

Aust J Crop Sci. 18(10):693-699 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.10.p89
Interference periods of sourgrass (Digitaria insularis) on maize crop
Ana E. Piazentine, Heytor L. Martins*, Juliana de S. Rodrigues, Willians C. Carrega, Pedro L.C.A. Alves.
Department of Biology Applied to Agriculture, Sao Paulo State University, Jaboticabal, SP, Brazil.
*Corresponding author: Heytor L. Martins 
 ORCID:
https://orcid.org/0000-0002-5786-2678
ORCID:
https://orcid.org/0000-0002-5786-2678
Abstract: Sourgrass (Digitaria insularis (L.) Fedde) is frequently reported as one of the main weeds in grain production, along with several reports of herbicide resistance. The objectives of this study were (1) to understand the length of time that the maize crop, cv. P4285VYHR PIONEER could coexist with a weed community dominated by D. insularis plants without suffering any negative impact on its final production, and (2) the period for which the crop needs to be free of this interference to ensure optimal production. The periods of weed interference and/or control were: 15, 30, 45, 60, 75, 90, 105, and 130 days after sowing maiz, to determine the period before interference (PBI), the total period of interference prevention (TPIP), and the critical period of weed interference (CPWI). A weed community predominantly containing D. insularis coexisting with the maize crop decreases yield by up to 86%, reduces plant height, the insertion height of the first ear, and the weight of 1000 grains. Maize can coexist with such a community (PBI) for 30 DAS, tolerating yield losses of up to 5%, without TPIP and, consequently, CPWI.
Keywords: Digitaria insularis (L.) Fedde. Zea mays L.. weeds. Coexistence. PAI.
Abbreviation: PBI_Period Before Interference, TPIP_Total Period of Interference Prevention, CPWI_Critical Period of Weed Interference, DAS_Days After Sowing.
Introduction
Maize is the second most widely cultivated crop in Brazil. According to CONAB (2024), it is estimated that the 2023/2024 crop will account for the production of 146,521.8 tons on 45,235.4 mil ha-1. Adopting technologies throughout crop production, such as no-till farming and genetically modified cultivars, especially those conferring herbicide resistance has modified weed management (Melo et al., 2017). Weed interference with maize crops can lead to yield losses ranging from 38 to 65% (Salgado, Pitelli and Alves, 2005; Gantoli et al., 2013; Kashiwaqui, 2016; Lopes-Ovejero et al., 2017).
Herbicide resistantweeds in grain-producing areas are not uncommon (Heap, 2024). The indiscriminate use of broad-spectrum control herbicides on genetically modified cultivars (Roundup Ready™ and LibertyLink®, respectively) has intensified the selection of weeds resistant to these herbicides, reducing the diversity of management techniques previously employed (Vencill et al., 2012).
Weeds are characterized by aggressiveness in competing for environmental resources, rapid development, and high reproductive capacity (Pitelli, 1985). Sourgrass (Digitaria insularis (L.) Fedde) is a native and aggressive perennial grass from the American continent that has emerged as one of the significant threats to agriculture in Brazil, mainly in the Southeast, Center-west, and Northeast regions (Lopez-Ovejero et al., 2017). Several cases of glyphosate-resistant weeds in Brazil and worldwide have been confirmed, mainly in grain-producing areas (Carvalho et al., 2011; Lopez-Ovejero et al., 2017). The resistant sourgrass biotype is more competitive than the susceptible one, as it sprouts in a broader temperature range (Martins et al., 2017; Mondo et al., 2010), tolerates water deficit and deeper sowing depths (Martins et al., 2017) and has a thicker epidermis (Barroso et al., 2015).
Adopting genetically modified maize plants has increased herbicide-resistant weeds, resulting in inefficiency in weed control and potential crop losses (Lisboa et al., 2019). Therefore, field studies to determine the degree and interference periods, if any, especially in the case of D. insularis, are critical to support this weed's management (Lisboa et al., 2019). Pitelli and Durigan (1984) defined three weed interference periods on crops: the period before interference (PBI), the total period of interference prevention (TPIP), and the critical period of weed interference (CPWI). The various stages of coexistence are labeled as the "critical time for weed removal" (CTWR), the "pre-interference period" (PIP), and the "critical weed control period" (CPWC) (Pitelli, 1985; Knezevic et al., 2002). CTWR refers to the timeframe in which the crop needs to be kept free from weeds to prevent significant interference-related losses. PIP delineates the period during which the primary crop and weeds can coexist without yield reduction. CPWC emerges when CTWR surpasses PIP, signaling the phase necessitating management to avert economic losses (Knezevic et al., 2002; Galon et al., 2018). Regression curves were used to obtain these periods, and they relate the crop yield as a function of the coexistence/control periods with the weeds (Kuva et al., 2000).
