

Aust J Crop Sci. 18(10):620-627 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.10.p45
Evaluate the physiological and sanitary quality of bean and maize seeds treated with Ocimum gratissimum extracts and eugenol-pure oil nanoemulsion
Juliana Trindade Lima1, Antonio Fernando de Souza2, Isabela Brunoro3, Letícia Martins De Araujo3, Hildegardo Seibert França*3
1Universidade Federal do Espírito Santo, Centro de Ciências Humanas e Naturais, Departamento de Ciências Biológicas, Programa de Pós-graduação em Biologia Vegetal, CEP 29075-910, Vitória, ES, Brasil
2Instituto Federal do Espírito Santo - campus Santa Teresa, CEP 29660-000, Santa Teresa, ES, Brasil
3Instituto Federal do Espírito Santo – campus Vila Velha, CEP 29106-010, Vila Velha, ES, Brasil
Abstract: The study aimed to assess the fungicidal potential of ethanolic and dichloromethane extracts from Ocimum gratissimum leaves and eugenol-pure nanoemulsion in the preservation and viability of bean (Phaseolus vulgaris L.) and maize (Zea mays L.) seeds during storage. Seeds were soaked in 5mg/mL ethanolic extract solution, 5mg/mL dichloromethane extract solution, and 5% eugenol nanoemulsion, followed by storage in plastic containers at room temperature for 60 days. Fungi presence was noted in the seeds, prompting evaluation. Methyl thiophanate was used as a positive control and water as a negative control. Vigor analysis used filter paper as a substrate pre- and post-storage. Effects of extracts on seed germination, germination speed index, first germination number, emergence, emergence speed index, root length, and stem length were also analyzed. The fungal diversity analysis demonstrated the presence of Aspergillus, Penicillium, and Rhizopus fungi in all treatments. For beans, eugenol nanoemulsion (4.4%) showed greater pathogen reduction, while for maize, ethanolic (40.4%) and dichloromethane (43.0%) extracts were more effective. Post-storage, bean seeds treated with ethanolic extract displayed a 98.6% germination rate, while maize seeds had an 84.6% rate. However, both dichloromethane extract and eugenol nanoemulsion negatively impacted seed vigor in both crops. Results suggest that ethanolic extract from O. gratissimum leaves could be a viable alternative for protecting seeds from fungi during storage while maintaining germination ability.
Keyword: allelopathic effect; eugenol; germination test; nanoemulsion; traditional communities.
Abbreviations AF_aqueous phase; BOD_Biological Oxygen Demand; DLS_dynamic light scattering; %E_ percentage of emerged seedling; EEtOH_ethanolic extract; ENE_eugenol nanoemulsion; FCG_First germination count; FDCM_dichloromethane extract; GSI_Germination Speed Index; GVI_germination viability index; HLB_hydrophilic-lipophilic balance; MT_fungicide Methyl thiophanate; NCS_nanostructured colloidal system; OP_oil phase; SEI_Seedling Emergence Index.
Introduction
Brazil is the world's third largest producer of maize (Zea mays L.) and beans (Phaseolus vulgaris L.), with a production of 105 million and 2.9 million tonnes respectively in 2021, according to (ONU, 2022). These foods are fundamental to Brazilian diets due to their high nutritional value, and important sources of protein, carbohydrates, vitamins, and minerals (Celmeli et al., 2018). Beans and maize creole seeds are an in vivo genetic heritage, as they have not been subjected to modern techniques of genetic modification. These seeds help to feed and provide economic stability to traditional communities (da Silva et al., 2017).
In agroecological farming, it is common practice to store seeds in PET bottles for domestic use and the next harvest. Nevertheless, farmers encounter many challenges in keeping these seeds physiologically viable due to inadequate storage conditions and susceptibility to pests and diseases. High temperature and humidity conditions during storage provide an environment for fungi to proliferate, compromising both germination and seed vigor. In addition, contaminated seeds can act as a source of infection in unfamiliar regions, potentially exacerbating the spread of diseases in the field (Stefanello et al., 2015; Garofolo et al., 2018; Leite et al., 2018).
A management challenge in agricultural pest control arises from the extensive use of chemical tools, leading to pathogen resistance and toxicity to non-target organisms (Lamichhane et al., 2020). Therefore, the use of plant extracts and essential oils is considered an ecologically safer alternative, with studies reporting their effectiveness in controlling phytopathogens (Onaebi et al., 2020). Ocimum gratissimum L., popularly known as alfavaca-cravo, is an aromatic herb with a rich diversity of secondary compounds, including phenolic compounds simple, flavonoids, terpenoids, fatty acids, tannins, and alkaloids with biological potential (Nassazi et al., 2020). This plant has been widely used in traditional medicine due to its bioactive properties, such as allelopathic, antioxidant, antibacterial, cytotoxic, and antifungal activity.
Table 1. Germination percentage (%G), germination speed index (GSI), first germination count (FGC), emergence percentage (%E), Seedling Emergence Index (SEI), root length, and stem length according to the treatments at each storage time for bean and maize creole seeds. EEtOH = ethanolic extract; FDCM = dichloromethane extract; ENE = eugenol nanoemulsion. Tukey test at p<0.05 probability.
