

Aust J Crop Sci. 18(09):518-527 (2024)
ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.09.p33
Guava ripening and quality in response to pre-harvest boron spraying
Vitor Hugo Artigiani Filho1*, Sarita Leonel1, Fernando Ferrari Putti2, Rafaelly Calsavara Martins1, Rafael Bibiano Ferreira1
1São Paulo State University, UNESP, School of Agriculture, Department of Crop Production, Zip Code 18.610-307. Botucatu, São Paulo, Brazil
2São Paulo State University, UNESP, School of Sciences and Engineering, Zip Code 17.602-496, Tupã, São Paulo, Brazil
Abstract
The physiological process of guava fruit ripening is difficult to manage in orchards. Boron (B) is a nutritional element with a physiological effect on fruit ripening through its role in ascorbate metabolism. This study evaluated the effects of single boron pre-harvest spraying on fruit ripening and post-harvest. The treatments consisted of B-monoethanolamine (MEA) sprays (11% B) at concentrations of 0 (control), 1, 2, and 3ml L-1. Foliar sprays were applied on guava trees cv. Cortibel RG, at the physiological stage Biologische Bundesanstalt, Bundessortenamt, und Chemische Industrie (BBCH) 78 (fruits at 80% of final growth). The study used a randomized block design with five replicates. Fruits were harvested 5, 8, 15, and 17 days after B-MEA spraying. The nutritional content was assessed in the leaves and fruits. The fruits were analyzed for ascorbic acid content (AA), total sugars, reducing and non-reducing sugars, titratable acidity, soluble solids, ripeness index, total phenolic compounds, total carotenoids and total anthocyanins. Moreover, the fruit weight, length and diameter were measured and yield were assessed. Fruit firmness and mass loss were evaluated post-harvest. The data showed an increase in ascorbic acid content in the fruit in all treatments with B-MEA sprays. Spraying B-MEA 3 ml L-1, or 180.84 g ha-1, increased the earliness and standardization of ripening, which took place over a shorter period of time, maintaining firmness and reducing post-harvest fruit mass loss.
Keywords: ascorbic acid; bioactive compounds; foliar spray; fruit maturation; nutritional content; Psidium guajava, L.
Abbreviations: AA_ ascorbic acid; ACC_ 1-aminocyclopropane 1-carboxylic acid; B_ boron; BBCH_ Biologische Bundesanstalt_ Bundessortenamt_ und Chemische Industrie; DAH_ days after harvest; DAS_ days after spraying; DCFI_ 2_6-dichlorophenolindolephenol-sodium; Fe_ iron; FD_ fruit diameter; FL_ fruit length; MEA_ monoethanolamine; Mn_ manganese; NaOH_ sodium hydroxide; RI_ ripening index; ROS_ reactive oxygen species; SS_ soluble solids; TA_ titratable acidity
In the management of guava orchards, there is no direct association between nutritional elements and effects on fruit ripening. Furthermore, no management practices currently exist to control the concentration, acceleration, or deceleration of the fruit ripening, nor to improve post-harvest-related variables such as shelf life. Guava is considered as a “super fruit” (Chang et al., 2018) due to its high nutritional value and antioxidant compounds, such as ascorbic acid, phenolic components, carotenoids, and lycopene (Ribeiro et al., 2020; Yousaf et al, 2021).
Guava orchards have a prolonged harvest period, highly perishable fruit production and, different flowering flushes, depending on the type of pruning adopted by the growers. Guava has a high moisture content and thin skin, being characterized as a climacteric fruit, depending on the cultivar (Azam et al., 2021; Yousaf et al., 2021).
Cortibel RG guava trees originated from seeds of an unidentified cultivar, possibly of Australian origin (Flori, 2016). The trees are vigorous and produce medium to large fruit with a rounded shape, rough skin, red pulp, and sweet flavor. Hence, the fruit is suitable for fresh market due to its good post-harvest conservation. The Cortibel RG cultivar is considered as a fruit with climacteric ripeness, which has not yet been studied (Mendonça et al., 2007). As a climacteric fruit, it shows respiratory peaks with an increase in ethylene production, which contributes to its perishability. These physiological reactions accelerate the ripening process of guavas and, consequently, there is a reduction in post-harvest shelf life with losses of around 15 to 22% and a limitation in the marketing of the fruit (Hiwasa-Tanase; Ezura, 2014; Dhara et al., 2017; Singh et al. 2017; Azam et al., 2021).
Harvesting guava is an expensive procedure, owing to labor and to the number of procedures necessary to collect the fruit at the ideal maturity stage for commercialization (Cavalini et al., 2015). Harvesting accounts for up to 83% of labor costs (Garcia et al., 2018).
Fruit ripening can be considered an oxidative process that involves enzymatic changes and alterations in antioxidant levels, the latter playing an important role in fruit ripening by promoting a balance in oxidative mechanisms, removing reactive oxygen species (ROS) (Jimenez et al., 2002), which cause damage to the plant. The authors report that AA, being a powerful antioxidant, actively participates in these processes.
As an enzyme cofactor, AA is essential for the synthesis of compounds that are important for fruit development and ripening, such as ethylene, gibberellic acid and flavonoids such as anthocyanins. Ascorbate is a cofactor for the enzyme ACC (1-carboxylic acid-1-aminocyclopropane) oxidase, which catalyses the last stage of ethylene biosynthesis in plants (Baldet et al., 2014).
B participates in many fundamental quality and standardization stages that influences fruit quality (Baranwal et al., 2017; Wei et al., 2018). It plays an important role in the synthesis of AA, and its deficiency changes metabolic and phenol oxidation processes, including AA levels in the plant. There is evidence of the association of B with ascorbate metabolism (Wei et al., 2018). The authors highlighted that B help increased ascorbate levels due to its effect on plasma membrane transport reactions, since B stimulates the ascorbate oxidoreductase enzyme. This enzyme catalyzes electron transfer to ascorbate. Thus, B deficiency in the plant would inhibit this process, thus reducing ascorbate levels.
The application of boron-monoethanolamine (MEA) has emerged as a promising technique, considering the hypothesis of better absorption efficiency by the tree. Ethanolamine or monoethanolamine is a molecule made up of an amine and a primary alcohol; therefore, when B combines with this organic chemical compound, it would be better absorbed by the leaves, since this molecule has the ability to solubilize the components of the cuticle (Singh et al., 2017).
Fruit ripening is an oxidative process that involves enzymatic changes and antioxidant levels that affect maturation by removing reactive oxygen species (ROS) that damage the plant. AA is an antioxidant that actively participates in these processes (Deng et al., 2022). Therefore, this study evaluated the effects of B as a guava fruit ripener. The obtained results provide reference for further B spraying evaluations to optimize cropping management in orchards. Moreover, they present a nutritional option for inducing the ripening process in guava fruit.
Nutritional content showed no variation in guava leaves, with the exception of iron (Fe) and manganese (Mn) (Table 1). Both positive and negative correlations were recorded for the interaction between B and Mn. Depending on the citrus cultivar studied, there was a linear increase in Mn content as the dose of B increased up to a certain limit, which, when exceeded, inverted the absorption curve (Papardakis et al., 2003). Reduced Fe content and increased Mn content in treatments with the highest B dosages (T3 and T4) could be explained by the Fe-Mn interaction, because there is competition for the absorption site of these two bivalent cations (Malavolta, 2006).
Macro and micronutrient analyses at 15 DAS showed no statistically significant difference between treatments (Table 2).
Yield performance and harvest flushing
There was no statistical difference between treatments for number of fruits, mean fruit weight, and yield. The average number and weight of fruits per tree, regardless of treatment, were 800 fruits weighing 135-140g. The average yield was 23.0 tons per hectare and is in line with the yields obtained for the Cortibel RG cultivar in commercial orchards (Mendonça et al., 2007).
The harvest took place over a total period of 12 days, in June 2022. This period was characterized by broad temperature variations and oscillation between typical winter cold fronts. After B spraying, there was a rapid increase in temperature, which accelerated the maturation process in all treatments evaluated (Figure 1). The trial would have to be evaluated on more than one harvest season in order to minimize the effects of annual climate change.
The assessment of the harvesting flushing identified that the harvest was concentrated at 17 DAS for fruits sprayed with 3ml l-1 of B-MEA (T4), suggesting a possible effect of the treatment on peel color changes, an indicator used in the field to identify the fruit to be harvested. This result showed the effect of B-monoethanolamine at the highest concentration evaluated, which allowed the harvest to be carried out earlier and in a shorter time interval (Figure 3).