It is suggested that D. insularis could negatively affect the growth and yield of maize, and this negative impact might be related to the period of coexistence (Timossi, 2009). Therefore, this study aimed to determine: (1) the length of time that the maize crop, cv. P4285VYHR PIONEER could coexist with a weed community dominated by D. insularis plants without suffering any negative impact on its final
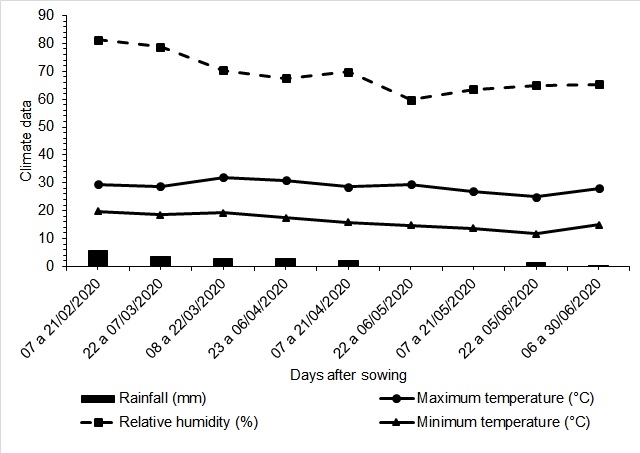
Figure 1. Climatic conditions during the experiment with the maize crop 'P4285VYHR PIONEER', rainfall (mm), relative humidity (%), maximum temperature (°C), and minimum temperature (°C).
Table 1. Experimental treatments for determining the interference periods in the maize crop 'P4285VYHR PIONEER'.
| Treatments | Coexistence periods (days) | Treatments | Control periods (days) |
| 1 | 0 - 15 | 9 | 0 - 15 |
| 2 | 0 - 30 | 10 | 0 - 30 |
| 3 | 0 - 45 | 11 | 0 - 45 |
| 4 | 0 - 60 | 12 | 0 - 60 |
| 5 | 0 - 75 | 13 | 0 - 75 |
| 6 | 0 - 90 | 14 | 0 - 90 |
| 7 | 0 - 105 | 15 | 0 - 105 |
| 8 | 0 –130 | 16 | 0 - 130 |
Table 2. List of weed species and respective international code* found in the maize crop 'P4285VYHR PIONEER',
| Family | Species | Code* | Common Name |
|---|---|---|---|
| Amaranthaceae | Alternanthera tenella Colla | ALRTE | Joyweeds |
| Asteraceae | Acanthosperum hispidum DC. | ACNHI | Bristly starbur |
| Commelinaceae | Commelina benghalensis L. | COMBE | Benghal dayflower |
| Convolvulaceae | Ipomoea sp. | - | Morning glory |
| Malvaceae | Sida glaziovii K. Schum. | SIDGZ | Fanpetals |
| Poaceae | Cenchrus echinatus L. | CCHEC | Southern sandbur |
| Poaceae | Digitaria insularis L. | DIGIN | Sourgrass |
*International code, according to the International Weed Society.
production (PBI), and (2) the period for which the crop needs to be free of this interference (TPIP) to ensure optimal production.
Results and Discussion
The weed community comprised seven weed species belonging to six botanical families (Table 2). The Poaceae family was represented by two species, Cenchrus echinatus L. and Digitaria insularis L. (Table 2). The low diversity in the weed flora is mainly due to the aggressive characteristics and adaptation of these plants to the no-till farming system (Timossi, 2009).
Regarding weed density (Figure 2), the coexistence periods presented the highest number of individuals in all evaluations, with a population peak of 16 plants m-2 at 30 and 45 DAS. There was a pronounced reduction at 60 DAS to 5 plants m-2, which continued until 75 DAS, while in the other evaluations, the number of individuals was constant (~6 plants m-2). Conversely, the control periods maintained a relatively stable weed density, with approximately 3 plants m2 in the initial three evaluations (15, 30, and 45 DAS). Subsequently, the number of plants decreased to 1 plant m2 in the following evaluation. No more weeds were found after this evaluation.