| Bean Seed Treatment | %G | GSI | SEI | root length (cm) | stem length (cm) | *FGC (%) | *%E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t=0) | (t = 60) | |||
| Negative Control | 96.0a | 97.1a | 37.3b | 27.0b | 37.3b | 27.0b | 8.7c | 11.53bc | 8.0c | 6.4b | 83.3ab | 83.4ab |
| Methyl thiophanate | 99.3a | 98.0a | 42.8a | 30.3ab | 42.8a | 30.3ab | 11.5b | 12.0bc | 3.2d | 7.6ab | 88.0a | 88.3a |
| EEtOH | 97.0a | 98.6a | 43.7a | 33.0a | 43.7a | 33.0a | 14.2a | 15.1a | 13.7a | 8.4a | 89.2a | 89.2a |
| FDCM | 96.7a | 98.1a | 26.6c | 21.4c | 26.6c | 21.4c | 12.0a | 12.8b | 10.9b | 8.0a | 75.0bc | 75.0bc |
| ENE | 92.8b | 97.0a | 44.5c | 49.8a | 24.3c | 19.7c | 8.0c | 10.4c | 7.8c | 7.1b | 68.4c | 68.3c |
| Maize Seed Treatment | %G | GSI | SEI | root length (cm) | stem length (cm) | *FGC (%) | *%E | |||||
| (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t = 0) | (t = 60) | (t =0) | (t = 60) | |||
| Negative Control | 80.7a | 78.5ab | 31.8b | 27.5a | 38ab | 50.6a | 9.9c | 19.1b | 8.0a | 9.2b | 82.6a | 18.0b |
| Methyl thiophanate | 77.3a | 62.7c | 39.5a | 8.6b | 43.3ab | 40.6b | 16.6b | 24.7a | 7.4a | 6.7c | 76a | 15.5b |
| EEtOH | 82.0a | 84.6a | 27.8b | 10.4b | 44a | 42.7ab | 19.6a | 24.3a | 7.6a | 10.4a | 77a | 20.4a |
| FDCM | 62.7b | 78.0ab | 22.0c | 9.2b | 34b | 45.3ab | 15.9b | 20.6b | 8.2a | 11.2a | 79a | 19.8a |
| ENE | 51.40c | 75.0b | 14.1d | 7.1b | 15.3c | 20.0c | 7.7d | 15.6c | 3.8b | 8.4b | 71a | 14.7b |
The in vitro antifungal activity of O. gratissimum against postharvest fungi has been studied by Olea et al., 2019; Silva, 2021. Fungicidal action has been linked to principal components in the oil, such as eugenol, linalool, and n-hexadecanoic acid (Mohr et al., 2017; Uchegbu et al., 2019). Our research group carried out a study that demonstrated the fungicidal properties of the ethanolic and dichloromethane extracts of O. gratissimum leaves against the phytopathogens Aspergillus sp. and Rhizopus sp. in vitro assays. Gas chromatography analysis revealed eugenol as the predominant compound in the extracts under investigation (Lima et al., 2022).
Eugenol, a yellowish liquid primarily found in clove, is an aromatic phenylpropanoid with diverse properties. Commonly used as an analgesic, it also has antifungal, insecticidal, and antimicrobial properties (Didehdar et al., 2022). Eugenol is insoluble in water and oxidizes when exposed to air. An effective strategy to mitigate this problem is the incorporation of this compound into a nanoemulsion, which is a tool to improve bioavailability and prevent the degradation of unstable compounds. Nanoemulsion formulations have been explored for their potential in antifungal control in grain storage systems (Silva et al., 2022).
This study aimed to evaluate the physiological and sanitary quality of bean (Phaseolus vulgaris L.) and maize (Zea mays L.) creole seeds treated with O. gratissimum extracts and eugenol oil nanoemulsion.
Results
Preparation of nanoemulsion from commercial eugenol-pure
The nanoemulsions were formulated using binary mixtures of emulsifiers, namaly polysorbate 80/sorbitan trioleate, polysorbate 20/sorbitan monooleate, and polysorbate 20/sorbitan trioleate, at Hydrophilic-Lipophilic Balance (HLBs) values of 15; 13; 11; and 10. Different concentrations of 5%, 10%, and 15% were employed. However, post-preparation, these formulations exhibited creaming or phase separation (Fig 1).
The emulsifier combination of polysorbate 80 and sorbitan monooleate, at the concentration of 15% of the oil phase with an HLB of 13, exhibited a distinct bluish light reflection attributable to the Tyndall effect - a characteristic feature of
nanoemulsion. Particle size analysis indicated a measurement of 247.5 nm, and the zeta potential was determined to be -8.9 mV (Fig 2).
Vigor analysis of seeds
The sanitary quality of bean and maize creole seeds was evaluated for the presence of fungal growth in each treatment. The Aspergillus sp., Penicillium sp., and Rhizopus sp. genus were found on both seeds, differing in quantity from each other (Fig 3).
There was an interaction between the treatments and the storage periods for the fungal genus evaluated in the bean creole seeds (Fig 4-a-c-e). Both natural (O. gratissimum leaves extracts) and synthetic (positive control) treatments reduced Aspergillus sp. incidence compared to the negative control, but the incidence of these fungi increased after storage of the seeds (Fig 4-a). In the initial phase, the ENE showed better efficacy against Aspergillus sp. (4.4% infestation) than other products (e.g., fungicide group with 11.5%) (Figure 2-a). After 60 days, the antifungal properties of the ENE (11.1%) were comparable to the positive control (16%) and superior to EEtOH- T1 (32.0%) and FDCM – T2 (24.0%) (see Figure 2-a). After sixty days, the fungicide (1.5%) had the most significant effect on Penicillium sp. followed by the ENE – T3 (8.3%), FDCM – T2 (20.0%), and EEtOH – T1 (27.8%) (Fig 4-c).
Seeds treated with the synthetic fungicide, EEtOH, and ENE showed a reduction in the proliferation of Rhizopus sp. during both periods of storage in comparison with the negative control (Fig 4-e). Only after storage, with a 5.6% infection rate, FDCM affected the growth of this phytopathogen, achieving an effect like to the fungicide (6.0%) and EEtOH – T1 (3.1%). These treatments resulted in a more significant reduction than ENE – T3 (11.5%) and the negative control (35.0%).
