated plants. Plant Communities and Their Environment, 8. IntechOpen. doi:10.5772/intechopen.85070.
Jacobsen MH, Tester K (2007) Sociology, nostalgia, utopia and mortality: A conversation with Zygmunt Bauman. Eur J Soc Theory. 10(2): 305-325.
Jensen KH, Berg-Sørensen K, Bruus H, Holbrook NM, Liesche J, Schulz A, Zwieniecki MA, Bohr T (2016) Sap flow and sugar transport in plants. Rev Mod Phys. 88: 035007 https://doi.org/10.1103/RevModPhys.88.035007.
Jiang HE, Li X, Zhao YX, Ferguson DK, Hueber F, Bera S, Wang YF, Zhao LC, Liu CJ, Li CS (2006) A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J Ethnopharmacol. 108(3): 414-422. https://doi.org/10.1016/j.jep.2006.05.034
Kassout J, Ater M, Ivorra S, Ros J, Paradis L, Terral JF (2021) Resisting aridification: adaptation of sap conduction performance in Moroccan wild olive subspecies distributed over an aridity gradient. Front Plant Sci. 12: 663721.
Kim HK, Park J, & Hwang I (2014) Investigating water transport through the xylem network in vascular plants. J Exp Bot. 65(7): 1895-1904. https://doi.org/10.1093/jxb/eru075
Lens F, Baas P, Jansen S, Smets E (2007) A search for phylogenetically informative wood characters within Lecythidaceae sl. Am J Bot. 94:483–502.
Lens F, Gleason SM, Bortolami G, Brodersen C, Delzon S, Jansen S (2022) Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 236: 2019-2036. doi:10.1111/nph.18447.
Levanic T, Cater M McDowell NG (2011) Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol. 31(3):298-308. doi: 10.1093/treephys/tpq111. Epub 2011 Mar 1. PMID: 21367747.
Li H (1974) An archaeological and historical account of Cannabis in China. Econ Bot. 28(4): 437-448. https://doi.org/10.1007/bf02862859
Maguire AJ, Kobe RK (2015) Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol Evol. 5(23): 5711-5721.
Miller NG (1970) The genera of Cannabaceae in the southeastern United States. J Arnold Arbor. 51(2):185–203.
Mirwais S, Kazmi SH, Hussain SI, Mirwais M, & Sharma A (2019) Hypothyroidism causing pericardial effusion: a case report. Cureus. 11(12).
Olson ME, Rosell JA (2013) Vessel diameter–stem diameter scaling across woody angiosperms and the ecological causes of xylem vessel diameter variation. New Phytol. 197: 1204–1213. https://doi.org/10.1111/nph.12097
O'Shaughnessy WB (1843) On the preparations of the Indian hemp, or Gunjah: Cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 5(123): 363. https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC2490264.
Pandey S (2021) Climatic influence on tree wood anatomy: A review. J Wood Sci. 67(1): 1-7. https://doi.org/10.1186/s10086-021-01956-w.
Petit G, Anfodillo T, Mencuccini M (2008) Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol. 177: 653–664. https://doi.org/10.1111/j.1469-8137.2007.02291.x.
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology. 87(7): 1733-1743.
Puntieri J, González AM (2023) Are the anatomical traits of stems and leaves good indicators of habitat specificity in closely related Myrtaceae species from Patagonia? Acta bot. bras. 37: e20230019. https://doi.org/10.1590/1677-941X-ABB-2023-0019.
UNODC (2022) World drug report 2022. Vienna: UNODC.
Rodriguez‐Zaccaro FD, Valdovinos‐Ayala J, Percolla MI, Venturas MD, Pratt RB, Jacobsen AL (2019) Wood structure and function change with maturity: Age of the vascular cambium is associated with xylem changes in current‐year growth. Plant Cell Env. 42(6): 1816-1831.
Rosell JA, Olson ME, Anfodillo T (2017) Scaling of Xylem Vessel Diameter with Plant Size: Causes, Predictions, and Outstanding Questions. Curr Forestry Rep. 3:46–59. https://doi.org/10.1007/s40725-017-0049-0
Sanad MNME, Campbell KG, Gill KS (2016) Developmental program impacts phenological plasticity of spring wheat under drought. Bot Stud. 57:1-35
Savo V, La Rocca A, Caneva G, Rapallo F, Cornara L (2013) Plants used in artisanal fisheries on the Western Mediterranean coasts of Italy. J Ethnobiol Ethnomed. 9: 9. https://doi.org/10.1186/1746-4269-9-9.
