

Aust J Crop Sci. 18(11):775-785 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.11.p192
Physiology, growth, and metabolism of seed-derived cocoa varieties in response to field stress conditions
Moses Kwame Aidoo1*, Esther Anokye1, Mikhail Tettey Agyemang1, Atta Ofori2, Alfred Arthur3 and Francis Kwame Padi2
1Physiology/Biochemistry Division, Cocoa Research Institute of Ghana, New Tafo - Akim, Eastern Region, Ghana
2Plant Breeding Division, Cocoa Research Institute of Ghana, New Tafo - Akim, Eastern Region, Ghana
3Soil Science Division Cocoa Research Institute of Ghana, New Tafo - Akim, Eastern Region, Ghana
*Corresponding author: Moses Kwame Aidoo  |
ORCID: https://orcid.org/0000-0003-0183-4771
|
ORCID: https://orcid.org/0000-0003-0183-4771
Abstract: A total of fifteen best seed-derived cocoa varieties known for their high combining abilities for vigour and yield were evaluated at the vegetative stage between 29 to 39 months after planting. Environmental factors, physiology, growth, carbohydrate, carbon, and nitrogen metabolism resilience of the varieties in response to field stress conditions during wet and dry main growing seasons were evaluated. The plants were then subjected to the environmental stress conditions (high temperature, low rainfall, and soil moisture content) at dry growing season as stress conditions, compared to wet season as unstress conditions (optimum temperature, high rainfall, and soil moisture). Physiology and growth of the varieties during wet season or in dry season conditions did not differ significantly but differed in their response to the seasons. The varieties exhibited a tight stomatal regulation and reduced photochemical efficiency at various magnitudes. Leaves relative chlorophyll content unchanged with high relative water content culminating in the reduction of electrolyte leakage during dry season stress conditions. The varieties accumulated soluble sugars, starch, non-structural carbohydrate, carbon and nitrogen under high temperature, low rainfall, and moisture content during dry season. Correlation analysis revealed strong relationships between the physiology, growth, and central carbon metabolism parameters. These findings were prominence in varieties AMAZ 15-15 x EQX 78, CRG 2029 x AMAZ 3-2, CRG 2029 x CRG 0314/102, CRG 9006 x AMAZ 3-2 and PA 150 x CRG 0314/102.
Keywords: Drought stress, field conditions, high temperature, metabolism, non-structural carbohydrate.
Abbreviations: C/N_Carbon nitrogen ratio; CRIG_Cocoa research institute of Ghana; DS_Dry season; MAP_Months after planting; NSC_Non structural carbohydrate; RCBD_Randomized complete block design; SS/S_Soluble sugars and starch ratio; WS_Wet season.
Introduction
The survival, growth and development of cocoa plants derived from seeds in the field are being threatening by the adverse environmental conditions. These environmental conditions include poor soil fertility, high temperatures, heat and drought stress. Drought and high temperature stresses have become the most important limiting factors to successful establishment and production of cocoa and its plantations in West Africa (Padi et al., 2013; Ofori et al., 2015). The adverse field conditions significantly reduce photosynthesis machinery and metabolism which in turn reduce the productivity of the plants (Alban et al., 2016). The genetic variation of cocoa germplasm, as planting materials that have the potential to adapt, grow and develop under changing environmental conditions for high productivity, has not been fully explored.
Recent reports on model predictions suggested that large parts of cocoa producing regions in West Africa will become unfit for production in the future (Läderach et al., 2013; Schroth et al., 2016). These claims are largely based on predicted temperature increases, which are expected to derive greater evaporative demand resulting in increasing incidence of water deficit or drought stress. This condition has the tendency to induce oxidative stress and reduce the efficiency of photosynthetic machinery of the cocoa plants culminating in reduced growth and development and eventually, trigger the death of the plants (Padi et al., 2013). Under unfavorable weather conditions, young cocoa continuously reduce their stomatal conductance for growth (Acheampong et al., 2015). However, knowledge on the adaptational mechanism of cocoa plants in mitigating the effects of changing field conditions such as water deficit and high temperatures on the physiology, growth and metabolism of cocoa varieties under field conditions are scarce (Lahive et al., 2019).
Carbon and nitrogen metabolism are basic processes essential for plant growth, development and survival under environmental stress (Rachmilevitch et al., 2006). These are crucially indicated by the level of non-structural plant
Table 1. Description of varieties used in the experiment by source and parentage. For full description see Lockwood and Gyamfi, (1979); Motamayor et al. (2008); Padi et al. (2015) and Padi and Ofori, (2016).
| Variety | Source / Parentage |
|---|---|
| AMAZ 15-15 x EQX 78 | AMAZ 15-15 is a clone selection from the Upper Amazon basin, belonging to the Iquitos genetic group. EQX 78 a clone selection of mixed parentage, but predominantly of the Scavina / Contamana genetic group. |
| CRG 2029 x AMAZ 3-2 | CRG 2029 is a clone selection from PA 7 x PA 150 belonging to the Marañón genetic group. AMAZ 3-2 is a clone selection from the Upper Amazon basin, belonging to the Iquitos genetic group. |
| CRG 2029 x CRG 0314/102 | CRG 0314/102 is a clone selection at CRIG derived from a Scavina (SCA 9) and Nanay (Pound 10) cross. |
| CRG 9006 x AMAZ 3-2 | CRG 9006 is a CRIG clone selection from a Guiana (GU 144C) and MAN 15-2, a clone of mixed parentage. |
| CRG 9006 x T17/524 | T17/524 is a clone derived from open pollinated seedling of clone IMC 53 (a clone of Iquitos origin). |
| GU 144C x EQX 78 | GU 144C is a clone belonging to the Guiana genetic group. |
| GU 144C x MAN 15-2 | MAN 15-2 is a clone of mixed parentage but predominantly of Purus genetic group of Motamayor et.al 2008. |
| PA 150 x CRG 0314/102 | PA 150 is a clone of Parinari origin, belonging to the Marañón genetic group. |
| PA 7 x AMAZ 3-2 | PA 7 is a clone of Parinari origin, belonging to the Marañón genetic group. |
| PA 7 x CRG 8914 | CRG 8914 is a clone selection done at CRIG derived from CAS 3 (of the Amelonado genetic group) and a Nanay clone. |
| SPD | SPD, acronym for Seed Production Division of Ghana Cocoa Board. This is the standard variety, composed of equal proportions of four crosses, derived from parental clones of Nanay, Iquitos and Marañón genetic groups. |
| T60/887 x CRG 0314/102 | T60/887 was derived from Marañón × Nanay (PA 7 × NA 32) cross made in Trinidad and collected by Posnette in 1944. |
| T63/967 x CRG 9006 | T63/967 was derived from a Marañón × Nanay (PA 35 x NA 32) cross collected by Posnette from Trinidad in 1944. |
| T65/238 x CRG 9006 | T65/238 is alone selection derived from Marañón (PA 7) and Iquitos (IMC 35) cross collected by Posnette from Trinidad in 1944. |
| T79/501 x AMAZ 3-2 | T60/887 was derived from Nanay × Marañón (NA 32 x PA 7) cross made in Trinidad and collected by Posnette in 1944. |
carbohydrate (Liu et al., 2018). Resourceful allocation of carbon and nitrogen patterns enhances plant tolerance to environmental stresses (Huang and Fu, 2000). The alteration of carbohydrates such as glucose, fructose, sucrose and maltose improve water absorption capacity of plants. These compounds serve as osmo-protectant of membranes and macromolecules by replacing water molecules, preventing the formation of intra-molecular hydrogen bonds that can reduce cellular dehydration and serve as source of energy (Rizhsky et al., 2004; Aidoo et al., 2017). Therefore, there is the need to explore, understand and select cocoa varieties that are efficient in allocating carbon and nitrogen in cocoa leaves, accumulating sugars and starch, which can be enhanced to improve the resilience of the crop in response to field stress conditions.