When analyzing Aspergillus sp. infestation in maize creole seeds, only ENE did not inhibit fungal growth during the initial period, resembling the negative control (Fig 4-b). However, after 60 days of storage period, all applied treatments showed a decrease in pathogen incidence. Among them, EEtOH-T1 was the most effective (40.4%), followed by FDCM-T2 (43.0%) and fungicide (61.3%), while ENE-T3 displayed the lowest efficacy (66.5%) (Fig 4-b).
Before storage, only EEtOH and FDCM treatments were able to reduce Rhizopus sp. infestation on maize seeds by 2.7% and

Fig 1. Emulsions prepared from 5% pure eugenol oil and the emulsifiers: A) Polysorbate 80/sorbitan trioleate B) Polysorbate 20/sorbitan monooleate at different HLBs (from right to left 10; 11; 13 and 15) C) Polysorbate 20/sorbitan trioleate at different HLBs (from right to left 10; 11; 13 and 15).
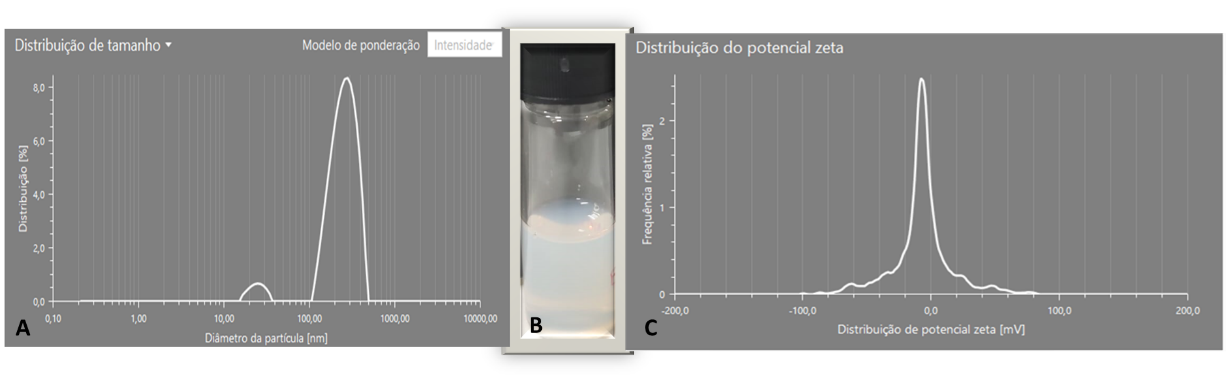
Fig 2. Emulsions from 5% pure eugenol oil and 20% polysorbate 80/sorbitan monooleate with HLB 13 (oil phase) and 75% aqueous phase. A) Dynamic light scattering (DLS) data; B) nanoemulsion; C) Zeta Potential data.
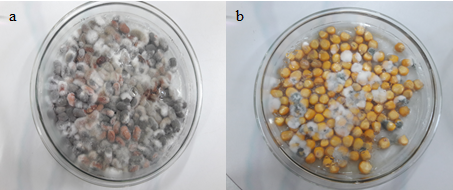
Fig 3. Incidence of fungi in bean (a) and maize (b) creole seed.
1.3%, respectively, with a significant difference from the negative control of 2.1% (Fig 4-f). Post-storage, the microorganism proliferated in all treatments except for ENE (9.0%), which displayed a decrease in infestation comparable to EEtOH (14.7%) and FDCM (3.4%). None of the products demonstrated a significant difference from the negative control in the inhibition of the growth of Penicillium sp. (Fig 4-d).
Physiological analysis of the seed after treatment
The study aimed to evaluate the storage conditions of creole bean and maize seeds treated with both natural (extracts from O. gratissimum leaves) and synthetic products (positive control). To this end, the initial water content of bean and maize seeds stored at levels between 7.3-8.4 % (Fig 5-a) and 11.7-13.2 % water (Fig 5-b), respectively, was measured. The results did not show any significant differences in humidity levels between the products used. However, a slight increase in the water content of the bean seeds after was observed storage (Fig 5-a).
Germination tests were conducted using five treatments: water, synthetic fungicide, ethanolic extract, dichloromethane extract, and eugenol nanoemulsion, on creole bean and maize seeds (Table 1). The tests were done over two storage periods: zero and sixty days. The data show significant interactions, which means that the efficacy of the treatments was related to the storage time of the seeds.
An interaction effect between the products and the storage time of the bean seeds was observed for the variables analyzed (Table 1). All treatments resulted in a similar germination (G) response, with values ranging from 92% to 99%. Before storage, only the treatment with the nanoemulsion presented a lower effect in comparison with the other treatments. During the first storage period, only EEtOH achieved a higher GVI than the negative control. Subsequently, EEtOH (51.1%), FDCM (51.6%), and ENE (49.8%) yielded better results than the fungicide (46.0%) and were comparable to the negative control (52.2%)
Root length increased after storage, with the highest mean values observed in EEtOH (15.1 cm) and FDCM (12.8 cm), while NEE had the lowest mean value of 10.4 cm compared to the negative control (11.53 cm). On the other hand, stem length decreased after storage, but the highest values were observed in EEtOH (8.4 cm), FDCM (8.0 cm), and fungicide (7.6 cm). For PCG and E, the factors were analyzed individually because there was no interaction between product and storage time. EEtOH and methyl thiophanate had the highest average FCG values with 89.2 % and 88.0 %, respectively. However, FDCM and eugenol nanoemulsion showed a decrease, with the latter exhibiting a significant decrease to 68.4%. Similar results were obtained for the variable E, indicating a decrease in GSI after storage for all the tested treatments. Eugenol nanoemulsion (19.7) and FDCM (21.4) had the lowest values, while EEtOH and fungicide had the highest averages of 33.0 and 33.3, respectively (Table 1).