Variations in shoot dry mass accumulation were noted across the interference periods (Figure 3), with exponential
accumulation observed during the coexistence periods. Regarding the control periods, the accumulation was minimal, on average 48.4 g m2, and no weeds were found in the last evaluations, as mentioned above. Oliveira et al. (2010) found the same dynamics in an experiment involving weed communities and maize hybrids.Throughout the coexistence period, weed density decreased while the dry mass of the weeds increased, indicating that the weeds in the plots continued to grow. The evaluations showed that the more aggressive species and those that sprouted or resprouted and established themselves first were the ones that dominated the area (Radosevich & Holt, 1984). Furthermore, for the control periods, the removal of weeds for increasing periods favored the maize development over weeds. This, coupled with the limited rainfall (Figure 1), a characteristic of second-crop rotations, slowed the resprouting and emergence of weeds, particularly after 60 DAS.
The weed community in the experiment showed that D. insularis (DIGIN) and Alternanthera tenella Colla (ALRTE) were the most important weeds with the highest relative importance (RI) (Figure 4). The weed diversity in the plots was initially high but decreased as the experiment progressed. Only A. tenella (ALRTE) and D. insularis (DIGIN) were present in the last two evaluations (105 and 130 DAS), and no weeds were found in the last three evaluations (90, 105, and 130 DAS). Timossi (2009) observed that in no-till
Figure 2. Weed community density as a function of coexistence and control periods in maize crop 'P4285VYHR PIONEER'.
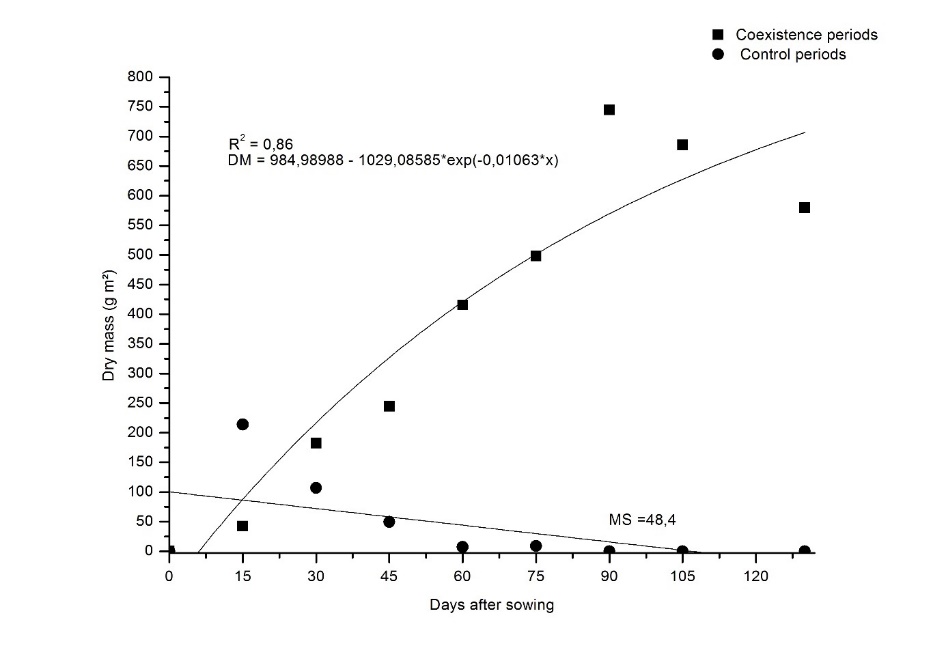
Figure 3. Shoot dry mass of the weed community in the maize crop 'P4285VYHR PIONEER' as a function of coexistence and control periods.
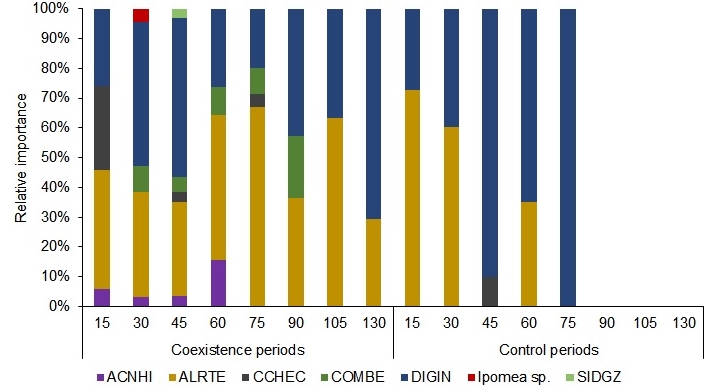
Figure 4. Relative importance of bristly starbur (ACNHI), joyweeds (ALRTE), southern sandbur (CCHEC), benghal dayflower (COMBE), sourgrass (DIGIN), and morning glory (Ipomoea sp.) as a function of coexistence and control periods in the maize crop 'P4285VYHR PIONEER'.
farming, areas infested with D. insularis sprouts, A. tenella had a significant presence. Even though D. insularis grows rapidly after 45 days, A. tenella has a high germination rate in both light (79%) and dark (69%), with the maximum germination at 28.2°C (Canossa et al., 2008).