Most of the research on the physiology of the cocoa plants has been conducted in the controlled environment at the seedling stage. For instance, Acheampong et al. (2015) established cocoa under heavy shade conditions in the greenhouse and reported the increase of photosynthesis at the seedling stage. A few efforts to mitigate the effects of drought in the field conditions have focused mainly on selecting seed-derived cocoa varieties with high percentage survival under low shade conditions and on soils with low organic matter content (Padi et al., 2013; Ofori et al., 2015). In order to understand the physiology, growth and metabolism of seed-derived established cocoa trees, it is important to setup experimental plots in the field, to enhance the need to evaluate the physiological responses of available cocoa germplasm to changing field conditions. This will elucidate the physiological processes underlying resilience of
cocoa plants to changing environmental conditions. In this study, a total of fifteen seed-derived cocoa varieties known for their high vigour and yield combining abilities (Padi et al. 2015; Padi and Ofori, 2016) were selected and evaluated. Ghana, Guiana, Trinidad and Upper Amazon Basin are the main parentage sources of the varieties (Lockwood and Gyamfi, 1979; Motamayor et al., 2008; Padi et al., 2015; Padi and Ofori, 2016). The objective of this study is to evaluate the physiological, growth and metabolism responses of seed-derived cocoa varieties under field stress conditions (high temperature, low rainfall and soil moisture content) during dry growing season as compared to unstress field conditions (optimal temperature, rainfall and soil moisture) during wet growing season. This study contributes to identifying varieties and their mechanisms that are resilient to high field temperature, low rainfall and soil moisture content, and the strategies for using those plants for new cropping systems.
Results
Monthly temperature, soil moisture content and properties
To understand the environmental factors, in which the cocoa varieties were grown, ambient temperature, rainfall and soil moisture content (Fig. 1) and their properties were evaluated (Supplementary Table 1). Monthly mean temperature for that period ranged from a minimum of 28.80 °C in September 2022 to a maximum of 34.73 °C in January 2023. The difference between the time points (June 2022 and January 2023) of the
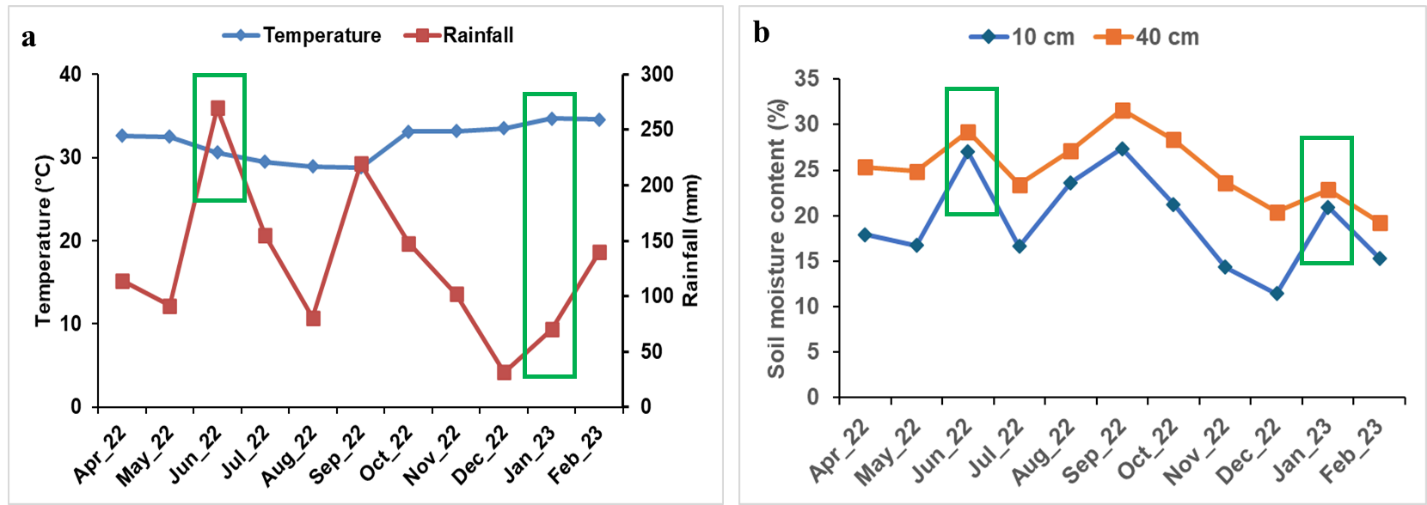
Fig. 1. Ambient temperature, rainfall (a) and soil moisture content (b) at 10 and 40 cm depth in the field during the experimental period. Green rectangles indicate time of taken data.