Discussion
Storage fungi are common in beans and corn creole seeds (Pinto et al., 2021). This study identified Aspergillus sp., Penicillium sp., and Rhizopus sp. in both creole seeds, but at different levels. The elevated levels of fungal infections during the storage phase could be related to the increased supply of nutrients and water in the seeds. The water content of these seeds before storage was more than 13%, which could have promoted the activity of the storage fungi (Nascimento and Moraes, 2011).
The incorporation of insoluble bioactive substances into aqueous media through nanoemulsions is a viable solution for microorganism control. This study employed the low-energy input method using vortex stirring, provided DLS results at the nanoscale level. A study using the high energy input ultrasound method and polysorbate 80 to formulate eugenol nanoemulsions yielded droplet sizes ranging from 19.21 to 42.82 nm with a zeta potential of -29.83 mV. The study aimed to refine the preparation process and demonstrate the efficacy of the method in producing stable nanoemulsions. The results demonstrate the potential of the technique as a means of producing uniform droplet sizes in nanoemulsions. Additional research on blends of polysorbate 80 with other sorbitan-derived surfactants showed increased stability and reduced colloidal droplet size (Peniche et al., 2022).
EEtOH and FDCM extracts were effective in reducing the proliferation of Rhizopus sp. and Aspergillus sp. in maize seeds during biological assays, supporting a previous in vitro study conducted by our research group (Lima et al., 2022). Eugenol nanoemulsion showed the greatest reduction in Aspergillus sp. and Penicillium sp. proliferation in bean seeds in both periods studied. Eugenol is a component of the essential oil derived from the leaves of O. gratissimum and has been characterized as the major constituent of both the ethanolic extract and the dichloromethane extract (Lima et al., 2022). Previous studies have demonstrated the efficacy of O. gratissimum in controlling several phytopathogenic fungi, including Colletotrichum lindemuthianum (Silva et al., 2022), Botryosphaeria rhodina, Rhizoctonia sp. and Alternaria sp. (; Faria et al., 2006), and Botrytis cinerea (Olea et al., 2019).
Therefore, the results of this study indicate that O. gratissimum extracts have fungicidal potential in the control of storage fungi mediated by eugenol, which may act synergistically with other secondary metabolites (Mann, 2012; Nguefack et al., 2012) and/or stimulate the defense system of bean and maize seeds against phytopathogens, justifying a different mechanism of action in plant species against the tested fungi (Colpas et al., 2009). The antifungal mechanism of eugenol is associated with the disruption of the plasma cell membrane, alteration of the transport of ions and ATP and the inhibition of the production of toxins (Olea et al., 2019; Ulanowska and Olas, 2021).
In the two storage periods evaluated in this study, EEtOH did no effect on the germination of the plant species. The results confirm those obtained by Araujo et al., 2018, who observed no effects on germination with the application of O. gratissimum extract on organic seeds of cherry tomato (Lycopersicon esculentum Mill.), and by Silva, 2021 on cowpea (Vigna unguiculata L. Walp). This may be due to the antimycotic effect of the extract, which prevents biochemical changes caused by fungi that could interfere with germination (Abiala et al., 2020; Silva, 2021). Furthermore, the antioxidant capacity of O. gratissimum EEtOH, observed during the preliminary studies of this research group, could prevent oxidative reactions that lead to the degradation of biomolecules fundamental to the germination process (Li et al., 2022; Lima et al., 2022).
The percentage of germination decreased when maize seeds were exposed to FDCM and eugenol nanoemulsion, causing to a reduction in bean seeds germination, However, this effect was mitigated after storage. Literature has already described O. gratissimum phytotoxicity observed in other species Euphorbia heterophylla (Martendal et al., 2018) and Lactuca sativa L. (Miranda et al., 2015), but the allelopathic study and concomitant protection of EEtOH, FDCM and eugenol nanoemulsion in bean and maize creole seeds during postharvest has not been documented.
The germination viability index (GVI) is a parameter for the assessment of seed vigor. In the present study, it was found that the application of products such as EEtOH, FDCM, and eugenol nanoemulsion led to a decrease in the GVI of maize seeds during storage. In the case of beans, only eugenol nanoemulsion affected the GVI. FDCM treatment showed a negative effect on PCG, E, and GVI variables. The results suggest that the allelopathic effect of O. gratissimum during storage most affects maize, and the allelopathic effect is mainly due to the FDCM and eugenol nanoemulsion products. These results are consistent with the observations of Ighodaro et al. (2010) on the allelopathic effect of O. gratissimum on commercial bean and maize seeds. The variation in plant toxicity to extracts may be attributed to the presence of seed coat, which acts as a barrier between embryo and environment, and selective seed coat permeability, shielding the inhibitory activity of phytotoxic compounds (Islam and Kato-Noguchi, 2014).