The diversity indexes (H') for both the coexistence and control periods are shown on table 3. The findings reveal that
weed flora diversity was greater during the coexistence periods. Additionally, the equitability index (E') suggests a harmonious balance within the community. It is worth noting that an E' score of 0 indicates that a single species dominates a site (Dajoz, 2005), a phenomenon observed solely during the control periods at 75 DAS.
Table 3. Shannon-Wiener diversity ('H') and Equitability (E') indexes of weeds as a function of the evaluation periods in the maize crop 'P4285VYHR PIONEER'.
| DAS | Coexistence period | Control period | ||
|---|---|---|---|---|
| H’ | E’ | H’ | E’ | |
| 15 | 1.14 | 0.57 | 0.66 | 0.66 |
| 30 | 0.73 | 0.31 | 0.69 | 0.69 |
| 45 | 1.07 | 0.41 | 0.69 | 0.69 |
| 60 | 1.28 | 0.64 | 0.56 | 0.56 |
| 75 | 0.45 | 0.23 | 0.00 | 0.00 |
| 90 | 0.98 | 0.62 | - | - |
| 105 | 0.68 | 0.68 | - | - |
| 130 | 0.67 | 0.67 | - | - |
(-) No weeds, DAS: Days after sowing; H': Shannon-Wiener diversity index; E': Equitability index,
Table 4. Effect of coexistence and control periods of weeds on maize 'P4285VYHR PIONEER' plant height (HGT), number of ears per plant (NEP), ear insertion height (EIH), 1000-grain weight (W1000g), and yield (Y).