Chemical quality of fruits
Variables related to the chemical analysis of the fruit were analyzed for their significant effects, and it was also proposed that they be classified into three metabolic stages (Figure 4). Thus, there was a significant effect at five DAS for AA and RI levels, which explained the results of the first metabolic stage. There were increasing and proportional gains according to B concentrations applied in each treatment, with T4 (3 ml L-1), presenting the highest AA and RI contents (Figure 5). In the first metabolic stage, AA levels differed between treatments in the following order: T4 ˃ T3 ˃ T2 ˃ T1 (Figure 5).
In the present study, at five DAS, the mean AA levels were different between treatments. However, this was not observed at 15 DAS, indicating the change to a second metabolic stage in the fruit ripening process.
The results of the second metabolic stage were obtained at 15 DAS, showing significant differences for the variables TA, SS, RI, reducing and non-reducing sugars, total phenolic compounds, and total carotenoids (Figures 6 and 7). The results demonstrated a different maturation process between treatments. There was an indication of maturation process acceleration in T4 (3 ml L-1), showing that T1 (control), T2 (1 ml L-1), and T3 (2 ml L-1) fruits entered the initial physiological maturation process later.
Analyses conducted zero days after harvest (0 DAH) showed that T4 (3ml L-1) already presented characteristics of the second metabolic step of maturation in terms of reducing and non-reducing sugar levels and total sugar content. There was also evidence of reduced total phenolic compounds, which are found at high concentrations in immature fruits.
The results of the evaluation at zero DAH showed advanced maturation process in T4 (3 ml L-1) evidenced by increased total soluble sugar content and increase SS, corroborating what was determined by Bashir and Abu-Goukh (2003), who reported that SS and sugars increased with maturation progression, with the most significant increase in sugar content occurring after the climacteric respiratory peak.
TA level showed different tree responses to B concentrations in T4 (3 ml L-1) (Figure 6). There was a significant effect on RI, AA, total sugars, non-reducing sugars, and total anthocyanins (Figure 6). T4 (3ml L-1) results showed that the fruit maturation process presented equal TA and RI values between treatments at five DAH. Moreover, it also showed that total phenolic compounds were also equal in this period, but at lower values than the ones identified at the beginning of the analyses.
Variables related to fruit pigmentation showed the same levels of total carotenoids at five DAH, regardless of B treatments. In the five-day interval between analyses, the metabolic rate eliminated the differences for this variable (Figure 4). This result corroborates with those of Jain et al. (2001), who reported increased carotenoid levels during guava ripening.
Regarding total anthocyanin levels, it was not possible to establish a response curve because the results were different between the evaluation stages. Ageorges et al. (2014) reported that anthocyanins begin to accumulate during maturation and stabilize or decrease near the harvest stage.
In the second metabolic stage, T1 (control), T2 (1 ml L-1), and T3 (2 ml L-1) showed the same TA, SS, and RI levels, differing from T4 (3 ml L-1). These differences suggest that maturation processes relate to starch (reserve sugar) transformation into soluble sugars. T4 (3 ml L-1) presented decreased AA and RI levels, since the highest values occurred in the previous stage. In this second stage, T4 (3 ml L-1) presented the highest values, with total, reducing and non-reducing sugars being highlighted in the maturation process.
The results of the third metabolic stage were obtained at 0 and 5 DAH. At this stage, there was a significant difference in soluble sugar and total anthocyanin levels.
In the third metabolic stage, some variables were replaced, showing the clear sequence of this dynamic fruit maturation metabolic process. There were increased non-reducing sugar, total sugar, and total anthocyanin levels, indicating that the ripening process was different between treatments and showing that T4 (3 ml L-1) presented different results compared to the other treatments, which showed evolution in later maturation parameters.
Post-harvest firmness and mass loss
There was interaction between storage time and treatments, with increasing B concentrations and greatest loss of mass in treatment T1 (control). T3 (2 ml L-1) and T4 (3 ml L-1) presented a lower percentage of mass loss than the other treatments.
There were statistical differences in the initial assessment of fruit firmness between the treatments. All the treatments reached the same fruit firmness at the end of the evaluation period (9 days), but at different times.
The slope of firmness curves showed that treatment T4 (3 ml -1) was similar to T1, but with lower values. Even though there were different stages of fruit maturation between treatments at the initial post-harvest evaluation, T4 (3 ml L-1) demonstrated no firmness loss difference.
T2 (1 ml L-1) and T3 (2 ml L-1) showed different results compared to T0 (control) and T4 (3 ml L-1). Graphical illustration presented different curves compared to T0, especially T3 (2 ml L-1), which showed an accelerated loss of firmness. In addition, treatments T2 (1 ml L-1) and T3 (2 ml L-1), both at the beginning of the evaluation, showed very hard flesh compared to treatments T1 (control) and T4 (3 ml L-1), indicating a later stage of ripeness compared to the other treatments.
Discussion
Guava (Psidium guajava) is a highly perishable fruit with a short shelf life as physic-chemical changes occur continuously and rapidly after harvest leading to heavy postharvest losses. Guava orchards have a long harvest period, with different flowering flushes depending on the pruning made by the farmers. The orchards are managed using two types of pruning. The management most commonly adopted by growers, including the orchard in this study, is staggered pruning by plot. In other words, the total cultivated area is divided into plots that are pruned alternately in order to improve the seasonality of the harvest and, consequently, the profitability of the orchards. This is an important cultural practice to avoid concentrating the harvest in a single period.
Cultural management practices in guava orchards do not have the option of using nutritional elements that affect fruit ripening. When they are used, it is usually through hormonal compounds. Boron application can be used in conjunction with Ethephon to promote fruit ripening, skin color and harvest timing (Brackman et al., 2016). This study assessed the possibility of introducing nutritional management, in addition to staggered pruning, by spraying with B-MEA, in order to reduce the time interval between harvests in each plot, enabling more efficient use of harvest labor and reducing costs.
The effects of B-MEA applied at the beginning of fruit ripening in a perennial crop such as guava were not aimed at productivity gains, since at this stage fruit set was already defined and, consequently, the number of fruits per tree and the average fruit weight. Boron does not produce ripening effects if the crop is not at the right stage of growth. It can only accelerate naturally occurring tree processes. If properly administered, boron applications can accelerate natural ripening processes (Islam et al., 2016).
Guava is a climacteric fruit with steep respiration peak and high rate of ethylene production that limits its postharvest shelf-life to three to four days at room temperature whereas, on the other hand refrigerated storage causes chilling injury (Ribeiro et al., 2020). After harvest, fruits characterized as climacteric continue the maturation process with respiratory peaks (Bassetto et al., 2005) that are often associated with peak AA concentrations (Baldet et al., 2014). The first "physiological sign" of the fruit's physiological ripeness was the increase in AA levels. Azam et al. (2021) reported that the post-harvest application of AA maintained the physiological and antioxidant responses of guava in ambient storage due to the maintenance of antioxidant enzyme activities in the fruit.
B-MEA at the highest concentration shortened the harvesting period duration, as well as reducing the time interval needed for harvesting. This result is very desirable for guava growers due to the possibility of increasing the fruit seasonality, being also reported by Arora & Singh (1972) in guava trees, and Brackmann et al. (2016) in apple trees. The pre-harvest spraying of boron makes it possible to harvest apples earlier due to the fact that it accelerates the fruit metabolism. In these trials, maturation was accelerated with B spraying.
B plays an important role in AA synthesis, and this is a cofactor for activating other maturation-related metabolic routes (Rogalla et al., 2002). AA is the reduced form of ascorbate, which, according to Gest et al. (2013), is an essential enzymatic cofactor for the synthesis of ethylene, gibberellic acid, anthocyanins, and flavonoids. Ascorbate is a cofactor for enzime 1-aminocyclopropane 1-carboxylic acid (ACC) oxidase, which catalyzes the last stage of ethylene biosynthesis. At this stage, maturation was accelerated (Baldet et al., 2014).
The T4 presented in the second metabolic stage decreased AA and RI contents. In this stage, another metabolic route may have been co-responsible for decreased AA levels because high B concentrations can damage plant tissues. Hence, we hypothesized for what was observed in T4 (3 ml L-1) is that the detox effect, also associated with ascorbate, overcame the stimulus provided by ascorbate on ACC oxidase. In addition, the protective effect against reactive oxygen species (ROS) prevailed. According to Pech et al. (2014), the antioxidant system that detoxifies ROS includes enzymes from the ascorbate glutathione cycle, which involve ascorbate peroxidase.