Schultes RE, Klein WM, Plowman T, Lockwood TE (1974) Cannabis: An example of taxonomic neglect. Bot Mus leaf Harv Univ. 23(9): 337-367.
Schweingruber FH, Rıha P, Dolezˇal J (2014) Variation in stem anatomical characteristics of Campanuloideae species in relation to evolutionary history and ecological preferences. PLoS ONE. 9(2): e88199. doi:10.1371/journal.pone.0088199
Skelton RP, Dawson TE, Thompson SE, Shen Y, Weitz AP, Ackerly D (2018) Low vulnerability to xylem embolism in leaves and stems of North American oaks. Plant Physiol. 177: 1066–1077. https://doi.org/10.1104/pp.18.00103.
Soriano D, Echeverría A, Anfodillo T, Rosell JA, Olson ME (2020) Hydraulic traits vary as the result of tip-to-base conduit widening in vascular plants. J Exp Bot. 71: 4232–4242. https://doi.org/10.1093/jxb/eraa157.
Sperry JS, Meinzer FC, & McCulloh KA (2008) Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant, Cell Environment. 31(5): 632-645.
Stevens CJ, Murphy C, Roberts R, Lucas L, Silva F, Fuller DQ (2016). Between China and South Asia: a middle Asian corridor of crop dispersal and agricultural innovation in the bronze age. Holocene. 26(10): 1541–1555. https://doi.org/10.1177/0959683616650268.
Tang K, Struik PC, Yin X, Thouminot C, Bjelková M, Stramkale V, Amaducci S (2016) Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind. Crops Prod. 87: 33-44. https://doi.org/10.1016/j.indcrop.2016.04.026.
Tietjen B, Schlaepfer DR, Bradford JB, Lauenroth WK, Hall SA, Duniway MC, Hochstrasser T, Jia G, Munson SM, Pyke DA, and Wilson SD (2017) Climate change-induced vegetation shifts lead to more ecological droughts despite projected rainfall increases in many global temperate drylands. Glob Change Biol. 23: 2743-2754. https://doi.org/10.1111/gcb.13598
Tyree M T, Zimmermann MH (2013) Xylem structure and the ascent of sap. Springer Science & Business Media.
Tyree MT, & Zimmermann MH (2002) Xylem Structure and the Ascent of Sap. Springer: Berlin/Heidelberg, Germany.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J (2015) Cannabinoids for medical use: a systematic review and metaanalysis. JAMA. 313: 2456-2473. https://doi.org/10.1001/jama.2015.6358.
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villa R (2004) The worldwide leaf economics spectrum. Nature. 428(6985): 821-827.
Wu Z, Zhou Z-K, Bartholomew B (2003) Cannabaceae. In: Wu Z, Raven PH (eds) Flora of China. Science Press, Beijing.
Zanne AE, Falster DS (2010) Plant functional traits – linkages among stem anatomy, plant performance and life history. New Phytol. 185(2): 348-351. https://doi.org/10.1111/j.1469-8137.2009.03135.x
Zhang H, Mittal N, Leamy LJ, Barazani O, Song B-H (2017) Back into the wild; applying untapped genetic diversity of wild relatives for crop improvement. Evol Appl. 10:5-24
Zimmermann MH (1983) Xylem structure and the ascent of Sap. Springer, Berlin.