| Treatments (DAS) | HGT (m) | NEP | EIH (cm) | W1000G (g) | Y (kg ha-1) |
|---|---|---|---|---|---|
| Coexistence periods | |||||
| 0 - 15 | 1.6 ab | 1.0 a | 66.4 a | 301.2 ab | 2063.7 ab |
| 0 - 30 | 1.5 abc | 1.0 a | 56.5 ab | 305.1 ab | 2029.5 ab |
| 0 - 45 | 1.4 bcd | 1.0 a | 48.1 ab | 287.5 abc | 1163.0 bc |
| 0 - 60 | 1.3 cd | 1.0 a | 48.4 ab | 246.8 cd | 394.2 cd |
| 0 - 75 | 1.2 d | 1.0 a | 49.3 ab | 227.3 d | 296.0 cd |
| 0 - 90 | 1.3 cd | 1.0 a | 50.9 ab | 214.6 d | 348.4 cd |
| 0 - 105 | 1.4 bcd | 1.0 a | 53.4 ab | 222.2 d | 374.1 cd |
| 0 - 130 | 1.3 d | 0.8 a | 39.4 b | 229.2 d | 200.8 d |
| Control periods | |||||
| 0 - 15 | 1.6 ab | 1.0 a | 62.8 a | 271.0 bc | 1600.7 ab |
| 0 - 30 | 1.6 ab | 1.0 a | 64.3 a | 291.7 ab | 2110.4 a |
| 0 - 45 | 1.7 a | 1.0 a | 67.1 a | 285.1 abc | 2126.5 a |
| 0 - 60 | 1.7 a | 1.0 a | 64.8 a | 289.6 ab | 2031.2 ab |
| 0 - 75 | 1.7 a | 1.0 a | 65.0 a | 301.2 ab | 2154.2 a |
| 0 - 90 | 1.6 a | 1.0 a | 64.0 a | 283.8 abc | 2064.2 ab |
| 0 - 105 | 1.7 a | 1.0 a | 68.8 a | 299.5 ab | 2207.6 a |
| 0 - 130 | 1.6 ab | 1.0 a | 61.2 a | 321.3 a | 2238.4 a |
| F treat | 14.01** | 1.00NS | 4.45** | 18.09** | 20.74** |
| F block | 1.71NS | 1.00NS | 0.96NS | 3.20* | 2.66NS |
| DMS (5%) | 0.22 | 0.32 | 21.37 | 41.11 | 941.66 |
| CV (%) | 5.81 | 12.70 | 14.34 | 5.86 | 25.11 |
**Significant at the 1% probability level (p < 0.01);* significant at the 5% probability level (01 =< p < 0.05).
Regarding plant height (Table 4), there was a reduction in the coexistence periods starting at 45 DAS compared to all control periods. Helving et al. (2020) observed a similar result for maize hybrids P2530™ and P3271H™ coexisting with Sida rhombifolia L., Euphorbia heterophylla L. and Ambrosia artemisiifolia L. starting at 7 and 14 days after crop emergence, respectively. Regarding the number of ears per plant, there was no significant difference for the interference periods, corroborating Kozlowski (2009). The insertion height of the first ear reduced at 130 DAS of coexistence compared to all control periods. Galon et al. (2008) also observed this decrease when the maize crop coexisted with Brachiaria plantaginea (Link) Hitchc. The 1000-grain weight showed a pronounced reduction starting at 60 DAS (coexistence period). This outcome could be attributed to the stress during the grain-filling period (Figure 1), a fact also observed by Safdar et al. (2016).
The maize grain yield results (Table 4) showed a substantial decrease (~85.6%) starting at 60 DAS (coexistence period) compared to the 130 DAS control period. The estimated upper limit values of the period before interference (PBI) were 30 and 34 DAS (Figure 5) for tolerable reduction of 5 and 10% in yield, respectively. The PBI obtained for a 5% loss aligns closely with findings from Balbinot et al. (2016), who reported a duration of 21 DAS. This finding also supports the results of Helvig et al. (2020), who observed durations of 33 and 31 days for the maize hybrids P2530™ and P3271H™, respectively, within a no-till farming system under black oat (Avena strigosa) straw. According to Boltzmann’s equation,
this represents a reduction from 2,137.4 to 2,030.5 kg ha-1 (5%) and 1,923.7 (10%) for these PBIs. However, comparing the extremes (the entire period on clean versus on dirty), the reduction in maize yield was up to 86 %, representing a total of 1,836.2 kg ha-1. This results emphasizes the need for effective control of a weed community predominantly composed of D. insularis and A. tenella. Tursun et al. (2016) and Wiqar et al. (2021) similarly noted a 72% reduction for an infestation predominantly consisting of Convolvulus arvensis L., Sorghum halepense (L.) Pers., Cyperus rotundus L., and Amaranthus retroflexus L.
Materials and Methods
Characterization of the area
The experiment was conducted in an agricultural production area in the municipality of Jaboticabal - SP, Brazil, located at 21º15'22 "S, longitude 48º18'58 "W, and altitude of 595 m from February to June 2020. According to Köeppen's international classification, the region's climate is of the Aw type. In the water season, summer rains predominate (Figure 1). The experiment was developed in an area with a history of predominant infestation of rhizomatous D. insularis, in a no-till farming system.
The area presents gently undulating topography and good drainage conditions, and the soil is classified as Eutrudox (Embrapa, 2018). The soil chemical analysis was of a composite sample up to 20 cm deep and showed a pH of 5.7, organic matter of 13 g dm3, 41 mg dm3 of P and K, Ca and
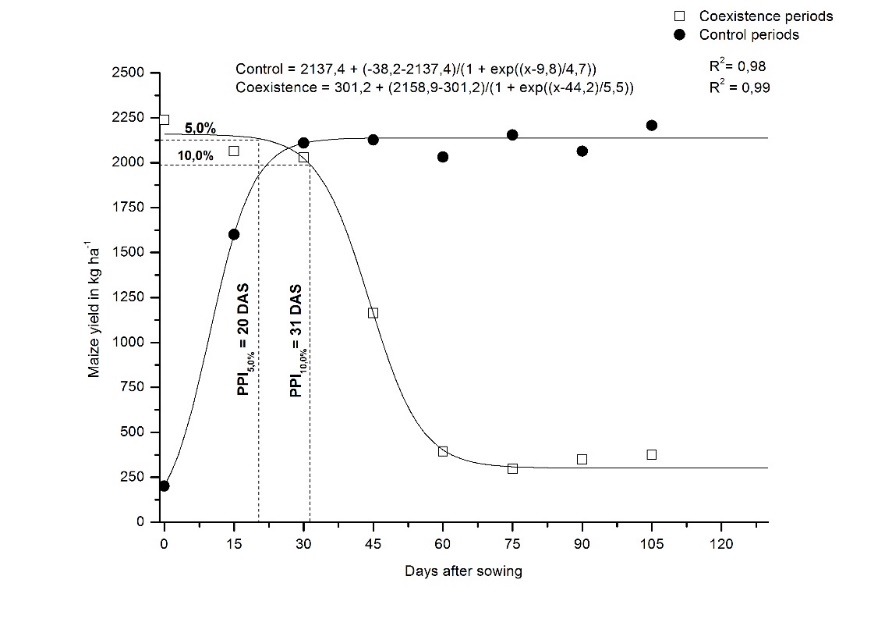
Figure 5. Maize 'P4285VYHR PIONEER' grain yield as a function of weed interference periods, with the estimated period before interference (PBI).