Srivastava & Singh (2005) reported the relationship between B and sugars such as mannitol and sorbitol, related to carbohydrate transport and, the presence of B-sorbitol and B-mannitol complexes in phloem sap.
As for metabolic reactions in the maturation process, non-reducing sugars would be the main sugars transported in the phloem, including sucrose linked to galactose, mannitol, and sorbitol molecules (Taiz et al., 2017). Therefore, the three sugars are related to B, acting in carbohydrate redistribution in polyol-producing species. Considering that both sorbitol and mannitol affect SS concentration in fruits, they act in carbohydrate translocation and replace simple sugars such as mannose and glucose during the ripening process (Singh & Pal, 2008).
The SS content, SS/TA ratio, and AA content influence organoleptic properties, thus influencing the nutritional value of guava fruits (Azzolini et al., (2004); Dhara et al., (2017).
B application reduces the respiration rate, therefore, the degradation of cellular structures would occur more slowly, increasing post-harvest fruit firmness (Islam et al., 2016). As a result, fruit quality would improve, resulting in longer shelf-life (Pereira et al., 2006). David et al. (2003) reported that B reduces tomato skin cracks, thus increasing post-harvest shelf life. Otherwise, Singh et al. (2007) found no significant differences in strawberry firmness after B spraying.
Although not all points of this study could be elucidated, we present results at all the different stages evaluated, which open up opportunities to continue or even search different approaches to analyze the interaction between B and ripening process.
The results indicated a positive effect of B on physiological processes related to guava ripening. The highest AA contents in T4 (3 ml L-1) reaffirmed the hypothesis of the potential use of B in the final stage of guava production. Furthermore, no signs of phytotoxicity related to the different B concentrations applied by foliar spray were identified in leaves or fruits.
Our results also identified beneficial effects in T4 (3 ml L-1) both related to maintaining fruit firmness and reducing post-harvest mass loss. These results can be useful in planning fruiting pruning for new orchards, identifying the possibility of spraying with B-MEA and determining the most appropriate crop management for the interval between harvests, post-harvest and assessing storage time.
The experiment was carried out in an orchard located in Valinhos, São Paulo, Brazil, (22º 55’ 51” S and 46º 58’ 39” W), and 715 m a.s.l. According to the Köppen-Geiger climate classification, the climate in the area is classified as Cfa (warm temperate climate), with a mean temperature of 20.6 °C and a mean annual rainfall of 1,462 mm (Setzer, 1966). Climatic, maximum and minimum temperature, and rainfall data during the experiment period are presented in Figure 1.
The soil was typical dystrophic red-yellow latosol, moderate A, medium texture, and clayey (LVAd6), according to the nomenclature of the Brazilian Soil Classification System (Santos et al., 2013).
Plant material and orchard management
The study analyzed 13-year-old red guava trees (Psidium guajava, L.) of the Cortibel RG cultivar, cultivated at a spacing of 4 x 3 m (800 trees ha-1), without irrigation. The trial was conducted in the 2021/2022 harvest season.
Prior to experimentation, soil samples were collected at a 0 to 20 cm depth to determine soil chemical analyses. The results indicated that pH (CaCl2) = 5.9, Organic Matter = 68 g/dm3, Presin = 364 mg/dm3, Al3+ = 0, H + Al = 21, K = 2.42 mmolc/dm3, Ca = 240 mmolc/dm3, Mg = 20 mmolc/dm3, Sum of bases = 262 mmolc/dm3, Cation exchange capacity = 283, and base saturation = 93%. There was no need for liming.
Fertilization was carried out as follows: after pruning, 2.000 g/tree of formulated 20-05-20; in full flowering, 1.500 g/tree of formulated 20-05-20; 30 days after the beginning of flowering, 1,500 g/tree of formulated 20-05-20; 60 days after the beginning of flowering, 2.000 g/ of formulated 15-05-30. No thinning or fruit bagging was carried out in the orchard. Fruiting trees were pruned in stages, by plot, at the end of February 2022.
Treatments and experimental design
The treatments consisted of foliar sprays of B-MEA with 11% B and a density of 1.37g/mL (Boroplus®). Spraying was carried out using electric backpack equipment with standardised pressurisation. The treatments corresponded to concentrations of 0 (control) (T1); 1.0 (T2); 2.0 (T3) and 3.0 ml L-1 (T4). The concentrations were equivalent to 0 g (control), 60.28 g, 120.56 g and 180.84 g ha-1 of B-MEA, at phenological stage 78 on the BBCH (Biologische Bundesanstalt, Bundessortenamt, und Chemische Industrie) scale (80% of final fruit growth), for the Psidium guajava L. species (Salazar et al., 2006).
The experimental design was a randomized blocks with four treatments, five replicates, and three trees per experimental plot, totaling sixty useful trees, in addition to ten guard trees for each treatment and twenty guard trees external to the trial.
Leaf and fruit nutritional content and harvest
Macro and micronutrient analysis was carried out on a leaf sample consisting of 25 pairs of leaves, collected five days after spraying (DAS) at the mean height of the canopy, considering the third or fourth expanded pair (Silva, 2009). Macro and micronutrient contents were evaluated in samples of fruits harvested and processed into guava pulp, at the time of harvest peak, at 15 DAS.
Yield performance
The total number of fruits harvested from each tree were counted, and the product of the total number of fruits and fresh weight of the fruits harvested per tree was considered the production per tree. The yield (t ha-1) was obtained considering a stand of 800 trees per hectare.
Harvest and sampling
The fruit was harvested at the stage where guava growers consider it to have reached physiological maturity, that is, firm, with green skin with an initial yellow color at the base and firm (Garcia, 1978; Ribeiro et al., 2020).
The fruit was sampled at the peak of the harvest (15 DAS). The samples were divided into three equal parts. A third of the samples were kept to evaluate firmness and weight loss over a total period of nine days, at three-day intervals. A third of the total sample were sent for immediate analysis of chemical variables, and another third was reserved to be analyzed five days after the first evaluation (Figure 2). The harvest flushing was determined by counting the fruits on each harvest date, at 5, 8, 15, and 17 DAS, and cumulatively throughout the process.
Fruit firmness, mass loss, length, and diameter
Firmness was determined by resistance to penetration using a PTR 500 fruit sclerometer. Mass loss (%ML) was calculated using equation 1:
$\%\ PM = 100 - (MA*\frac{100}{MI}$). (Equation 1)
Where: %ML = percentage of mass loss, MA = mass on the day of evaluation, and MI = initial mass (day zero). Fruit length and fruit diameter, in mm, were also evaluated.
Chemical analyses
Titratable acidity and the percentage of citric acid, was evaluated using the sodium hydroxide (NaOH) titration method (IAL, 2008). The potential of Hydrogen (pH) was measured using a pH meter (Digital DMPH-2) (Association of Official Analytical Chemists (AOAC), 2019). Soluble solids content was obtained using a digital refractometer and expressed in ºBrix. Ripening index (RI) is the relationship between SS and TA.
Sugars (total, reducing, and non-reducing) were determined using a spectrophotometer and the methodology described by Somogy with adaptation by Nelson (1944). AA content was determined using the modified Tillmans titration method with reduction of the 2,6-dichlorophenolindolephenol-sodium indicator by AA (MAPA, 2021). Total phenolic compound levels were determined using a spectrophotometer at 750 nm, after one hour of reaction with acetone extractor (50%), according to the methodology described by Singleton et al. (1999), and the results were expressed in ug gallic acid 100 ml-1.
Total carotenoid and total anthocyanin contents were determined using a spectrophotometer at 470 nm and 537 nm, respectively, after 1 hour of reaction with acetone extractor (80%) and tris (hydroxymethyl) aminomethane (20%) solution, according to the method described by Sims and Gamon (2002). The results were expressed in mg 100-1.
Statistical analysis
The Anderson-Darling test was used to analyze data normality in all sets of results. Homoscedasticity was verified using the variance equation test (or the Levene’s test). The data were subjected to analysis of variance at a significance level of 0.05 error probability by the F test. The means, when significant, were subjected to the Tukey test at 5% error probability using the R software. The graphs were created using the SigmaPlot software (Systat Software Inc., San Jose, CA, USA).
Pre-harvest foliar B-MEA spraying at a concentration of 3 mL L-1 (180.84 g B-MEA ha-1) resulted in harvest anticipation and concentration, thereby maintaining post-harvest fruit firmness and decreasing mass loss in ‘Cortibel RG’ guava fruits.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPQ Processes 302611/2021-5 and 302848/2921-5).