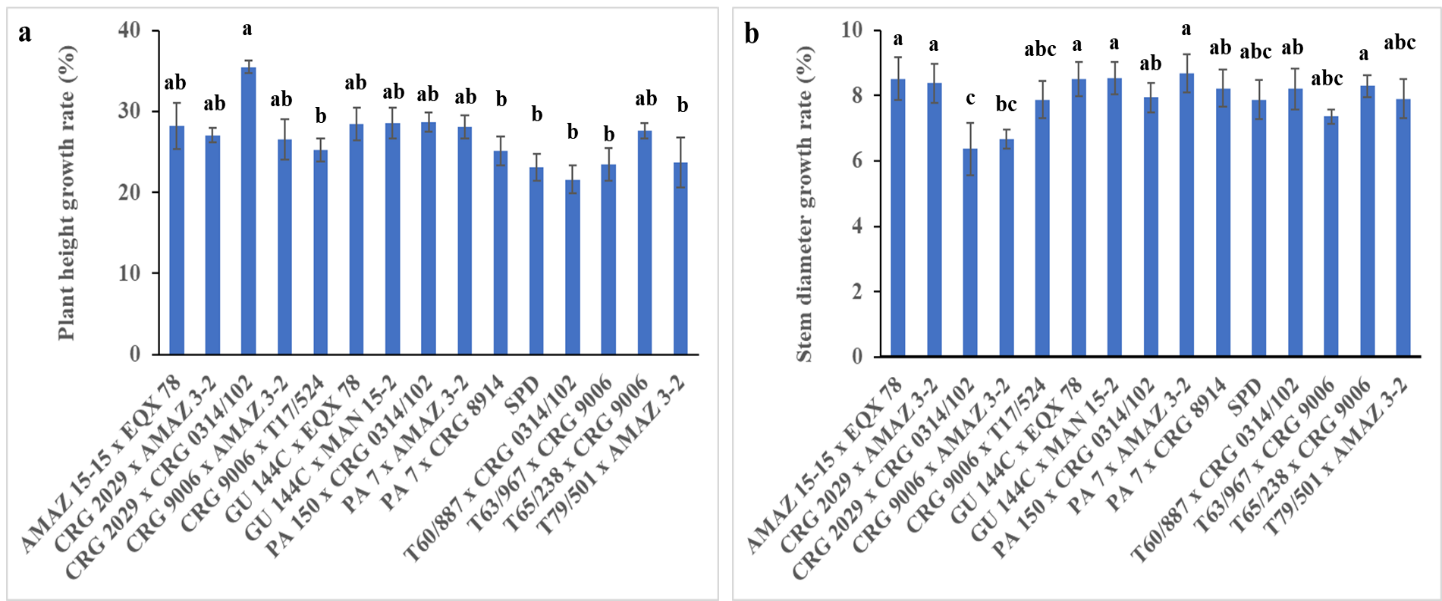
Fig. 2. Six months growth rate of stem diameter (a) and plant height (d) of some seed-derived cocoa varieties in response to field high temperature and low soil moisture conditions during vegetative phase recorded at 23 and 29 months after planting (MAP). Values are means of 4 replicates with standard error bars (n≤4; p≤0.05). The means were separated using Fisher pair wise comparison. Different letters indicate significant differences among the varieties.
study was 4.10 °C (Fig. 1a). The rain recorded during the study ranged from 31.60 mm in December 2023 to 270.00 mm in June 2022 (Fig. 1a). The soil moisture assessed at 10 and 40 cm depth in the plot followed a similar trend. The soil moisture values recorded for 10 cm depth were lower, compared to that of 40 cm soil depth in all the months. June (wet) and January (dry) are the months, in which sampling and data were taken, having high and low soil moisture content respectively at both depth (Fig. 1b). The soil texture determined in the experimental plot was sandy loam (Supplementary Table 1). Soil carbon content, available P and exchangeable K, Mg and Ca are the soil chemical properties that fall below the critical levels for cocoa cultivation (Supplementary Table 1).
Plant height and stem diameter growth rate of seed-derived cocoa varieties
The growth rate was determined after 6 months (April to October 2022). It was insignificantly different in both plant height and stem diameter growth rates due to the varietal effect (Fig. 2a and b). The three varieties with best plant height and growth rate were CRG 2029 x CRG 0314/102 (35.48%), PA 150 x CRG 0314/102 (28.68%) and GU 144C x EQX 78 (28.56%).
The tallest plants (CRG 2029 x CRG 0314/102) had the lowest stem diameter growth rate (6.37%). The varieties that occupied the best three top positions regarding stem diameter growth rate were PA 7 x AMAZ 3-2 (8.68%), GU 144C x MAN 15-2 (8.53%) and AMAZ 15-15 x EQX 78 (8.52%). The only variety among the top performed three varieties in both plant height and stem diameter growth rate was GU 144C x MAN 15-2, which was second and third best in stem diameter and plant height growth rates, respectively (Fig. 2a and b).
Leaf functional traits and relative water content of seed-derived cocoa varieties
Leaf area was measured during wet and dry seasons to understand the shoot development in response to field conditions during wet and dry growing seasons. The variation recorded for the leaf area did not show significant differences in comparing the varietal and the seasonal effects (Supplementary Table 2 and 3). The leaf area during the wet season ranged from 84.45 in variety CRG 2029 x CRG 0314/102 to 99.61 cm2 in variety PA 7 x AMAZ 3-2. In dry season, 6 out of the 15 varieties had a high leaf area compared to that of the wet season. The highest among them were variety CRG 2029 x AMAZ 3-2 with a leaf area of 100.63 cm2 (Supplementary Table
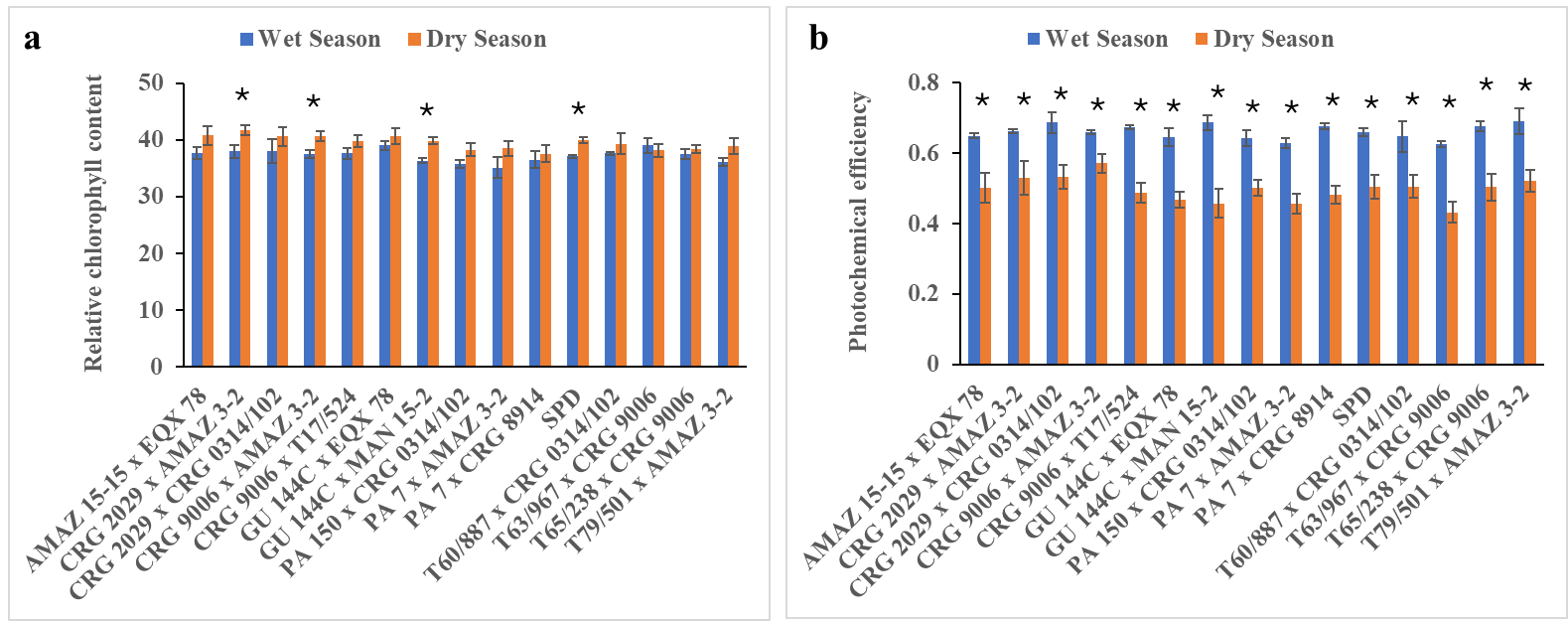
Fig. 3. Relative chlorophyll content (a) and photochemical efficiency (b) of some seed-derived cocoa varieties in response to field high temperature and low moisture content conditions during vegetative phase recorded in 2022/2023 wet and dry growing season. Values are the means of 4 replicates with standard error bars. Bars with asterisks indicate significance (p ≤ 0.005; student t-test) between wet and dry seasons.