Mg of 3, 24 and 8 mmolc dm3, respectively, with a base saturation percentage (V%) of 61. The planting fertilization was 100 kg ha-1 04-28-08 (NPK), and the covering fertilization was 100 kg ha-1 of urea 30 days after sowing. Figure 1 shows the climatic conditions during the experiment.
Experimental design
Maize cv. P4285VYHR PIONEER was sown after the first crop of soybeans (TMG 7063 IPRO) (with a 130-day cycle) in a direct planting system, with 0.90 m spacing between rows, after the area's desiccation with glyphosate (Zap QI 620® - 2.0 L ha-1 p.c.) + 0.5 v/v mineral oil. The transgenic cultivar presents an early cycle, Leptra®, Herculex®, LibertyLink®, YieldGard®, Agrisure Viptera®, Roundup Ready™ technologies, and a recommended stand of 55,000 pl/ha-1 (Pioneer, 2021). No spraying with insecticides or fungicides was conducted during the experiment.
Two models were used to determine the critical period of interference prevention: (a) initially dirty (coexistence periods) and (b) initially clean (control periods) (Kuva et al., 2001). Thus, the treatments comprised eight increasing periods for each model, being 15, 30, 45, 60, 75, 90, 105, and 130 days after sowing maize (Table 1). The crop coexisted with the weeds that emerged during the increased periods. After each period, the weeds were removed by manual weeding, and the plots were kept weed-free until the crop cycle ended. This model aimed to determine the period before interference (PBI). The second model (control periods) aimed to determine the total period of interference prevention (TPIP) (Pitelli, 1985). The plots were kept weed-free for increasing periods, and at the end of each period, the weeds that emerged in the area were allowed to grow freely in the plots until the crop cycle ended.
The experimental design was in randomized blocks with four repetitions in a 2x8 factorial scheme, with two interference models (coexistence and control periods) and eight periods of coexistence and control (15, 30, 45, 60, 75, 90, 105, and 130 days after sowing (DAS) of maize). The plots comprised three sowing rows spaced 0.90 meters apart, each six meters long, totaling 16.2 m2. The central row was considered the useful area for sampling and evaluation, disregarding 0.5 m from the extremities, totaling 4.5 m2.
Phytosociological assessment and maize productivity
A phytosociological survey was carried out at the end of each weed coexistence period with the maize crop. The weeds within a 0.25m2 sample frame, randomly placed twice on each plot (0.5m2 sample area), were identified based on specialized literature, separated by species, and counted. Plants were cut close to the ground, conditioned in paper bags, and dried in a forced-air circulation oven for 96 hours (70°C) to obtain the dry mass using a 0.01 g precision scale. The importance value index (IVI) (relative frequency + relative density + relative dominance), the relative importance (RI) (Mueller-dombois and Ellemberg, 1974), the Shannon-Wiener diversity index (H'), and the equitability index (E') (Pinto-Coelho, 2000) of each species were calculated. Regarding the maize plants, ten plants per replication were randomly selected. The height (m), number of ears per plant, the insertion height of the first ear, and the weight of 1000 grains were recorded. The data were submitted to an analysis of variance, and when significant, the means were compared using Tukey's test at 5% (p<0.05) probability.
To determine maize yield, the center row of each plot was harvested when grain moisture approached 13%. The ears were threshed, the grains were weighed on a 0.01 g precision scale, and the yield was extrapolated to a hectare.
Data analysis
The normality of the data and homoscedasticity of the variances were verified by the Lillierfors and Bartlett’s test at the significance level α = 0.05, verifying that the data do not fit the premises for the analysis of variance. The yield analyses for each model (initial periods of weed control or coexistence) were separately processed, and the results were subjected to regression analysis according to the Boltzmann model (Kuva et al., 2000):
$$y = A2 + \frac{(A1 - A2)}{1 + e^{(\frac{x - zo}{dz})}}$$
Where: Y = maize yield depending on the control or coexistence periods; X = upper limit of the control or coexistence period; A1 = maximum yield obtained in plots kept clean throughout the cycle; A2 = minimum yield obtained in plots maintained in coexistence throughout the cycle; (A1 – A2) = yield loss; X0 = upper limit of the control or coexistence period, which corresponds to the intermediate value between maximum and minimum yield and dx = parameter that indicates the speed of yield loss or gain.