References
Ageorges A, Cheynier V, Terrier N (2014) Polyphenols. In: Nath P, Bouzayen M, Matto AK, Pech JC (orgs.) Fruit Ripening: Physiology, Signaling and Genomics. CABI, Boston, MA. p 151-177.
AOAC (2019) Official methods of analysis. Method Nº. 967.21. Washington: Association of Official Analytical Chemists. 21st ed.
Arora JS, Singh JR (1972) Responses of Guava (Psidium guajava L.) to Boron Spray's. J. Japan Soc. Hort. Sci. 41(3): 239-244.
Azam, M, Hameed L, Qadri R, Shaghef E, Aslam A, Khan MI, Shen J, Zhang J, Naffes M, Ahmad I, Ghani M, Chen J, Anjun N. (2021) Postharvest ascorbic acid application maintained physiological and antioxidant responses of Guava (Psidium guajava L.) at ambient storage. Food Sci. Tech. 41(3): 748-754.
Azzolini M, Jacomino AP, Spoto MHF (2004) Estádios de maturação e qualidade pós-colheita de goiabas ‘Pedro Sato’ (Ripening stages and post-harvest quality of 'Pedro Sato' guavas). Rev. Bras. Frutic. 26(1): 29-31.
Baldet P, Ferrand, C, Rothan C (2014) Vitamins in Fleshy Fruits. In: Nath P, Bouzayen M, Matto AK, Pech JC (orgs.) Fruit Ripening: Physiology, Signalling and Genomics. CABI, Boston, MA, p 127-150.
Baranwal D, Tomar S, Singh JP, Maurya JK (2017) Effect of foliar application of zinc and boron on fruit growth, yield and quality of winter season guava (Psidium guajava L.). Int. J. Curr. Microbiol. Appl. Sci. 6(9): 1525-1529.
Bashir HA, Abu-Goukh ABA (2003) Compositional changes during guava fruit ripening. Food Chem. 80(4): 557-563.
Bassetto E, Jacomino AP, Pinheiro AL, Kluge RA (2005) Delay of ripening of ‘Pedro Sato’ guava with 1-methylcyclopropene. Postharvest Biol. Technol. 35(3): 303-308, 2005.
Brackmann A, Thewes FR, de Oliveira Anese R, Linke Junior W (2016) Preharvest boron application and its relation with the quality of ‘Galaxy’ apples after harvest and controlled atmosphere storage. Ciênc. Rural. 46(4): 585-589.
Cavalini, FC, Jacomino, AP, Trevisan, MJ, Miguel ACA (2015) Ponto de colheita e qualidade de goiabas ‘Kumagai’ e ‘Paluma’ (A. Harvest point and quality of 'Kumagai' and 'Paluma' guavas). Rev. Bras. Frutic. 37(1): 064-072.
Chang SK, Alasalvar C, Shahidi F (2018) Superfruits: Phytochemicals, antioxidant efficacies and health effects - a comprehensive review. Crit Rev in Food Sci. 59(10): 1580-1604.
Chaudhary DR, Shukla LM (2004) Boron adsorption and desorption in arid soils of India. Agroch. 48: 141-152.
David JM, Sanders DC, Nelson PV, Lengnick L, Sperry WJ (2003) Boron improves growth, yield, quality, and nutrient content of tomato. J. Am. Soc. Hortic. Sci. 128(3): 441-446.
Dengh H, Xia H, Guo Y, Liu X, Lin L, Wang J, Xu K, Lv X, Hu R, Liang D (2022) Dynamic changes in ascorbic acid content during fruit development and ripening of Actinidia latifolia (an ascorbate-rich fruit crop) and the associated molecular mechanisms. Int. J. Mol. Sci. 23(10):5808.
Dhara P, Patel NL, Tanveer A, Apeksha P, Kumar V (2017) Effect of pre-cooling packaging material on chemical and sensory quality of guava fruits [Psidium guajava (Linn.)] cv Allahabad Safeda. Environ. Ecol. 35(1): 64-69.
Flori J (2016) Principais variedades de goiaba (Main guava varieties). Campo & Negócios hortifruti. Informe técnico p.72–73.
Garcia JLM (1978) Matéria prima. In: Medina JC, Garcia JLM, Kato K, Martin ZJ, Vieira LF, Renesto OV (eds) Goiaba: da cultura ao processamento e comercialização (Guava: from cultivation to processing and marketing). ITAL, Campinas, p 47-59.
Garcia DM, da Costa AF, Galeano EAV, Rossi DA, Bárbara WP de F, Egger VA (2018) Análise de custos de produção da goiabeira: Um estudo de caso em Venda Nova do Imigrante, ES (Analysis of guava production costs: A case study in Venda Nova do Imigrante, ES). Revista Científica Intelletto. 3(especial): 33-42.
Gest N, Gautier H, Stevens R (2013) Ascorbate as seen through plant evolution: the rise of a successful molecule? J. Exp. Bot. 64(1): 33-53
Hiwasa-Tanase K, Ezura H (2014) Climacteric and Non-climacteric Ripening. In: Nath P, Bouzayen M, Matto AK, Pech JC (orgs.) Fruit Ripening: Physiology, Signalling and Genomics. CABI, Boston, MA. p 1-14.
IAL - Instituto Adolfo Lutz (2008) Métodos físico-químicos para análise de alimentos. (4th ed.). IAL, São Paulo. 533 p.
Islam M, Mele M, Baek JP, Kangh HM (2016) Cherry tomato qualities affected by foliar spraying with boron and calcium. Horti. Environ. Biotechnol. 57: 46-52.
Jain N, Dhawan K, Malhotra SP, Siddiqui S, Singh R (2001) Compositional and enzymatic changes in guava (Psidium guajava L.) fruits during ripening. Acta Physiol. Plant. 23: 357–362.
Jimenez A, Creissen G, Kular B, Firmin J, Robinson S, Verhoeyen A, Mullineaux P (2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 214(5): 751-758.
Malavolta E (2006) Manual de nutrição mineral de plantas (Manual of plant mineral nutrition). Editora Agronômica Ceres, São Paulo. P 638.
MAPA. Ministério da Agricultura, Pecuária e Abastecimento. Método de Tillmans modificado (Modified Tillmans' method) (2021) Accessed on: May 22, 2021.
Mendonça RD, Ferreira KS, de Souza LM, Marinho CS, Teixeira SL (2007) Caracteristicas físicas e químicas de goiabas ‘Cortibel 1’ e ‘Cortibel 4’ armazenadas em condições ambientais (Physical and chemical characteristics of 'Cortibel 1' and 'Cortibel 4' guavas stored under ambient conditions). Bragantia. 66(4):685-692.
Natale W, Rozane DE, Prado RM (2012) Diagnose foliar nas culturas da goiaba e da carambola (Foliar diagnosis in guava and star-fruit crops.). In: PRADO RM (ed.) Nutrição de Plantas – Diagnose Foliar em Frutíferas. Fcav/Unesp, Jaboticabal. p 411-441.
Nelson N (1944) A photometric adaptation of somogi method for determination of glicose. Journal Biological Chemistry. 156:375-380.
Papardakis IE, Dimassi KN, Therios IN (2003) Response of two citrus genotypes to six boron concentrations: concentration and distribution of nutrients, total absorption, and nutrient use efficiency. Aust. J. Agr. Res. 54(6):571-580.
Pech JC, Bouzayen M, Latché A (2014) Cellular, Metabolic and Molecular Aspects of Chromoplast Differentiation in Ripening Fruit. In: Nath P, Bouzayen M, Matto AK, Pech JC (eds.) Fruit ripening: physiology, signalling and genomics. CABI, Boston, MA, p 28-47.
Pereira T, Almeida Carlos L, Gonçalves de Oliveira J, Rodrigues Monteiro A (2006) Influência das condições de armazenamento nas características físicas e químicas de goiaba (Psidium guajava), cv. ‘Cortibel’ de polpa branca (Influence of storage conditions on the physical and chemical characteristics of white guava (Psidium guajava) cv. ‘Cortibel’). Revista Ceres. 53(306):276-284.
Ribeiro LR, Leonel S, Souza JMA, Garcia EL, Leonel M, Monteiro LNH, Silva MS, Ferreira RB (2020) Improving the nutritional value and extending shelf life of red guava by adding calcium chloride. LWT. 30:109655.
Rogalla H, Romheld V (2002) Effects of silicon on the availability of boron: possible effects on the phenol pathway and on the redox status in Cucumis sativus L. In: Goldbach HE, Rerkasem B, Wimmer MA, Brown PH, Thellier M, Bell RW (eds.). Boron in plant and animal nutrition. Springer Science + Business Media, New York. p 205-211.