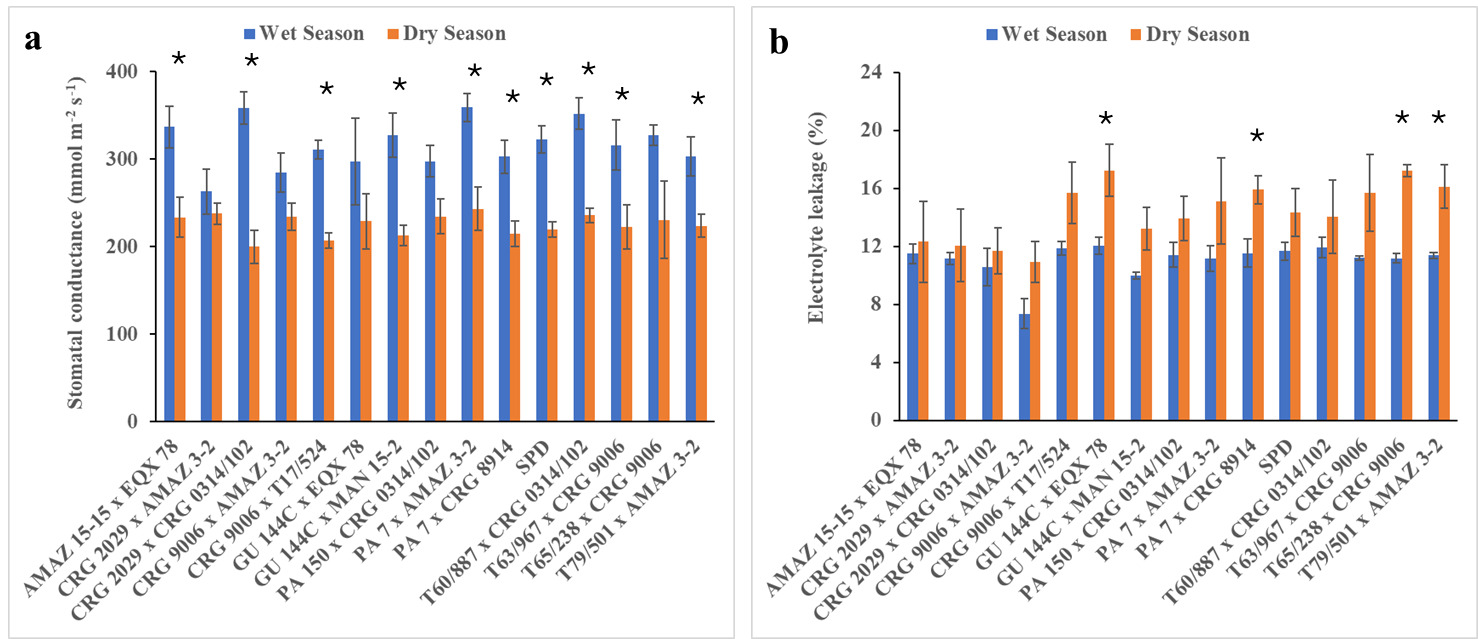
Fig. 4. Stomatal conductance (a) and electrolyte leakage (b) of some seed-derived cocoa varieties in response to field high temperature and low soil moisture content conditions during vegetative phase recorded in 2022/2023 wet and dry growing season. Values are the means of 4 replicates with standard error bars. Bars with asterisks indicate significance (p ≤ 0.005; student t-test) between wet and dry seasons.
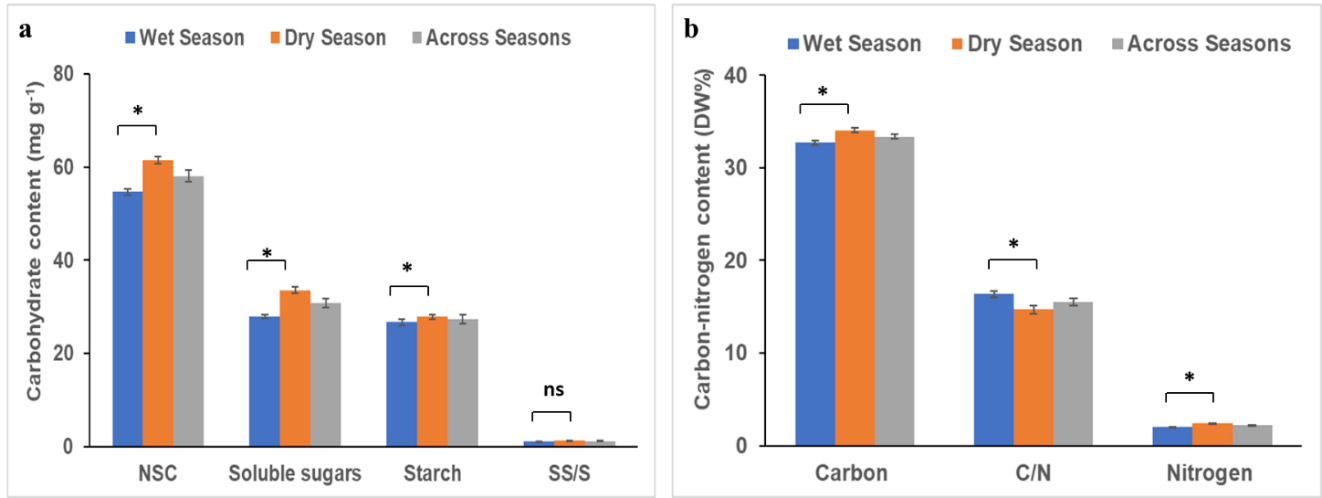
Fig. 5. Change of carbohydrate content (a) and carbon nitrogen (b) in across varieties and seasons (wet and dry). Values are means of four replicates of 15 seed derived cocoa varieties with standard errors (± SE; n≤4). Bars with asterisks indicate significance (p ≤ 0.005) between them and ns means insignificant.