Allelopathic effects of Ocimum extract may be caused by flavonoids and terpenoids, as suggested by previous studies (Ighodaro et al., 2010; Miranda et al., 2015; Martendal et al., 2018). In this study, the use of eugenol nanoemulsions
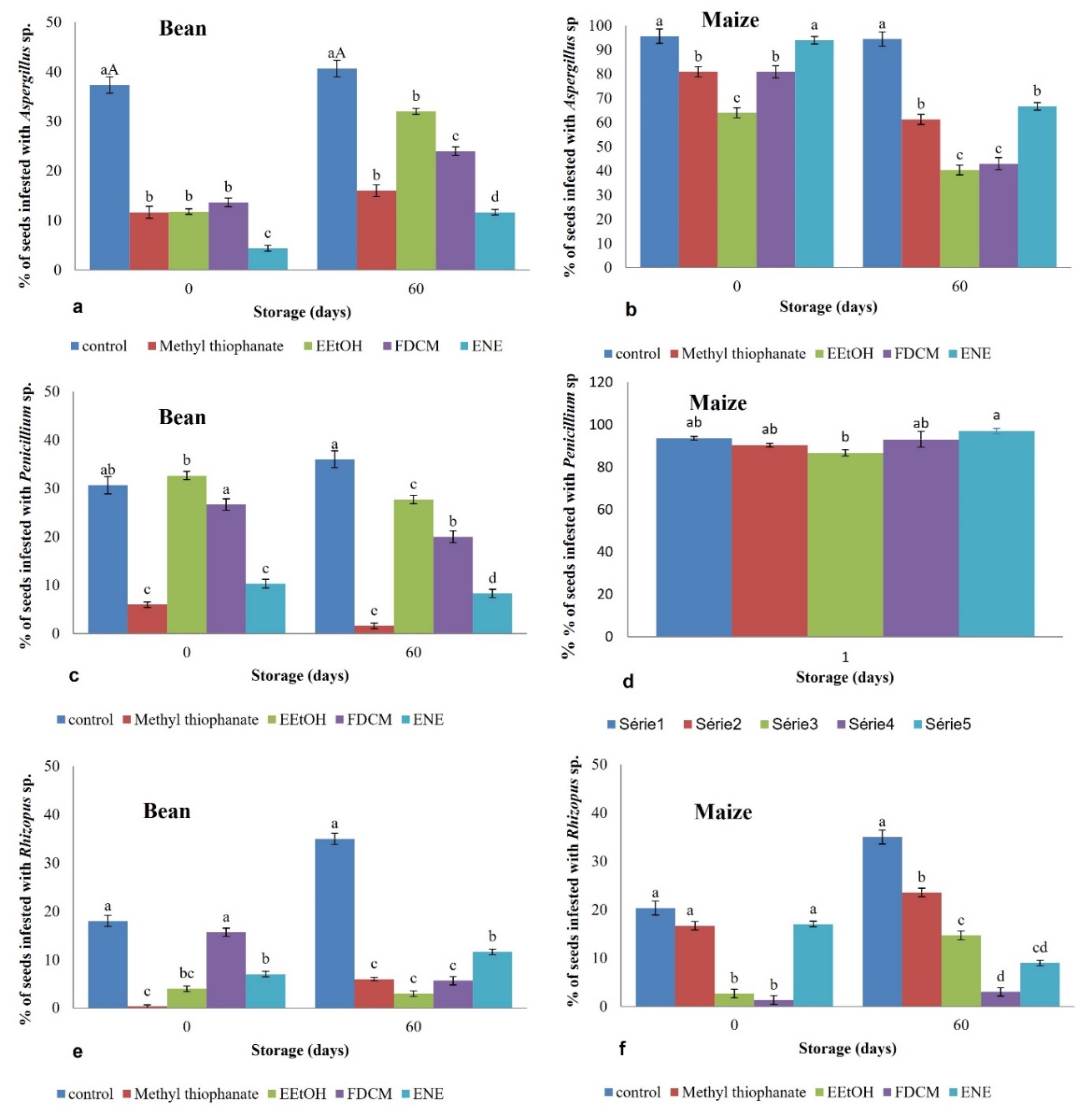
Fig 4. Fungal incidence (%) was observed on bean (a-c-e) and maize (b-d-f) creole seeds treated with different products and stored for two periods (0 and 60 days). The averages marked with the same small letter are not different for each product according to the Tukey test at 5% probability. EEtOH refers to ethanolic extract, Methyl thiophanate to synthetic fungicide; FDCM to dichloromethane extract; and ENE to eugenol nanoemulsion. The Tukey test at 5% significance level (p < 0.05) was then used to compare means.
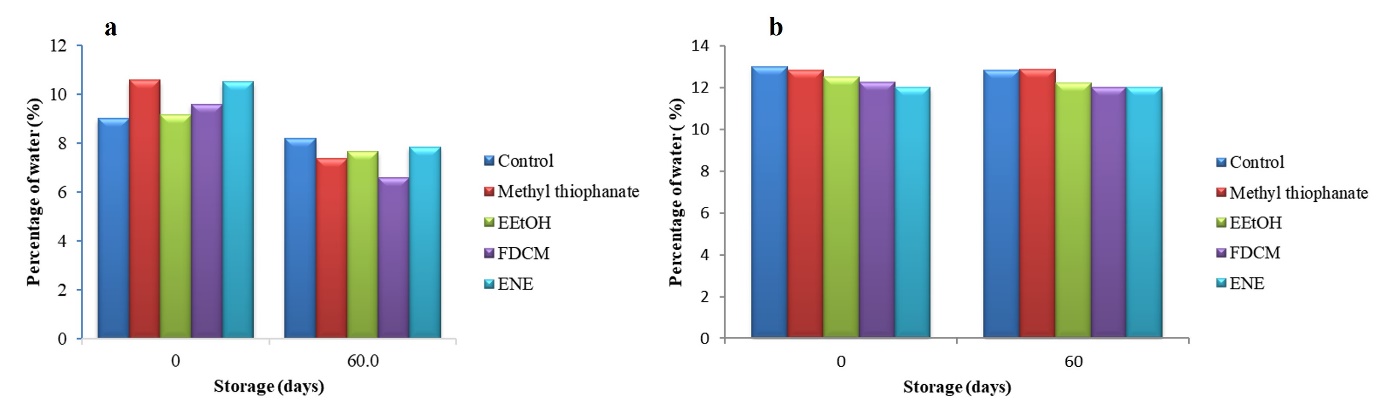
Fig 5. Percentage of water in beans (a) and maize (b) creole seeds stored in 0 and 60 days.
resulted in reduced growth of both aerial and root parts of the seed, and this effect was maintained in maize roots even after 60 days of storage. Miranda et al. (2015) reported a decrease in germination, growth vigor index, and aerial and root length after exposure to O. gratissimum essential oil and its major constituent (eugenol) in Lactuca sativa l. Our results
are consistent with their findings and emphasize a marked effect from eugenol.