Salazar DM, Melgarejo P, Martínez R, Martínez JJ, Hernández F, Burghera, M (2006) Phenological stages of the guava tree (Psidium guajava L.). Sci. Hortic. 108(2): 157-161.
Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Cunha TJF (2013) Sistema brasileiro de classificação de solos (Brazilian system of soil classification). Embrapa, Brasília. p 353.
Setzer J (1966) Atlas climático e ecológico do estado de São Paulo (Climate and ecological atlas of the state of São Paulo). CIBPU, São Paulo.
Silva FC da (2009) Manual de análises químicas de solos, plantas e fertilizantes (Manual of chemical analysis of soils, plants, and fertilizers). Brasília, DF: Embrapa Informação Tecnológica; Rio de Janeiro: Embrapa Solos.
Sims DA, Gamon J A (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sensing of Environment. 81(2–3):337-354.
Singh J, Prasad N, Singh S (2017) Postharvest treatment of guava (Psidium guajava L.) fruits with boric acid and NAA for quality regulation during ambient storage. International Journal of Bio-resource and Stress Management. 8(2):201-206.
Singh R, Sharma RR, Tyagi SK (2007) Pre-harvest foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (Fragaria×ananassa Duch.). Sci. Hortic. 112(2):215-220.
Singh SP, Pal RK (2008) Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharvest Biol. Technol. 47(3):307-314.
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299:152-178.
Srivastava AK, Singh S (2005) Boron nutrition in citrus-current status and future strategies – review. Agr. Rev. 26(3):173-186.
Taiz L, Zeiger E, Moller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal (Plant physiology and development). 6th.edn. Artmed Editora, São Paulo p 858.
Wei C, Ma Z, Liu Y, Oiao J, Sun G (2018) Effect of boron on fruit quality in pineapple. AIP Conference Proceedings. 1956:020006.
Yousaf AA, Abbasi KS, Ahmad A, Hassan I, Sohal A, Qayyum A, Akram MA (2021) Physico-chemical and nutraceutical characterization of selected indigenous guava (Psidium guajava L.) cultivars. Food Sci. Technol. 41(1):47-58.
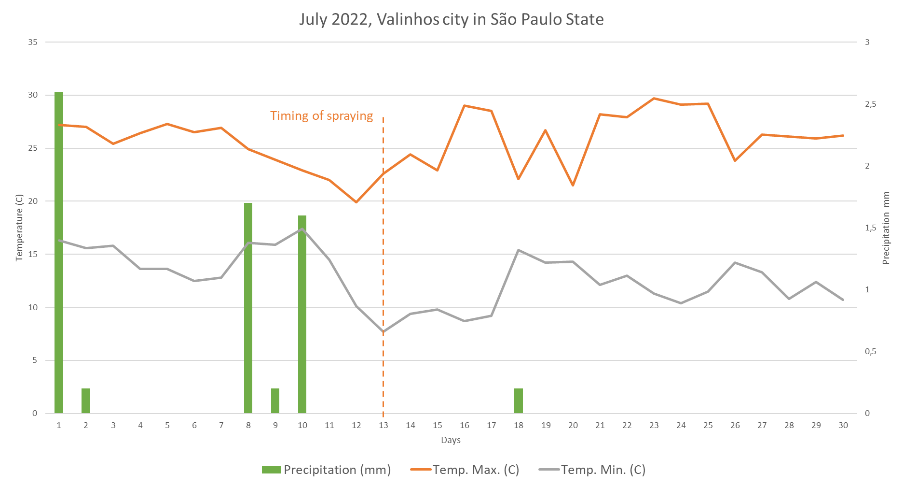
Figure 1. Maximum and minimum temperatures in Valinhos, São Paulo, Brazil, in June 2022. Data provided by the National Institute of Meteorology (INMET), collected by the automatic station; identified as V0516.
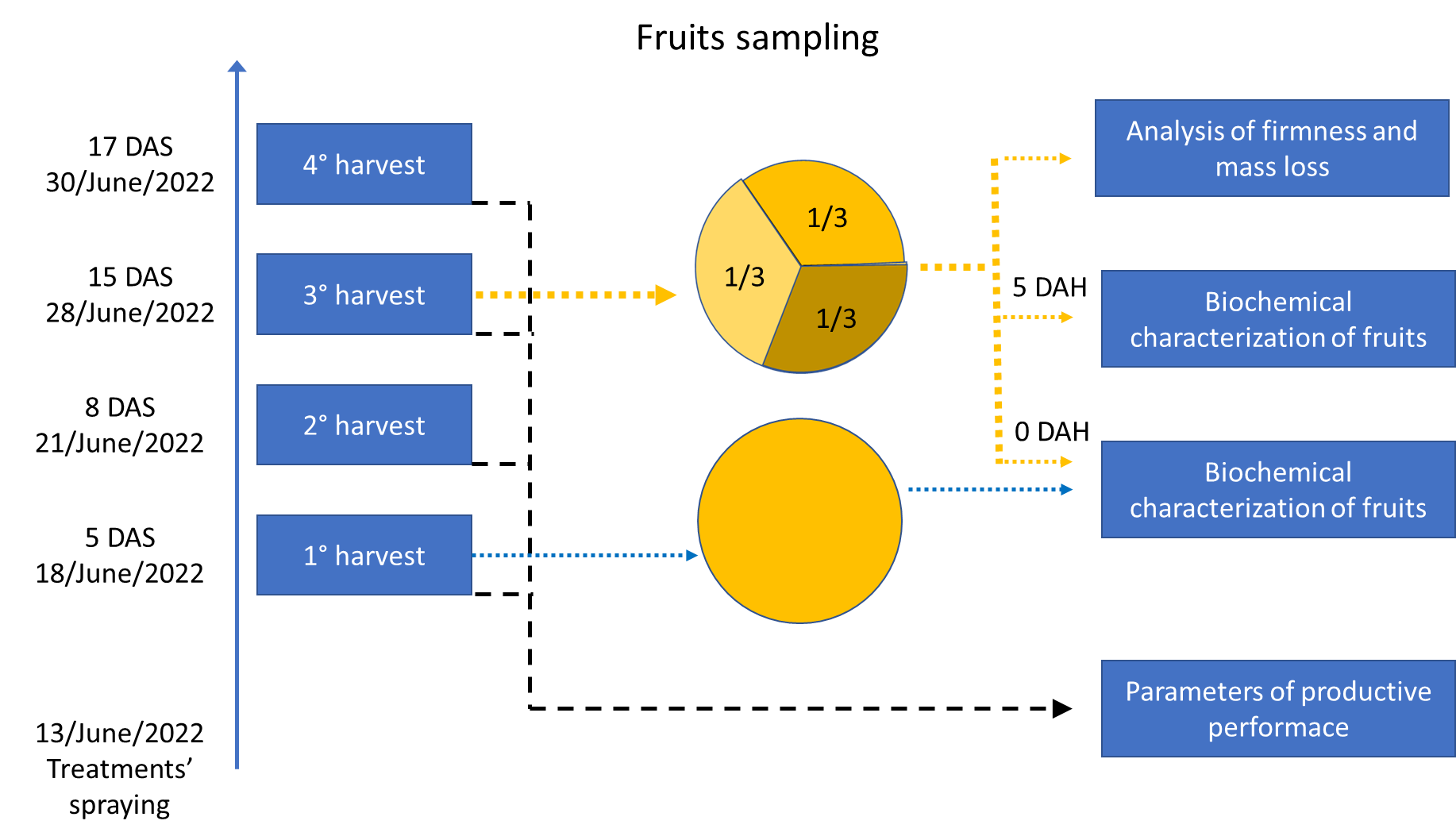
Figure 2. Schematic representation of fruit sampling and analysis at different harvest and spraying stages. DAS, days after spraying, DAH, days after harvest.
Table 1. Nutrient content of Cortibel RG guava leaves, five days after spraying.
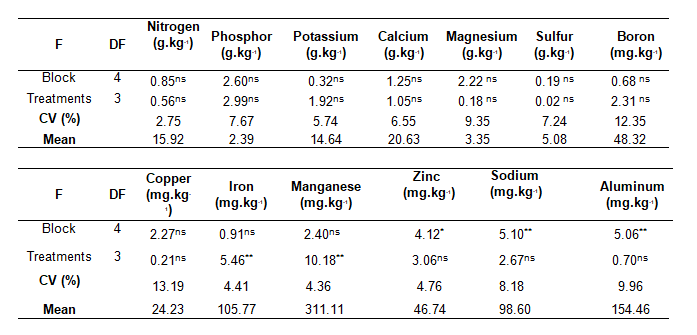
ns = not significant; * = significant at 5%; **; DF = degrees of freedom; CV = coefficient of variation.