Table 2. The concentration of soluble sugar, starch, non-structural carbohydrate (NSC) and ratio of soluble sugar to starch for the 15 seed derived cocoa varieties. Values are means of four replicates and standard error (SE; n ≤ 4) during the wet season (WS) and dry season (DS). Different letters indicate significant differences among the varieties within a season (Fisher pair wise comparison; p≤0.05).
Seed-derived cocoa varieties |
Soluble sugars (mg g-1) |
Starch (mg g-1) |
NSC (mg g-1) |
Soluble sugars starch ratio | ||||
|---|---|---|---|---|---|---|---|---|
| WS | DS | WS | DS | WS | DS | WS | DS | |
| AMAZ 15-15 x EQX 78 | 22.73ab | 27.85a | 26.24b | 31.67ab | 48.97bcd | 59.52ab | 0.89cd | 0.88a |
| CRG 2029 x AMAZ 3-2 | 28.55ab | 29.70a | 29.90ab | 31.34ab | 58.45abcd | 61.04ab | 1.00abcd | 0.95a |
| CRG 2029 x CRG 0314/102 | 34.33a | 31.86a | 19.62b | 25.95abc | 53.95abcd | 57.81ab | 1.97ab | 1.21a |
| CRG 9006 x AMAZ 3-2 | 20.46b | 27.85a | 19.70b | 27.79abc | 40.17d | 55.63ab | 1.03abcd | 1.04a |
| CRG 9006 x T17/524 | 25.88ab | 37.58a | 27.84b | 34.75a | 53.71abcd | 72.33a | 0.92bcd | 1.08a |
| GU 144C x EQX 78 | 23.70ab | 34.47a | 28.46b | 32.47ab | 52.16abcd | 66.94ab | 0.87cd | 1.07a |
| GU 144C x MAN 15-2 | 33.35a | 35.82a | 20.26b | 28.81abc | 53.60abcd | 64.63ab | 1.72abc | 1.24a |
| PA 150 x CRG 0314/102 | 25.77ab | 30.79a | 30.15ab | 24.29abc | 55.91abcd | 55.08ab | 0.96abcd | 1.45a |
| PA 7 x AMAZ 3-2 | 24.92ab | 27.60a | 33.24ab | 28.67abc | 58.16abcd | 56.27ab | 0.79cd | 0.96a |
| PA 7 x CRG 8914 | 33.81a | 37.56a | 18.14b | 24.08abc | 51.95abcd | 61.65ab | 1.99a | 1.57a |
| SPD | 24.81ab | 38.21a | 44.04a | 29.91ab | 68.84a | 68.12ab | 0.57d | 1.39a |
| T60/887 x CRG 0314/102 | 31.42ab | 39.57a | 31.73ab | 31.42ab | 63.15abc | 70.99ab | 1.01abcd | 1.38a |
| T63/967 x CRG 9006 | 34.34a | 36.42a | 30.73ab | 22.19bc | 64.71ab | 58.61ab | 1.19abcd | 1.67a |
| T65/238 x CRG 9006 | 24.84ab | 36.88a | 20.35b | 27.83abc | 45.19cd | 64.71ab | 1.36abcd | 1.34a |
| T79/501 x AMAZ 3-2 | 30.29ab | 31.99a | 20.68b | 17.08c | 50.97abcd | 49.07b | 1.48abcd | 1.91a |
| Means | 27.95 | 33.61 | 26.72 | 27.88 | 54.66 | 61.49 | 1.18 | 1.28 |
| SE | 2.52 | 4.25 | 3.02 | 2.36 | 3.72 | 4.47 | 0.30 | 0.22 |
WS-Wet season; DR-Dry season; SE-Standard error.
Table 3. The concentration of carbon, nitrogen and ratio of carbon to nitrogen for the 15 seed derived cocoa varieties. Values are means of four replicates and standard error (SE; n ≤ 4) during the wet season (WS) and dry season (D). Different letters indicate significant differences among the varieties within a season. (Fisher pair wise comparison; p≤0.05).
| Seed-derived cocoa varieties | Carbon (DW%) | Nitrogen (DW%) | Carbon nitrogen ratio | |||
|---|---|---|---|---|---|---|
| WS | DS | WS | DS | WS | DS | |
| AMAZ 15-15 x EQX 78 | 34.37a | 32.95ab | 2.08ab | 2.41ab | 17.39ab | 13.76bc |
| CRG 2029 x AMAZ 3-2 | 32.57ab | 34.47ab | 2.10ab | 2.47ab | 15.81ab | 14.00bc |
| CRG 2029 x CRG 0314/102 | 31.59ab | 33.69ab | 1.79b | 2.42ab | 17.63a | 13.92bc |
| CRG 9006 x AMAZ 3-2 | 32.88ab | 34.76ab | 2.11ab | 2.81a | 15.79ab | 12.53c |
| CRG 9006 x T17/524 | 34.17a | 34.13ab | 2.16ab | 2.39ab | 16.08ab | 14.34a |
| GU 144C x EQX 78 | 32.52ab | 34.52ab | 2.03ab | 2.47ab | 16.08ab | 14.13abc |
| GU 144C x MAN 15-2 | 32.32ab | 34.56ab | 1.92ab | 2.42ab | 17.35ab | 14.39abc |
| PA 150 x CRG 0314/102 | 33.83a | 33.59ab | 2.26a | 2.32ab | 15.34ab | 14.58abc |
| PA 7 x AMAZ 3-2 | 32.57ab | 32.81b | 2.09ab | 2.41ab | 15.96ab | 13.77bc |
| PA 7 x CRG 8914 | 29.37b | 33.93ab | 1.77b | 2.08b | 16.66ab | 18.44ab |
| SPD | 32.86ab | 33.54ab | 1.99ab | 2.31ab | 16.79ab | 14.68abc |
| T60/887 x CRG 0314/102 | 32.71ab | 34.22ab | 1.94ab | 2.30ab | 17.05ab | 15.10abc |
| T63/967 x CRG 9006 | 33.34ab | 34.91ab | 2.04ab | 2.09b | 16.79ab | 18.94a |
| T65/238 x CRG 9006 | 31.93ab | 35.05a | 2.09ab | 2.53ab | 15.77ab | 14.16abc |
| T79/501 x AMAZ 3-2 | 33.44ab | 33.74ab | 2.22ab | 2.51ab | 15.16b | 13.53bc |
| Means | 32.70 | 34.06 | 2.04 | 2.39 | 16.37 | 14.69 |
| SE | 0.87 | 0.89 | 0.16 | 0.18 | 1.33 | 1.73 |
WS-Wet season; DR-Dry season; SE-Standard error
Table 4. The physiological and leaf traits of some seed-derived cocoa varieties in response to field conditions during vegetative phase recorded in 2022/2023 wet and dry growing season. Values are means of 60 replicates with standard error. Different letters indicate significant differences between wet and try season.