Regression equations were used to determine the weed interference periods for the reference levels of 5.0 and 10.0% yield reduction compared to the weed-free treatment. Therefore, the OriginPro v. 8.5 software (OriginalLab Corporation, USA) was used.
Conclusions
A community predominantly containing D. insularis coexisting with the maize crop decreases Pioneer cv. P4285VYHR yields up to 86% by reducing plant height, the insertion height of the first ear, and the weight of 1000 grains. Pioneer maize cv. P4285VYHR can live with such a community for 30 DAS, tolerating yield losses of up to 5%.
Acknowledgments
We want to thank the Weed Control Laboratory team for their support in developing this research. The Coordination for the Improvement of Higher Education Personnel (CAPES) for the grant to AEP and HLM, and the National Council for Scientific and Technological Development (CNPq) for the research grant to PLCAA.
References
Barroso AAM, Galeano E, Albrecht AJP, dos Reis FC, Victoria Filho R (2015) Does Sourgrass leaf anatomy influence glyphosate resistance?. Comunicata Scientiae, 6(4), 445–453. https://doi.org/10.14295/cs.v6i4.1124
Balbinot CR, Dariva PA, Sordi A, Lajús CR, Cericato A, Luz GL, Klein C (2016) Período crítico de interferência de plantas daninhas na cultura do milho. Unoesc & Ciência. 7(2):211-218.
Canossa RS, Oliveira Jr RS, Constantin J, Braccini Al, Biffe DF, Alonso DG, Blainski E (2008) Temperature and light in the germination of fire extinguisher seeds (Alternanthera tenella). Planta Daninha. 26(4):745-750.
Carvalho LB, Cruz-Hipolito H, González-Torralva F, Alves PLCA, Christoffoleti PJ, De Prado, R (2011) Detection of sourgrass (Digitaria insularis) biotypes resistant to glyphosate in Brazil. Weed Science. 59(2):171−176.
Companhia Nacional de Abastecimento - Conab. Acompanhamento da safra brasileira de grãos, quinto levantamento, safra 2020/21. Fevereiro, 2021. Brasília: Conab; 2021.v.8.
Dajoz, R. Ecologia Geral. Rio de Janeiro, Editora Vozes, 1978, 472p.
Empresa Brasileira de Pesquisa Agropecuária – Embrapa. Sistema brasileiro de classificação de solos. 5th. ed. Brasília: Embrapa, 2018.
Galon L, Pinto JJO, Rocha AA, Concenço G, Silva AF, Aspiazú I, Ferreira EA, França AC, Ferreira FA, Agostinetto D, Pinho CF (2008) Interference periods of Brachiaria plantaginea in corn in the southern region of Rio Grande do Sul. Planta Daninha. 26(4):779-788.
Galon L, Bagnara MAM, Gabiatti RL, Júnior FWR, Basso FJM, Nonemacher F, Agazzi LR, Randuz LL, Forte CT (2018) Períodos de interferência de plantas daninhas infestando a cultura do milho. Jornal de Ciência Agrícola 10: 197-205.
Gantoli G, Ayala VR, Gerhards R (2013) Determination of the Critical Period for Weed Control in Corn. Weed Tech. 27:63-71.
Heap I. Herbicide Resistant Weeds in Corn Globally. Disponível em http://www.weedscience.org/Pages/crop.aspx. Acesso em: 18 April 2024.
Helvig EO, Pinheiro KKG, Dranca AC, Silva AAP, Mendes MC, Maciel CDG (2020) Interference periods of weeds in maize in no tillage and conventional systems at high altitudes. Planta Daninha. 38:1-10.
Kashiwaqui MM. Dinâmica de nematóides e eficiência do manejo químico do capim-amargoso em culturas de soja e milho resistentes ao glifosato. 2016. 64 f. Dissertação (Mestrado em Ciências Agrárias) - Universidade Estadual de Maringá, Umuarama, PR, 2016.
Knezevic SZ, Evans SP, Blankenship EE, Van Acker RC, Lindquist JL (2002) Período crítico para controle de ervas daninhas: O conceito e análise de dados. Ciência de Ervas Daninhas50: 773-786.