Table 2. Nutrient content of Cortibel RG guava fruit 15 days after
spraying, with different foliar B-MEA concentrations.
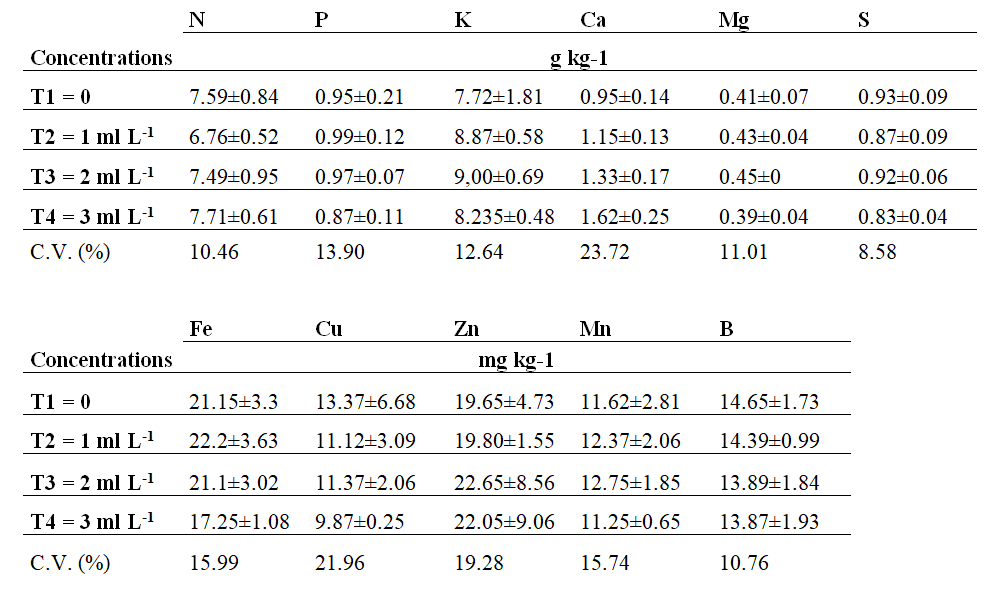
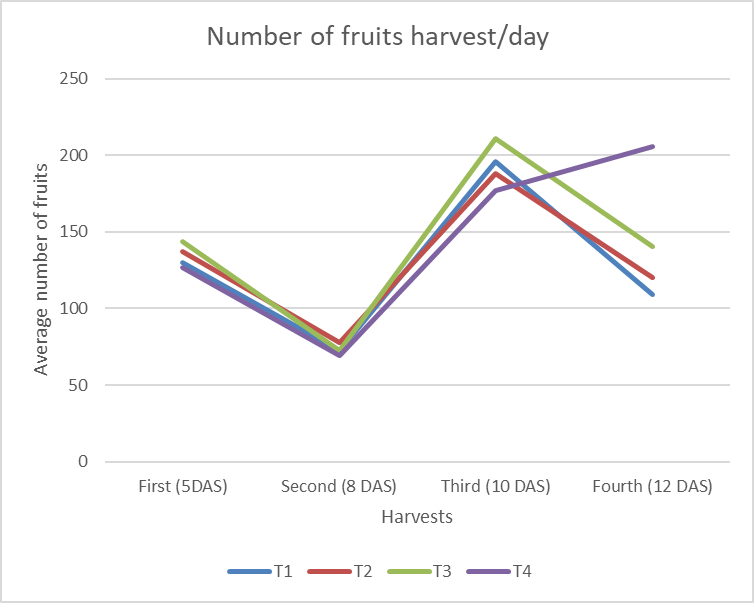
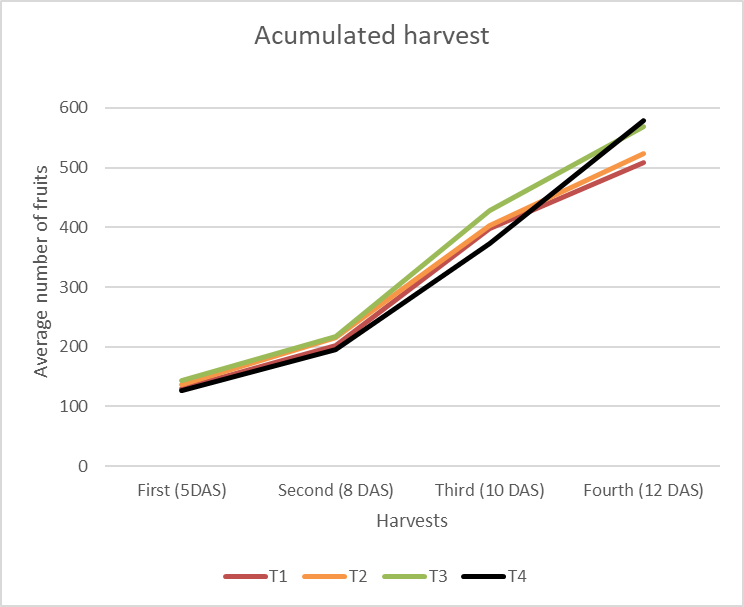
Figure 3. Harvest flushing of Cortibel RG guava trees at different days after spraying (DAS). The number of fruits harvested per day and the accumulated number during the period between harvests are shown.
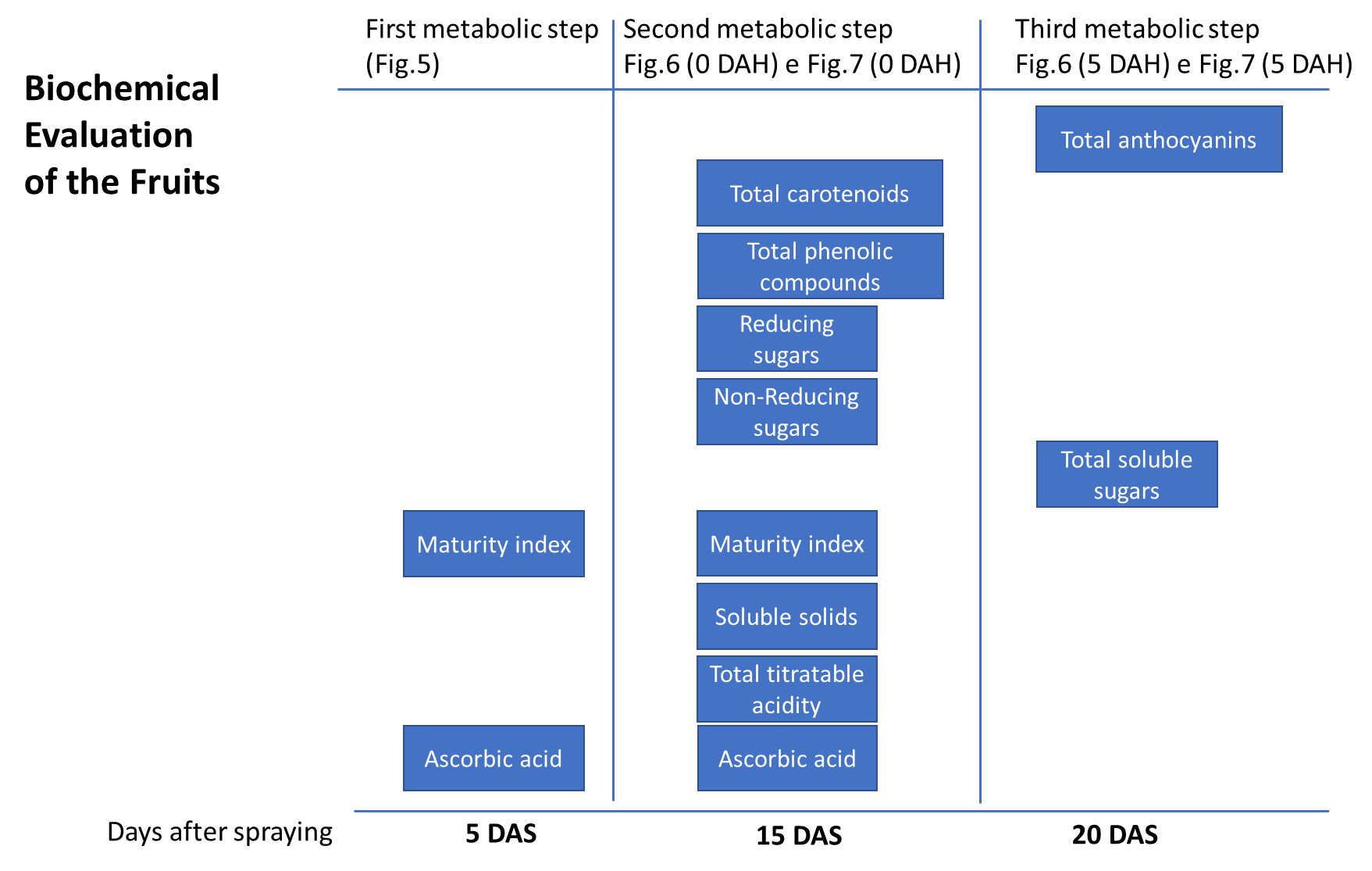
Figure 4. Flowchart identifying the biochemical markers involved in the guava fruit ripening process at each metabolic stage. The analysis considers the variables that showed statistical differences in the three metabolic steps analyzed. DAS, days after spraying; DAH, days after harvest.