| Parameters | Wet season | Dry season |
|---|---|---|
| Relative chlorophyll content | 37.28±0.29b | 39.53±0.32a |
| Photochemical efficiency | 0.66±0.006a | 0.50±0.009b |
| Stomatal conductance (mmol m-2 s-1) | 309.20±6.86a | 231.60±5.99b |
| Relative water content (%) | 81.74±0.33a | 78.29±0.71b |
| Leaf area (cm2) | 92.57±2.01a | 90.68±1.68b |
| Leaf dry mass (g) | 1.38±0.04a | 1.17±0.03b |
| Specific leaf dry mass (g cm2) | 0.015±0.0002a | 0.013±0.0002b |
| Electrolyte leakage (%) | 11.43±0.17b | 14.01±0.56a. |
2). Leaf dry mass, which showed significant seasonal effect, was mostly higher among the varieties in the wet season compared to the dry season (Supplementary Table 2 and 3). Only varieties CRG 2029 x AMAZ 3-2 and CRG 2029 x CRG 0314/102 had a high leaf dry mass during the wet season compared to the dry season. In the dry season among the varieties leaf dry mass reduced and ranged from 0.95 to 1.42 g in varieties T79/501 x AMAZ 3-2 and CRI 2029 x AMAZ 3-2 respectively (Supplementary Table 2). The relative water content determined for the varieties in response to field conditions were mostly higher in the varieties during wet season compared to that of the dry season (Supplementary Table 2). Seasonal effect showed significant differences (Supplementary Table 2 and 3). The variety CRG 2029 x CRG 0314/102 had the lowest relative water content of 78.54% and variety T63/967 x CRG 9006 had the highest of 84.30% in the wet season while in dry season lowest (72.01%) was recorded in variety PA 7 x AMAZ 3-2 and the highest (83. 61%) in variety PA 150 x CRG 0314/102 (Supplementary Table 2).
Relative chlorophyll content and photochemical efficiency of seed-derived cocoa varieties
The relative chlorophyll content among the varieties was mostly high during the dry season compared to the wet season. These differences were significant in the seasonal effect (p ≤ 0.001; Fig. 3a; Supplementary Table 3). In the wet season relative chlorophyll content compared among the varieties was high in variety GU 144C x EQX 78 (39.07) closely followed by variety T63/967 x CRG 9006 (39.03) and lower in variety PA 7 x AMAZ 3-2 (35.10), while in dry season ranged between 37.60 to 40.76 in varieties PA 7 x CRG 8914 and AMAZ 15-15 x EQX 78, respectively (Fig. 3a). The photochemical efficiency of photosystem II (measured as Fv/Fm) was generally high in all the varieties during wet season compared to dry season and these differences were significant (p ≤ 0.001). No significant varietal effect differences were observed (Supplementary Table 3). The field conditions of the wet season increased the Fv/Fm values, ranging from 63% in varieties T63/967 x CRG 9006 and PA 7 x AMAZ 3-2 to 69% in the following varieties CRG 2029 x CRG 0314/102, GU 144C x MAN 15-2 and T79/501 x AMAZ 3-2. The lower Fv/Fm values recorded in dry season compared to the wet season, ranging from 43% in variety T63/967 x CRG 9006 to 57% in variety CRG 9006 x AMAZ 3-2 (Fig. 3b; Supplementary Table 3).
Stomatal conductance and electrolyte leakage of seed-derived cocoa varieties
A significant varieties × season interaction was observed on the stomatal conductance. High stomatal conductance was observed among the varieties during the wet season (Fig. 4a; Supplementary Table 3). The top three best varieties with high stomatal conductance during the wet season are PA 7 x AMAZ 3-2; CRG 2029 x CRG 0314/102 and T65/238 x CRG 9006 with 359, 358 and 351 mmol m-2 s-1, respectively (Fig. 4a). The dry season field conditions reduced stomatal conductance of the varieties. The reduction observed in the dry season ranged from 120 (CRG 2029 x CRG 0314/102) to 238 mmol m-2s-1 (CRG 2029 x AMAZ 3-2; Fig. 4a). The levels of leaked ion of the leaves of cocoa varieties measured to determine their membrane stability were less in all the varieties in the wet season compared to dry season (Fig. 4b). The varietal and seasonal effects were significantly different (Supplementary Table 2). Variety CRG 9006 x AMAZ 3-2 had the lowest (7.36%) membrane leakage during the wet season. The highest (17.24 %) in the dry season was recorded in variety GU 144C x EQX 78 and T65/238 x CRG 9006 (Fig 4b).
Shift in leaf carbohydrate metabolism of seed-derived cocoa varieties
The cocoa varieties showed significant varieties × season interaction on starch, non-structutral carbohydrate (NSC) and soluble sugars to starch (SS/S) ratio. The varietal effect for starch and NSC differed significantly uder both growing seasons, while soluble sugars and SS/S were only significant under dry season. Seasonal effect was only significant under soluble sugars and NSC (Tables 2 and Supplementary Table 3). The concentration of the compounds were generally high during the dry season compared to the wet season (Table 2). Varieties CRG 9006 x T17/524 (34.75 mg g-1) and GU 144 x EQX 78 (32.47 mg g-1) are the first and second varieties with high starch content during the dry season, respectively. Non-structural carbohydrate also increased in all the varieties except varieties PA 150 x CRG 0314/102, PA 7x AMAZ 3-2, SPD, T63/967 x CRG 9006 and T79/501 x AMAZ 3-2, ranging between 49.07 and 72.33 mg g-1 in the dry season compared to the wet season (Table 2). Soluble sugars to starch ratio mostly increased in the dry season and ranged from 0.88 to 1.65 (Table 2).