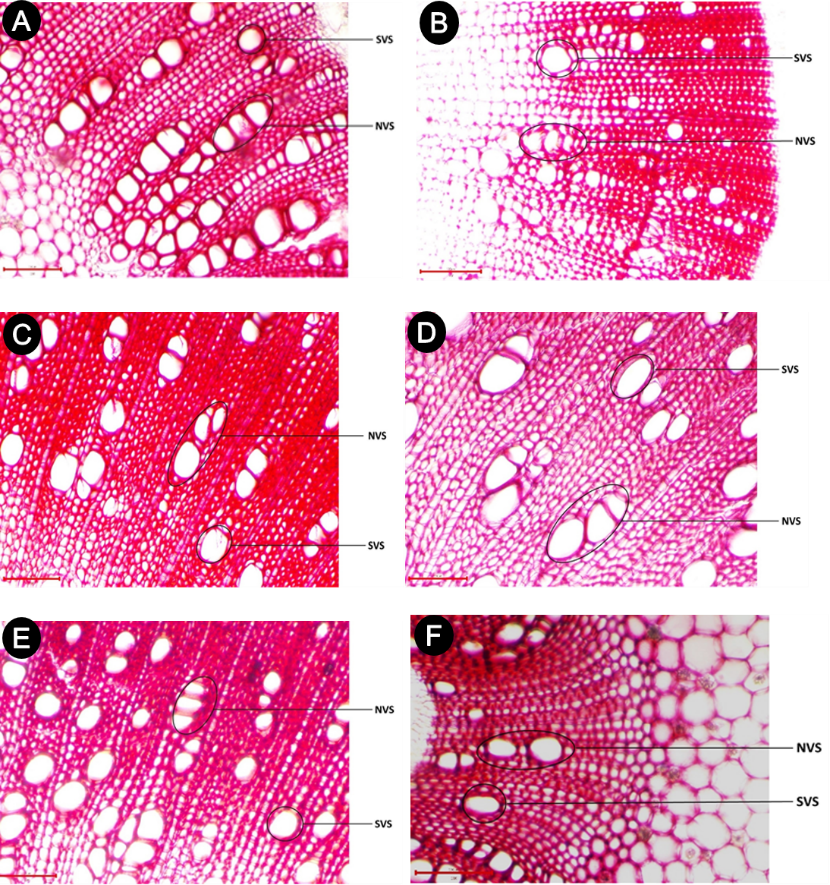
Figure 1. Images of stem cross-sections taken from the lower part of six Cannabis sativa cultivars. A: ‘Avocat’, B: ‘Beldiya’, C: ‘Critical Plus’, D: ‘Khardala’, E: ‘Mexicana’, F: ‘Industriel’. SVS: vessel size; NVS: number of vessels joined in radial files.
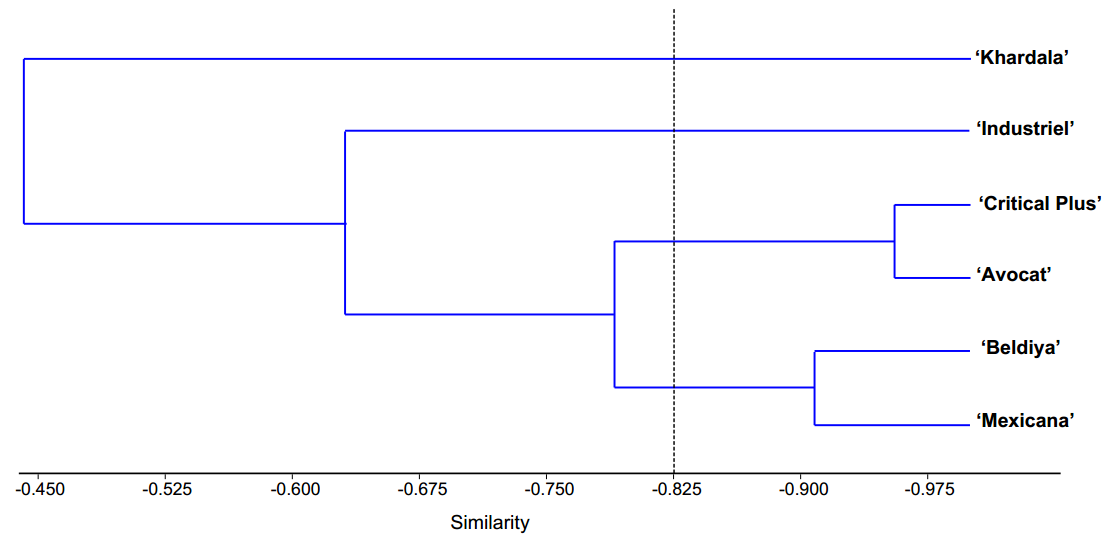
Figure 2. Dendrogram of the cluster grouping of the six cannabis cultivars based on similarity of eco-anatomical traits.