To be a potential biocontrol agent, the extract with pathogen-fighting ability must have no harmful effects on seeds or seedlings. The present study shows that EEtOH successfully maintained the physiological state of bean seeds during storage. In addition, it promoted root and aerial growth of both plants during the two evaluated periods, which confirms the findings of (Abiala et al., 2020) who observed that maize seedlings treated with aqueous extracts of O. gratissimum had improved germination and growth, indicating a positive response of maize seedlings to aqueous extracts of O. gratissimum. Furthermore, the positive response of EEtOH in the present study may be attributed to the low concentration and proportion of phytochemicals based on the findings of Martendal et al., 2018; Mekky et al., 2019; Silva, 2021. However, the allelopathic effect of FDCM and eugenol nanoemulsion was found to affect both seeds. In particular, the inhibitory effect of eugenol nanoemulsion persisted in both storage periods, as expected due to its superior physicochemical stability and biological efficacy of bioactive compounds (Luo et al., 2020).
Material and Methods
Preparation of the plant extracts
Leaves of O. gratissimum were obtained from the Fazenda Experimental Engenheiro Reginaldo Conde (FERC), in Viana/ES, at the Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural (INCAPER). The species was identified by researchers from the Federal University of Espírito Santo (UFES). The exsiccate was deposited in the herbarium of the Botany Department of the UFES under the code VIES 36175.
The leaves were dried in an oven with circulating air at a temperature of 40 °C for 24 hours and then crushed. The ethanolic extract (EEtOH) was obtained by maceration of the leaves in 96% ethanol at a ratio of 1:10 (plant: ethanol) and kept at room temperature, protected from light. After filtration, the ethanol was evaporated using a rotary evaporator. The recovered solvent was added to the remaining leaf and the maceration was repeated until the complete exhaustion of the plant drug had been achieved. The concentrated residue obtained (called EEtOH) was stored in an amber-colored glass vial under refrigeration at 4 ºC. Part of the EEtOH was suspended in a mixture of ethanol with water (8:2), which was extracted with the organic solvent dichloromethane to obtain the dichloromethane extract (FDCM), after solvent elimination.
Preparation of nanoemulsion from commercial eugenol-pure
Pure eugenol oil was purchased commercially and employed to develop a nanoemulsion through the low-energy process (Peniche et al., 2022). The formulation of the eugenol nanoemulsion (ENE) involved combining the oil phase (OP) and aqueous phase (AF) based on the following formula:
% total (final weight of 2g)= OF5% eugenol oil + X% Emulsifiers+ AF95% - X% Emulsifiers,
being X% = 20%, 15%, 10% e 5%.
Surfactant mixtures of polysorbate 20 (HLB 16.7), polysorbate 80 (HLB 15), sorbitan trioleate (HLB 1.8) and sorbitan monooleate (HLB 4.3) with different final hydrophilic-lipophilic balance (HLBf) values of 15, 13, 11 and 10 were used for the preparation of the nanoemulsion. To determine the gram amount of each surfactant (ma and mb), was considered the HLBf value, the desired percentage/total mass of surfactant (mt = ma + mb) in the solution, and the HLB values of the emulsifiers used (HLBa and HLBb) were taken into account, employing theformula:HLBf = (HLBa . ma) + ((HLBb . mb))/ (ma + mb).
Subsequently, the AP was added to the OP with constant stirring using a vortex apparatus, to obtain a nanostructured colloidal system (NCS), that exhibited slightly blue reflection due to the Tyndall effect. The most stable NCS were identified using a Litezer 500 instrument to evaluate dynamic light scattering (DLS) and zeta potential. The nanostructures of the essential oils were diluted from 5 % to 1 % using ultra-pure water at a temperature of 25 °C.
Seed treatments
The creole seeds of the bean (Phaseolus vulgaris L.) and of the maize (Zea mays L.) were obtained from the germplasm bank of the Federal Institute of Espirito Santo, Campus of Santa Teresa, ES. The seeds were then immersed for 15 minutes in three different treatments: T1) 5.0 mg/mL ethanolic extract (EEtOH) of O. gratissimum leaves, T2) 5.0 mg/mL dichloromethane extract (FDCM) of O. gratissimum, and T3) 5% eugenol nanoemulsion (ENE). The commercial fungicide Methyl thiophanate (MT) was used as a positive control at a concentration of 0.8 mg/mL. Distilled water was used as a negative control for seed immersion. Seeds were naturally dried on plastic trays for 24 hours after treatment. To simulate storage practices in agroecological systems, the seeds were then transferred to hermetically sealed plastic containers and stored for 60 days at ambient conditions. The seeds were evaluated on two occasions: immediately after the treatments were applied and after the 60 days of storage.
Analysis of creole seeds vigor
The seed vigor test was performed using filter paper as a substrate. Four replicates of 25 seeds were distributed in Petri dishes on three sheets of filter paper moistened with distilled water. The Petri dishes were kept in a B.O.D. (Biological Oxygen Demand) incubator at a temperature of 25°C under a lighting regime with a 12-hour photoperiod. After 24 hours, the seeds were frozen for 24 hours and then returned to the incubator for 10 days. The fungi were identified, and the damaged seeds were counted under a stereoscopic microscope (Neergaard, 1977). The result was expressed as a percentage of infested seeds, by the recommendations of the seed analysis rules (Brasil, 2009b).