Alterations of leaf carbon-nitrogen metabolism of seed-derived cocoa varieties
The changes that occurred in the cocoa varieties leaf contents of carbon, nitrogen and carbon to nitrogen ratio (C/N) in response to changing field conditions were not significant (p ≤ 0.05) during wet or dry seasons, except for the carbon levels (Table 3 and Supplementary Table 2). Varieties × season interaction and varietal effect were not significantly different but seasonal effect showed significance (Supplementary Table 2). The leaf carbon content of varieties ranged from 29.37 to 34.37 DW% in PA 7 x CRG 8914 and AMAZ 15-15 x EQX 78, respectively, during the wet season (Table 3). The levels of nitrogen content detected in the leaves of the varieties ranged from 1.77 (PA 7 x CRG 8914) to 2.26 DW% (PA 150 x CRG 0314/102) at the wet season. The estimated C/N ratio also ranged from 15.16 to 17.63 in T79/501 x AMAZ 3-2 and CRG 2029 x CRG 0314/102, respectively (Table 3). In dry season, carbon and nitrogen were almost high, compared to wet season. The highest carbon content (35.05 DW%) was observed in T65/238 x CRG 9006 in dry season. The highest nitrogen content (2. 81 DW%) was recorded in CRG 9006 x AMAZ 3-2. Carbon to nitrogen ratio ranged from 12.76 to 18.94 in AMAZ 15-15 x EQX 78 and T63/967 x CRG 9006, respectively, also detected in the dry season (Table 3).
Physiology, functional leaf traits and metabolism of cocoa varieties across seasons
Contrast analysis was performed on physiology, functional leaf traits (Table 4), total change of carbohydrate and carbon-nitrogen metabolism in the varieties (Fig. 5). This enhanced the understanding of the overall magnitude of physiology, growth and metabolism of cocoa varieties during wet and dry growing seasons. All the physiological and leaf functional traits were significantly different across the varieties in wet and dry growing seasons (Table 4). Relative chlorophyll content and electrolyte leakage were the only physiological parameters that reduced in wet season compared to the dry season (Table 4). Non-structural carbohydrate, soluble sugars and starch were significantly higher in dry season. Soluble sugars to starch ratio were the only compound that did not show significance in comparing wet and dry seasons (Fig. 5a). The leaf contents of carbon, nitrogen and C/N ratio were all significantly different in two seasons. Carbon and nitrogen were high and C/N low during the dry season (Fig. 5b).
The relationship of all parameters of seed-derived cocoa varieties
The correlation analysis revealed either positive or negative relationships between all the parameters evaluated in the varieties response to different field conditions (Table 5). Soluble sugars positively correlated to NSC and soluble sugars to starch ratio and negatively correlated to photochemical efficiency, specific leaf mass and relative water content. Starch correlated positively and negatively to NSC and soluble sugars to starch ratio, respectively. Non-structural carbohydrate was also correlated positively and negatively to carbon and photochemical efficiency, respectively. Carbon correlated positively to nitrogen, relative chlorophyl content and negative to photochemical efficiency, relative water content and stomatal conductance. Nitrogen was the only parameter that correlated to more parameters positively or negatively (Table 5). The correlation between carbon nitrogen ratio and leaf area, leaf dry mass, stomatal conductance was positive and negative between relative chlorophyll content. Relative chlorophyll content showed positive correlation between photochemical efficiency and stomatal conductance. Photochemical efficiency, leaf area, leaf dry mass, membrane stability and relative water content showed at least two either positive or negative correlation among other parameters (Table 5).
Discussion
The limited physiological understanding of plant during transition of vegetative phase under changing environmental conditions in the field is a major challenge in envisaging its full productivity (Acheampong et al., 2015; Aidoo et al., 2019). For this challenge, it is imperative to know the ambient temperature, rainfall, soil moisture content as well as soil physical and chemical properties, of which the plants are grown and how these environmental factors affect their growth and development. To answer this question field ambient temperature, rainfall, soil moisture content, soil physical and chemical properties, physiology, growth and carbon central metabolism of seed-derived cocoa varieties were evaluated. The soil moisture levels recorded were higher at 40 cm soil depth compared to 10 cm depth (Fig. 1). In June 2022, more soil moisture was recorded compared to January 2023 (Fig. 1). This condition might have influenced by the high rainfall and optimum temperature, which are mostly associated with June and on contrary with January (Fig 1).
Field conditions influenced plants growth and development. The top three cocoa varieties with highest plant height and stem diameter were exceptionally faster in growth when exposed to field conditions after six months intervals. However, the reduction of growth in varieties SPD, T60/887 x CRG 0314/102, CRG 2029 x CRG 0314/102 and CRG 9006 x AMAZ 3-2 is an indication that the plants were negatively affected by the field conditions. This result agrees with the findings of Ofori et al. (2015), when they reported that cocoa plants cultivated in different location with relatively low soil moisture and rainfall reduced growth. The high growth observed in some of the varieties might be because of precocity and vigor of the plants and their ability to establish under different field conditions (Edwin and Master, 2005). Under changing field conditions, cocoa plants in the vegetative phase either show more growth in height or an increase in stem diameter as they develop above-ground biomass (Fig. 2).
High temperature, low rainfall and soil moisture during January 2023 reduced leaf area and leaf dry mass of the varieties (Table 4). The high leaf growth performance of AMAZ 15-15 x EQX 78, CRG 2029 x AMAZ 3-2, CRG 2029 x CRG 0314/102, CRG 9006 x AMAZ 3-2 and T63/697 x CRG 9006, when the soil moisture was low during January 2023 (compared to June 2022) (Supplementary Table 2) might have been due to plants vigour, contributing to bigger leaves development. This might have resulted from adequate partition biomass to leaf traits, and this confirm a mechanism of tolerance in respond to changing environmental conditions. The allocation of biomass in the building of leaf, directly depends on the type of the variety and the environmental conditions, in which the plants have been exposed to and this is a mechanism to improve plants tolerance in responding to extreme environmental conditions (Aidoo et al., 2017).
Depletion of leaf chlorophyll content of variety T63/967 x CRG 9006 in response to water stress is reported to have been linked to reduced photosynthesis of plants (Fig. 3a; Hailemichael et al., 2016). However, the accumulation or retention of chlorophyll pigments of the rest of the varieties under unfavorably field conditions may be a mechanism to mitigate against the harmful effects of the field stress (Fig. 3a). This may also indicate reduced or slow growth and development of the varieties under field stress conditions. The photochemical efficiency was inhibited in the varieties in January 2023 where Fv/Fm was drastically reduced because of low soil moisture content (Fig. 3b). This is an indication of underutilization of absorbed light during low soil moisture (Zhuang et al., 2020).