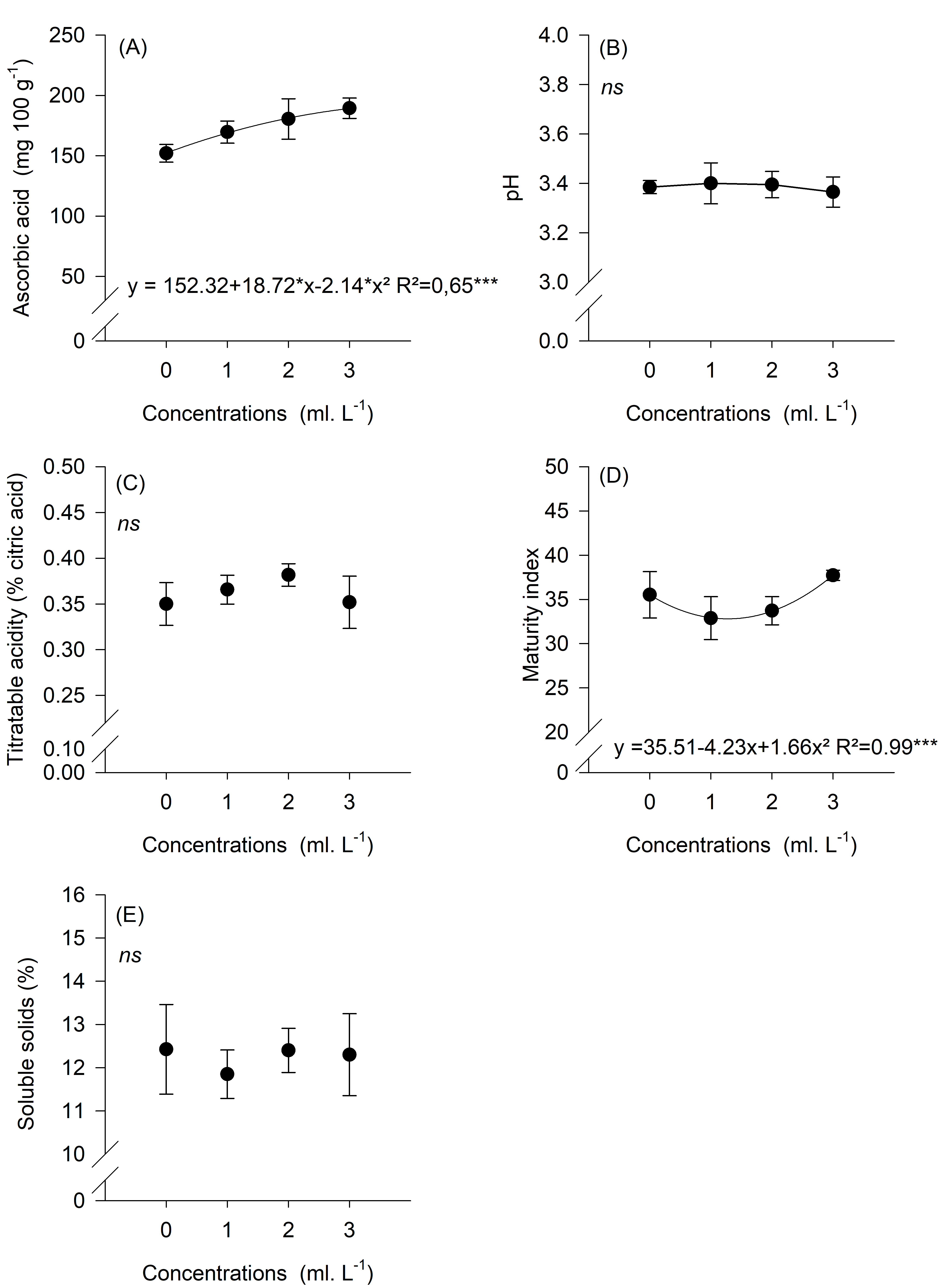
Figure 5. Mean ascorbic acid content, titratable acidy, soluble solids, and ripening index of Cortibel RG guava fruit, five days after spraying.
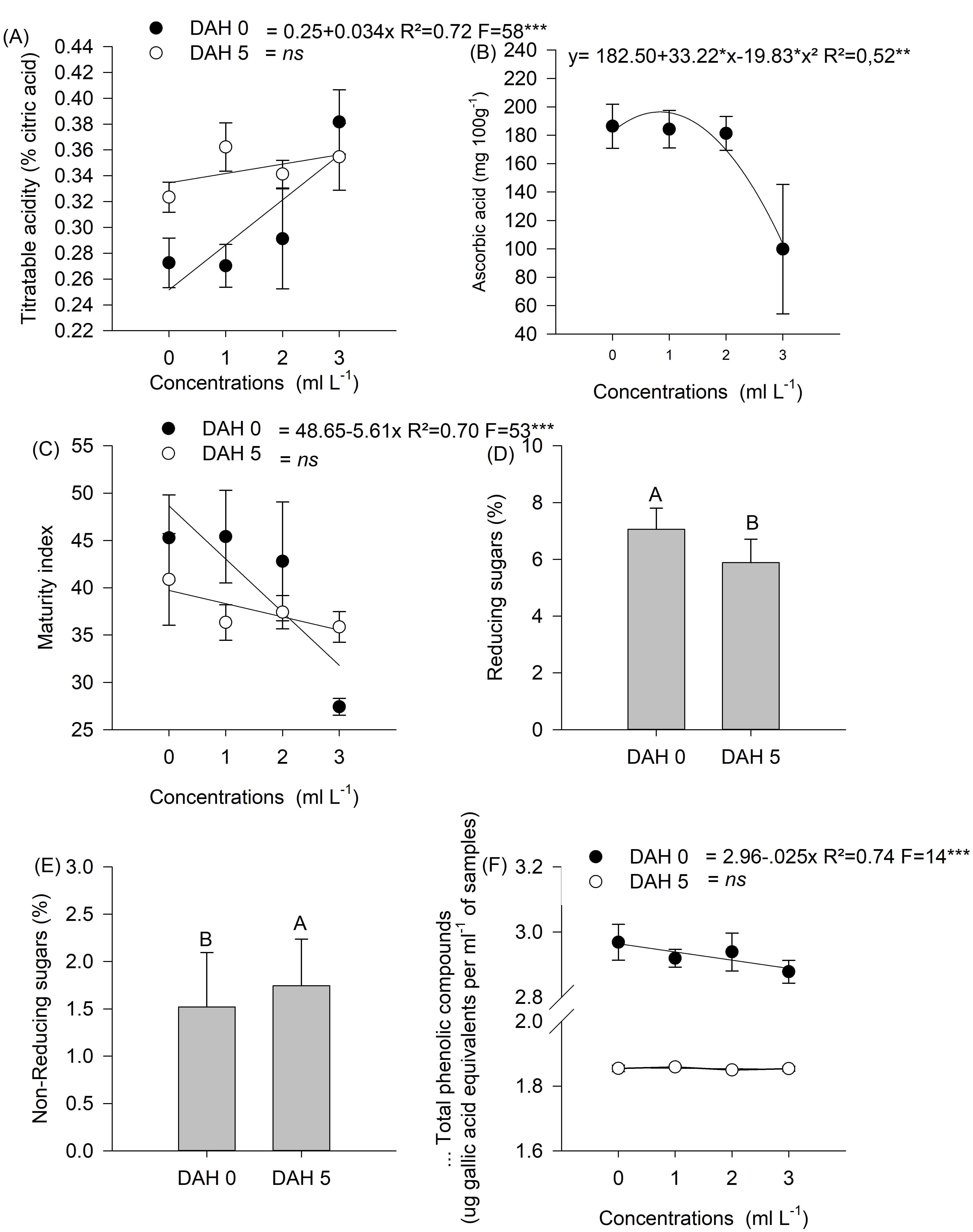
Figure 6. Results of the analyses carried out at zero and five days after harvest (DAH) for titratable acidity, ascorbic acid, ripening index, reducing sugars, non-reducing sugars, and total phenolic compounds.