Physiological Quality of seeds
The water content of the seeds was assessed before and after storage, according to the regulations for the analysis of seeds (Brasil, 2009a). The seeds were subjected to the oven method at 105 ± 3 °C for 24 h, with two replicates of 25 seeds. The result was expressed as a percentage (%) of water content (ratio between the weight of water in the seed and the total seed mass).
The germination test was carried out according to the rules for seed analysis (Brasil, 2009a). A total of four replicates were used, each containing 25 seeds, which were placed on two sheets of germination paper and covered with an additional sheet, forming rolls that were moistened with distilled water at a ratio of 2.5 parts of water to one part of the weight of the paper. These rolls were then placed in polyethylene bags and stored in a B.O.D. at 25°C under a 12-hour light-12-hour dark cycle. Seedlings were evaluated daily after test establishment and results were expressed as percent germination (%G).
The first count of the germination test (FCG) was carried out simultaneously with the germination test. In this test, the seedlings were counted on the fifth day (beans) and the fourth day (maize) after seeding, and the results were expressed as a percentage (Brasil, 2009a)
The Germination Speed Index (GSI) was evaluated daily during the germination test, starting from the day when the first seeds emitted a radicle until the last day counted, according to the Rules for Seed Analysis (BRASIL, 2009a). For the calculation, the formula proposed by (Maguire, 1962) was used.
$GSI\ = \frac{G1}{T1} + \frac{G2}{T2} + \ \cdots\ + \frac{Gi}{Ti}$Where GSI is the germination speed index, while G1 to Gi is the number of seedlings that have germinated each day, and T1 to Ti is the time in days.
Seedling emergence was performed with four replicates of 25 seeds per replicate, sown individually in cells with "sand" substrate and placed in polystyrene containers in the germination chamber. The assessment was made 12 days after seeding to determine the percentage of emerged seedlings (%E).
The Seedling Emergence Index (SEI), according to the protocol established by the Rules for Seed Analysis (Brasil, 2009a), was determined along with the seedling emergence evaluation, recording the number of seedlings daily from the first to the tenth day. The value of the SEI was calculated according to the formula of (Maguire, 1962).$SEI\ = \frac{E1}{T1} + \frac{E2}{T2} + \ \cdots\ + \frac{Ei}{Ti}$
SEI = Seedling Emergence Index; E1 to Ei is the number of emergences for each day, and T1 to Ti is the time in days.
Statistical analysis
The study used a fully randomized experimental design consisting of a 5x2 factorial with five products and two storage periods. The data were analyzed using analysis of variance, with the product x storage period interaction split when significant. The Tukey test at 5% significance level (p < 0.05) was then used to compare means. In cases where the interaction was not significant, a single-factor analysis was carried out.
Conclusion
Overall, the results suggest that due to the presence of eugenol and other fungicidal components in the extract composition, EEtOH treatment from O. gratissimum could be a viable alternative for protecting creole seeds from fungi during storage while maintaining their germination ability. This extract has the potential to be a biodegradable solution for the resistance of pathogens in the organic farming system. However, it is necessary to carry out additional research to analyze the nanoemulsions of the extract and the extracts of O. gratissimum that can optimize and extend the bioactivity of the product.
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship – Financial Code 001; to the Espírito Santo Research and Innovation Support Foundation (FAPES) APES/SEAG (Processo 76449130/16) and Federal Institute of Espirito Santo (IFES) by financial support from PRODIF.
References
Abiala MA, Akanmu AO, Oribhaboise AC, Aroge T (2020) Combined effects of Ocimum gratissimum and soil-borne phytopathogenic fungi on seedling growth of quality protein maize. J. Adv. Biol. Biotechnol. 23(3): 25–32.
Araujo EF, Mauri AL, Araujo RF, Amaro HTR, Silva DJH, Santos DDCF (2018) Physiological and sanitary quality of organic tomato seeds treated with clove basil extracts. Comun. Sci. 9(1): 6–33.
Brasil (2009a) Ministério da Agricultura, Pecuária e Abastecimento. Manual de Análise Sanitária de Sementes (1st ed.). Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília, DF: MAPA/ACS, 200p.
Brasil (2009b) Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília, DF: MAPA/ACS, 395 p.
Celmeli T, Sari H, Canci H, Sari D, Adak A, Eker T, Toker, C (2018) The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy. 8(9): 166.
Colpas TF, Schwan-Estrada KRF, Stangarlin JR, Ferrarese ML, Scapim, CA, Bonaldo SM (2009). Induction of plant defense responses by Ocimum gratissimum L. (Lamiaceae) leaf extracts. Summa Phytopathol 35: 191-195.
Faria TJ, Ferreira RS, Yassumoto L, Pinto SJR, Ishikawa NK, Melo BA (2006) Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz. Arch. Biol. Technol. 49: 867–871.
da Silva PM, Antunes IF, Feijó CT, Bevilaqua GAP (2017) Transgênicos e erosão genética: o paradoxo da (in) segurança alimentar. Agroecología. 12(2): 81-87.
Didehdar M, Chegini Z, Shariati A (2022) Eugenol: A novel therapeutic agent for the inhibition of Candida species infection. Front. Pharmacol. 13: 872127.
Garofolo AC, Risso IA, Barbosa D (2018) Conservação on farm de variedades crioulas em bancos familiares localizados em assentamentos rurais em território da cidadania no estado do Rio de Janeiro. Cadernos de Agroecologia.13(1).
Ighodaro OM, Agunbiade SO, Akintobi O (2010) Phytotoxic and antimicrobial activities of flavonoids in Ocimum gratissimum. Life Sci. J. 7(3): 45–48.
Islam AKMM, Kato-Noguchi H (2014) Phytotoxic activity of Ocimum tenuiflorum extracts on germination and seedling growth of different plant species. Sci. World J. 2014.