Kozlowski LA (2009) Critical period of weed interference in corn based on crop phenology. Planta Daninha. 20(3):365-372.
Kuva MA, Pitelli RA, Christoffoleti PJ, Alves PLCA (2000) Períodos de interferência das plantas daninhas na cultura da cana-de-açúcar. I Tiririca. Planta Daninha, v. 18, n. 2, p. 241-251.
Lisboa LAM, Viana RS, Ribeiro FV, Figueiredo PAM, Ramos SB (2019) Initial development of the peanut tree under different weed densities, competition with Urochloa. Revista de Agricultura Neotropical, Cassilândia-MS. 6(2):45-51.
Lopez Ovejero RF, Takano HK, Nicolai M, Ferreira A, Melo MSC, Cavenaghi AL, Christoffoletti PJ, Oliveira RS (2017) Frequency and dispersal of glyphosate resistant sourgrass (Digitaria insularis) populations across Brazilian agricultural production areas. Weed Science. 65(2):285-94.
Martins JF, Barroso AAM, Alves PLCA (2017) Effects of environmental factors on seed germination and emergence of glyphosate resistant and susceptible sourgrass. Planta Daninha. 35(1):1-8.
Melo MSC, Rocha LJFN, Brunharo CACG, Silva DCP, Nicolai M, Christoffoleti PJ. Alternativas para o controle químico do capim-amargoso resistente ao glifosato, com herbicidas registrados para as culturas de milho e algodão. Revista Brasileira de Herbicida. 16(3):206-215.
Mondo VHV, Carvalho SJP, Dias ACR, Marcos Filho J (2010) Efeitos da luz e da temperatura na germinação de sementes de quatro espécies de plantas daninhas do gênero Digitaria. Revista Brasileira de Sementes. 32(1):131-7.
Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. 1th. ed. New York: John Wiley e Sons, 1974.
Oliveira RP, Alves PLCA, Nepomuceno M, Yamauti MS (2010) Influência do arranjo de plantas em dois híbridos de milho safrinha nas relações de interferência com a comunidade de plantas daninhas. Agrária. 5:450-459.
Pinto-Coelho RM. Fundamentos em ecologia. 1th. ed. Porto Alegre: Artes Médicas Sul, 2000.
Pioneer. 2020. Características da cultivar de milho P4285VYHR. Disponível em: https://www.pioneer.com/content/dam/dpagco/pioneer/la/br/pt/files/880.3_lay_guia_milho_ver_uo_safrinha_2020_18.pdf. Acesso: 18 de mar. de 2021.
Pitelli RA, Durigan JC (1984) Terminologia para períodos de controle e convivência de plantas daninhas em culturas anuais e semestrais. In: Congresso Brasileiro de Herbicidas e Plantas Daninhas, 15, 1984, Belo Horizonte. Resumos. Piracicaba: SBHED, p.3.
Pitelli RA Interferências de plantas daninhas em culturas agrícolas. Informe Agropecuário. v. 11, n. 129, p. 16-27, 1985.
Pitelli RA (1987) Competição e controle de ervas daninhas em áreas agrícolas. Série Técnica, Piracicaba, v.4, n.12, p. 1-24.
Radosevich SR, Holt JS (1997) Weed ecology: implications for vegetation management. 2th. ed. New York: John Wiley & Sons, 1997.
Safdar ME, Tanveer A, Khaliq A, Maqbool R (2016) Critical competition period of parthenium weed (Parthenium hysterophorus L.) in maize. Crop Protection. 80:101-107.
Salgado T, Pitelli RA, Alves PLCA (2005) Weed interference on maize (Zea mays) under no tillage system. Journal of Environmental Science and Health, Part B, v. 40, n. 1, p. 181-184.
Timossi PC (2009). Manejo de brotos de Digitaria insularis em milho plantio direto. Planta Daninha. 27(1):175-179.
Tursun N, Datta A, Sakinmaz MS, Kantarci Z, Knezevic SZ, Chauhan B S (2016) The critical period for weed control in three corn (Zea mays L.) types. Crop Protection. 90:59-65.
Vencill WK, Nichols RL, Webster TM, Soteres JK, Mallory-Smith C, Burgos NR, Johnson WG, McClelland, MR (2012) Herbicide resistance: toward an understanding of resistance development and the impact of herbicide-resistant crops. Weed Science. 60(SP1), 2-30.
Wiqar, B, Noori, MS, Amini, SY (2021) Effects of weed management on agronomic performance and productivity of hybrid maize (Zea mays L.). Journal of Agriculture and Applied Biology. 2(2):70 - 75.