Table 1. Main characteristics of the studied C. sativa cultivars. THC: Tetrahydrocannabinol.
| Cultivar | ‘Avocat’ | ‘Beldiya’ | ‘Critical Plus’ | ‘Khardala’ | ‘Mexicana’ | ‘Industriel’ |
|---|---|---|---|---|---|---|
| Origin | Unknown | Morocco | Netherlands | Unknown | Unknown | Spain |
| Year of introduction | 2012 | Since 7 th century | 2015 | 2010 | 2011 | 2016 |
| Main use | Hashish production | Kif and Hashish production | Hashish production | Hashish production | Hashish production | Fiber production |
| Sexuality | Dioecious | Dioecious | Dioecious | Dioecious | Dioecious | Dioecious |
| Mean plant height (cm) | 121.48 | 95.92 | 111.98 | 92.25 | 133.56 | 138.6 |
| Sowing time | April-May | Mars-April | April-May | April-May | April-May | April-May |
| Harvest time | September-October | July-August | September-October | September-October | September-October | September-October |
| Sowing to flowering (months) | 3-4 | 2-3 | 3-4 | 3-4 | 3-4 | 3 |
| Sowing to harvest (months) | 6 | 5 | 6 | 6 | 6 | 6 |
| THC concentration | High | Medium | Very High | Very High | High | Low |
| Voucher | BAH 3065, BAH 3069, BAH 3071 |
BAH 3081, BAH 3083, BAH 3084 |
BAH 3088, BAH 3091 |
BAH 3111, BAH 3113 |
BAH 3122, BAH 3125 |
BAH 3095, BAH 3099 |
Table 2. Eco-anatomical trait results per cultivar. Data are presented as means ± confidence intervals (CI). Values sharing the same letter are not significantly different at p < 0.05. DM: stem section diameter; DVS: Stem vessel density; NVS: number of vessels joined in radial files; SVS: stem vessel size. CI: confidence interval. V: number of vessels.
| Cultivars | Height (cm) Mean ± CI |
DM (cm) Mean ± CI |
DVS (v/mm2) Mean ± CI |
SVS (µm2) Mean ± CI |
NVS Mean ± CI |
CD Mean ± CI |
|---|---|---|---|---|---|---|
| ‘Avocat’ | 121.48 ± 2.61 c | 5.6 ± 0.37 ab | 95.7 ± 0.26 ab | 1551.4 ± 191.6 ab | 2.9 ± 0.43 a | 4230.5 ± 1332 a |
| ‘Beldiya’ | 95.92 ± 1.41 a | 2.8 ± 0.15 b | 79.3 ± 0.21 b | 1147.1 ± 140.2 ab | 2.7 ± 0.26 a | 2600.2 ± 621 a |
| ‘Critical Plus’ | 111.98 ± 2.23 b | 4.0 ± 0.31 ab | 85.3 ± 0.16 ab | 1403.4 ± 135.9 ab | 2.5 ± 0.23 a | 3884.8 ± 1240 a |
| ‘Industriel’ | 138.6 ± 2.24 d | 3.8 ± 0.25 ab | 120.6 ± 0.23 a | 923.2 ± 85.2 b | 2.7 ± 0.34 a | 1034.3 ± 198 a |
| ‘Khardala’ | 133.56 ± 2.36 d | 5.7 ± 0.47 a | 104.0 ± 0.48 ab | 1847.5 ± 281.6 a | 2.6 ± 0.25 a | 12553.1 ± 4860 a |
| ‘Mexicana’ | 92.25 ± 0.85 a | 4.4 ± 0.27 ab | 98.0 ± 0.18 ab | 1304.6 ± 97.1 ab | 2.7 ± 0.28 a | 2091.7 ± 322 a |
Table 3. Pearson correlations among measured eco-anatomical traits. DM: stem section diameter; DVS: Stem vessel density; VS: number of vessels joined in radial files; SVS: stem vessel size. *P < 0.05, **P < 0.01, ***P < 0.001.
| Height | DM | DVS | SVS | NVS | CD | |
|---|---|---|---|---|---|---|
| Height | – | |||||
| DM | 0.19164*** | – | ||||
| DVS | 0.22973*** | -0.604* | – | |||
| SVS | 0.085345 | 0.765** | -0.806*** | – | ||
| NVS | 0.035118 | -0.123 | 0.287 | -0.181 | – | |
| CD | 0.15844*** | 0.7482** | -0.882*** | 0.922*** | -0.376 | – |