Lamichhane JR, You MP, Laudinot V, Barbetti MJ, Aubertot JN (2020) Revisiting sustainability of fungicide seed treatments for field crops. Plant Disease. 104(3): 610–623.
Leite K, Bonome LTS, Moura GS, Franzener G (2018) Óleos essenciais no tratamento de sementes de Phaseolus vulgaris L. durante o armazenamento. Revista Verde de Agroecologia e Desenvolvimento Sustentável. 13(2): 186.
Li W, Niu Y, Zheng Y, Wang Z (2022) Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Frontiers in Plant Science. 13: 1–9.
Lima J T, Souza AF, França HS (2022) Ocimum gratissimum L.: A natural alternative against fungi associated with bean and maize seeds during storage. Agronomia Colombiana. 40 (3): 395–402.
Luo Y, Wang Q, Zhang Y (2020) Biopolymer-based nanotechnology approaches to deliver bioactive compounds for food applications: a perspective on the past, present, and future. J. Agric. Food Chem. 68(48): 12993–13000.
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. Madison, 2(1):176-177.
Mann A (2012) Phytochemical constituents and antimicrobial and grain protectant activities of Clove Basil (Ocimum gratissimum L.) grown in Nigeria. International Journal of Plant Research. 2(1): 51–58.
Martendal CO, Mantovanelli GC, Reis B, Cavaleiro C, Iwamoto ELI, Bonato CM (2018) Effects of Ocimum gratissimum L. extract on the germination, respiration, and growth of Euphorbia heterophylla L. Allelopathy Journal. 45(1): 29–44.
Mekky MS, Hassanien AMA, Kamel EM, Ismail AEA (2019) Allelopathic effect of Ocimum basilicum L. extracts on weeds and some crops and its possible use as new crude bio-herbicide. Annals of Agricultural Sciences. 64, 211–221.
Miranda CASF, Graças CM, Carvalho MLM, Machado SMF, Souza G.M, Andrade SJ, Teixeira ML (2015) Atividade alelopática de óleos essenciais de plantas medicinais na germinação e vigor de aquênios de alface. Semina: Ciências Agrarias. 36(3): 1783–1798.
Mohr FBM, Lermen C, Gazim ZC, Gonçalves JE, Alberton O (2017) Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genet. Mol. Res. 16(1): 1-10.
Nascimento WMOD, Moraes MHD (2011) Fungos associados a sementes de açaí: efeito da temperatura e do teor de água das sementes durante o armazenamento. Revista Brasileira de Sementes. 33: 415–425.
Nassazi W, K’Owino IO, Makatiani J, Wachira S (2020) Phytochemical composition, antioxidant and antiproliferative activities of African Basil (Ocimum gratissimum L.) leaves. Asian Journal of Applied Chemistry Research. 6(4):1–18.
Neergaard P (1977) Seed pathology Vols. 1 and 2. The MacMillan Press.
Nguefack J, Tamgue O, Dongmo JBL, Dakole CD, Leth V, Vismer HF, Amvam ZPH, Nkengfack AE (2012) Synergistic action between fractions of essential oils from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Penicillium expansum. Food Control. 23(2): 377–383.
Olea AF, Bravo A, Martínez R, Thomas M, Sedan C, Espinoza L, Zambrano E, Carvajal D, Silva-Moreno E, Carrasco H (2019) Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules. 24(7): 1239.
Onaebi C, Onyeke C, Osibe D, Ugwuja F, Okoro A, Onyegirim P (2020) Antimicrobial activity of Ocimum gratissimum L. and Carica papaya L. against postharvest pathogens of avocado pear (Persea americana Mill.). J. Plant Pathol. 102(2): 319–325.
ONU. (2022). Food and Agriculture Organization. https://fenix.fao.org/faostat/internal/en/#data
Peniche T, Duarte JL, Ferreira RMA, Sidônio IAP, Sarquis RSFR, Sarquis ÍR, Oliveira AEMFM, Cruz RAS, Ferreira IM, Florentino AC, Carvalho JCT, Souto RNP, Fernandes CP (2022) Larvicidal effect of Hyptis suaveolens (L.) poit. essential oil nanoemulsion on Culex quinquefasciatus (Diptera: Culicidae). Molecules. 27 (23): 8433.
Pinto KM, Noronha DA, Mosser LM (2021) Qualidade sanitária de sementes crioulas de feijão no agreste de Pernambuco. Brazilian Journal of Agroecology and Sustainability. 3(1).
Silva AA, Pereira FAC, Souza EA, Oliveira DF, Nobre DAC, Macedo WR, Silva GH (2022) Inhibition of anthracnose symptoms in common bean by treatment of seeds with essential oils of Ocimum gratissimum and Syzygium aromaticum and eugenol. Eur. J. Plant Pathol. 163(4): 865–874.
Silva CM (2021). Extratos naturais na qualidade sanitária e fisiológica de sementes de feijão-caupi. Tese da Universidade Federal de Viçosa (Pós-Graduação em Fitotecnia), 82f.
Stefanello R, Muniz MFB, Nunes UR, Dutra CB, Somavilla I (2015) Physiological and sanitary qualities of maize landrace seeds stored under two conditions. Ciência e Agrotecnologia. 39(4): 339–347.
Uchegbu RI, Akalazu JN, Sokwaibe CE (2019) An evaluation of the chemical compositions and antifungal activity of Ocimum gratissimum (Nchuanwu) leaves against some plant pathogens. Asian Journal of Applied Chemistry Research. 2(3-4): 1–7.
Ulanowska M, Olas B (2021) Biological properties and prospects for the application of eugenol - a review. Int. J. Mol. Sci. 22(7): 3671.