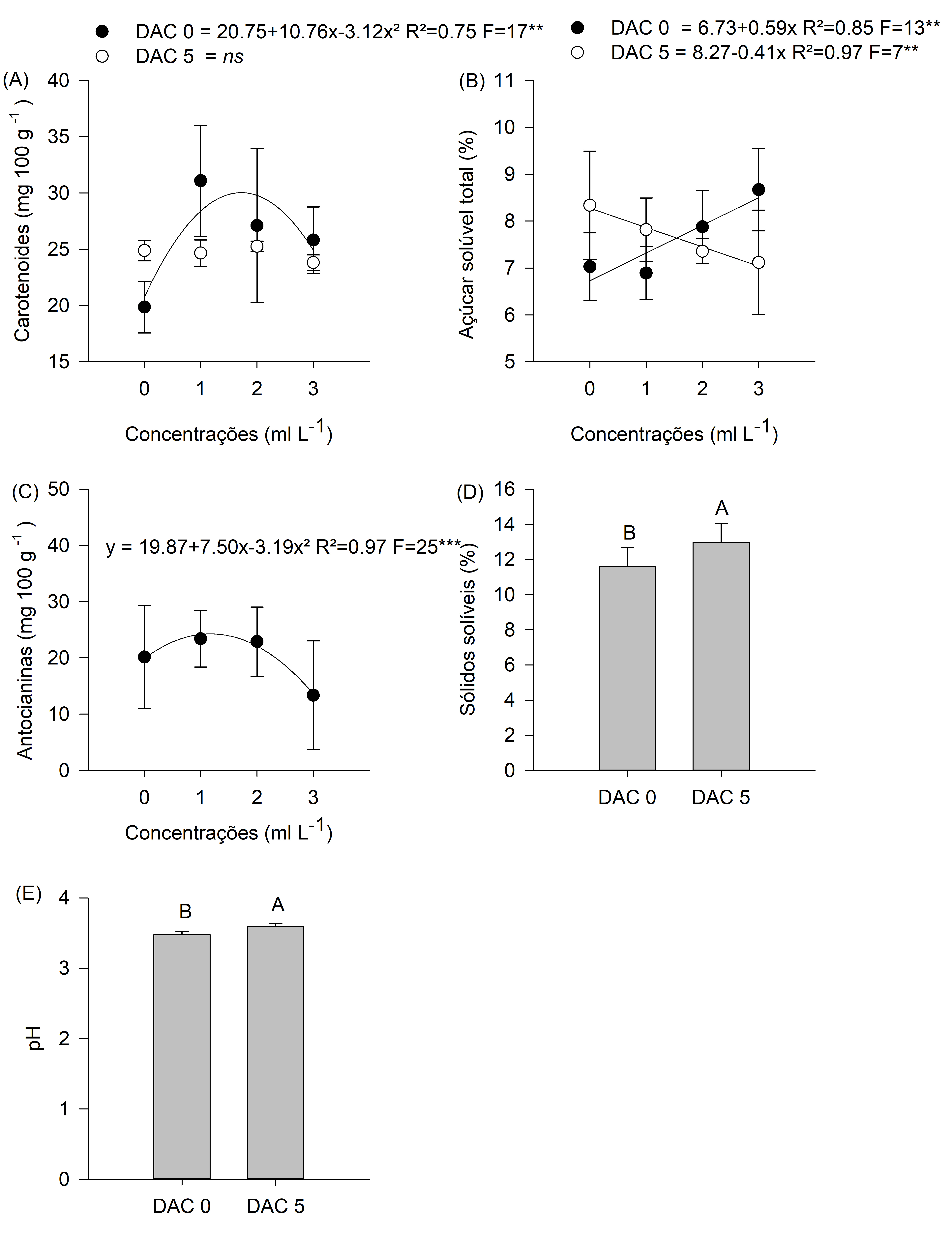
Figure 7. Results of the analyses carried out at zero and five days after harvest (DAH) for total carotenoid content, total soluble sugars, total anthocyanins, soluble solids, and pH in Cortibel RG guava fruit.
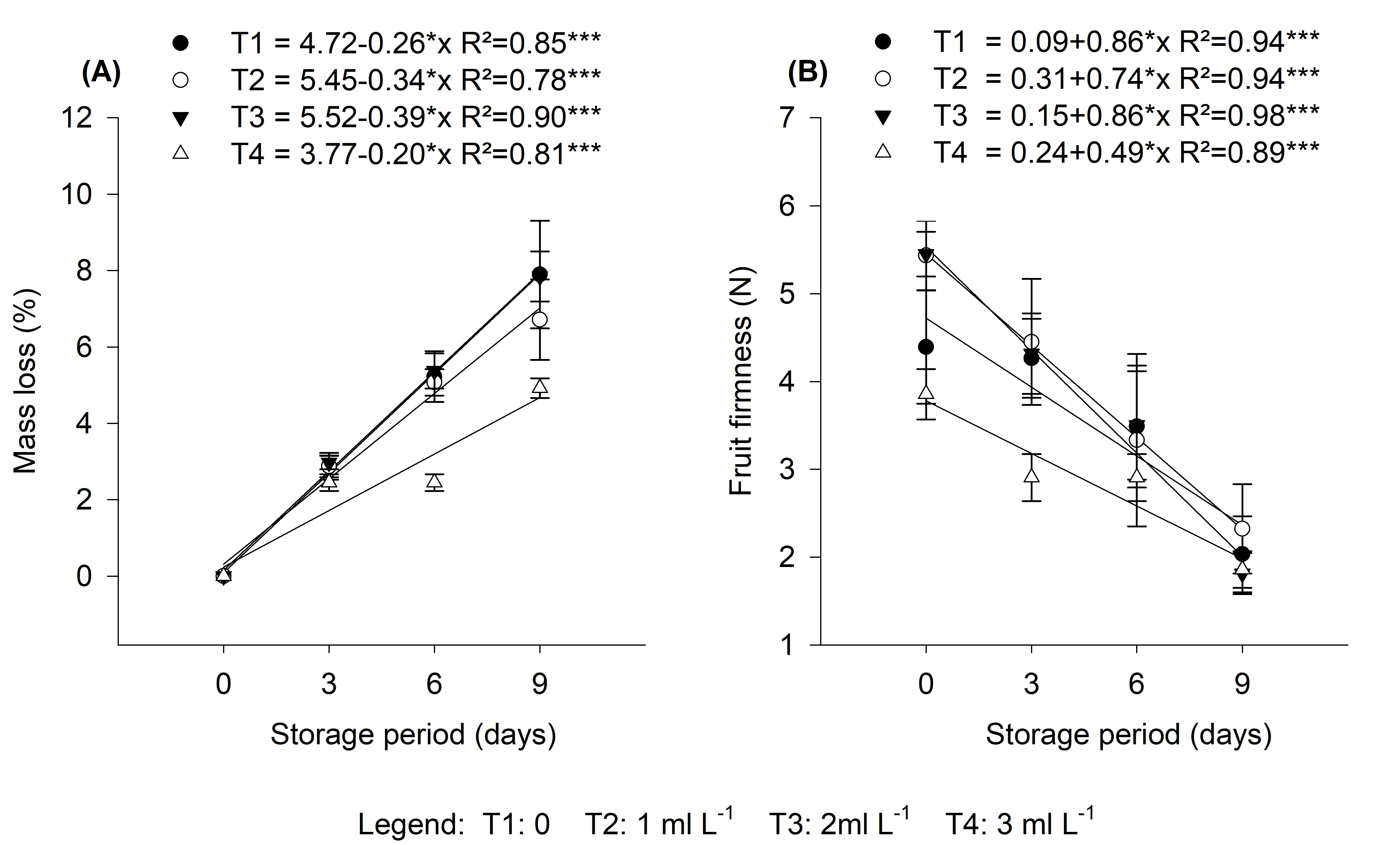
Figure 8. Relationship between storage period, mass loss, and fruit firmness in Cortibel RG guava fruit samples collected at 15 days after spraying.