The change in stomatal conductance measured in high rainfall and soil moisture content in June 2022 and low soil moisture in January 2023 did not show significant varietal variations (Fig. 4a). The tight regulation of stomatal conductance of all the varieties during low soil moisture levels might explain the plant’s ability to close their stomata in response to environmental stresses, resulting in reduction of photosynthesis (Sperling et al., 2014). The closure of the stomata may reduce loss of soil water which eventually could have collapsed the whole plant (Joshi et al., 2022). Plant cellular water levels are important for determining tolerant varieties to changing field conditions such as water deficit and high temperature stress (Chowdhury et al., 2017). Electrolyte leakage reduced in varieties CRG 2029 x AMAZ 3-2, CRG 2029 x CRG 0314/102 and CRG 9006 x AMAZ 3-2 under field low soil moisture and high temperature conditions (Fig. 4b). This is an indication that the plants might have developed leaf surface wax and accumulated metabolites (Table 2 and 3) to protect and improve the membrane stability of the plant leaves (Fig. 4b). It is well-known that reduction in electrolyte leakage under various environmental stress in the field is an important plants tolerance mechanism (Mushtaq et al. 2022). The high electrolyte leakage recorded in the rest of the varieties (Fig. 4b) is an indication that varieties were more affected by the stress in the field.
The reduction in leaf relative water content (Supplementary Table 2) in some of the varieties may be due to water stress and this has also been reported by De Almeida (2016) and Zambrano et al. (2021) in seed-derived cocoa varieties. Plants grown under water stress conditions showed a reduced leaf relative water content than those grown under non-stress water conditions. However, the cells of seed-derived cocoa varieties CRG 2029 x CRG 0314/102 and PA 150 x CRG 0314/102 were the only varieties that had high cellular relative water
Table 5. Correlation between parameters evaluated during wet and dry growing cycles, S-starch; SS-soluble sugars; NSC-Non-structural carbohydrate; SS/S-soluble sugars to starch ratio; C-carbon; N-nitrogen; C/N-carbon to nitrogen ratio; Chl-chlorophyll content; PE-photochemical efficiency; LA-leaf area; LDM-leaf dry mass; MS-membrane stability and RWC-relative water content. Values with asterisks indicate significance (p ≤ 0.005).
| SS | S | NSC | SS/S | C | N | C/N | Chl | PE | LA | LDM | MS | RWC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | -0.09 | ||||||||||||
| NSC | 0.70* | 0.65* | |||||||||||
| SS/S | 0.66* | -0.72* | -0.01 | ||||||||||
| C | 0.15 | 0.16 | 0.23* | -0.05 | |||||||||
| N | 0.10 | 0.11 | 0.16 | -0.07 | 0.31* | ||||||||
| C/N | -0.04 | -0.09 | -0.09 | 0.07 | 0.04 | -0.89* | |||||||
| Chl | 0.06 | 0.087 | 0.11 | -0.08 | 0.21* | 0.30* | -0.22* | ||||||
| PE | -0.25* | -0.07 | -0.24* | -0.02 | -0.31* | -0.31* | 0.14 | -0.33* | |||||
| LA | 0.04 | 0.137 | 0.13 | -0.05 | 0.05 | -0.19* | 0.19* | 0.01 | 0.15 | ||||
| LDM | -0.11 | 0.14 | 0.02 | -0.14 | -0.06 | -0.26* | 0.20* | -0.08 | 0.39* | 0.79* | |||
| MS | 0.22* | 0.004 | 0.17 | 0.10 | 0.08 | 0.05* | -0.05 | 0.04 | -0.48* | -0.15 | -0.25* | ||
| RWC | -0.25* | -0.05 | -0.23 | -0.09 | -0.20* | -0.24* | 0.14 | -0.15 | 0.28* | -0.22* | -0.02 | -0.22* | |
| Gs | -0.17 | -0.03 | -0.54 | -0.04 | -0.26* | -0.37* | 0.25* | -0.33* | 0.53 | -0.01 | 0.21* | -0.28* | 0.22* |
content during low moisture content recorded in the field (Supplementary Table 2). Similar responses have been found in other cocoa plants under water stress conditions compared to non-water stress (De Almeida et al., 2016; Zambrano et al., 2021). This result may suggest that the metabolic activities (Table 2 and 3) and water uptake capacity of those seed-derived cocoa varieties are high and can rapidly recover from low soil moisture stress (Chowdhury et al., 2017).
Non-structural carbohydrates (NSC) which constitute soluble sugars and starch in plants are the main photosynthates. It plays a crucial role in regulating plants physiological modifications in response to drought and high temperature stress (Ai et al., 2017). In this study, the high level of NSC, soluble sugars and starch in the leaves of varieties CRG 9006 x T17/524 and GU 144 x EQX 78 during dry season (Table 2; Fig. 5a) agrees with the work by Galvez et al. (2011) on leaves of aspen under water stress. They suggested that the accumulation of NSC might have been used to maintain osmotic pressure and hydraulic functions. These functions can protect cellular proteins from dehydration to improve the field stress tolerant of plants and enhanced growth (O’Brien et al., 2014). The reduced concentration of non-structural carbohydrate and starch in the tissues of the varieties during the wet season may reflect the vigor and rapid growth observed in this study (Fig. 2). The high level of soluble sugar to starch ratio observed at the wet season indicates that, more carbohydrates were invested in the growth of the crop as indicative of high uptake and synthesizing of more carbon (Table 2 and 3).
In this study, high levels of carbon were found in most of the leaves of the cocoa varieties during the dry season (Table 3; Fig. 5b). These high carbon contents might have resulted from high capacity of carbon uptake rate of young leaves and roots (Njoroge et al., 2023), and remobilization of absorbed carbon from the senescing old leaves to young leaves (Wang et al., 2023). The accumulation of nitrogen in the plant organs plays a critical role in improving growth, tolerance and alleviation of the detrimental effects associated with field stress conditions (Waraich et al., 2021). High nitrogen levels found in this study during the dry season (Table 3; Fig. 5b) agreed with that of Querejeta et al. (2022) on the variation in leaf nitrogen per area of dryland tree species. The group linked their findings to tighter stomatal regulation of transpiration and water use efficiency of the trees. The ratio of carbon and nitrogen metabolism in plant cells is essential in regulating the growth of plants (Aoyama et al., 2014). In comparing the seasons, most of the varieties had a reduced carbon to nitrogen ratio during dry season (Table 3; Fig. 5b,). This indicates that the varieties efficiently utilized nitrogen and balanced carbon to nitrogen ratio in response to changing field conditions.
Correlation analysis demonstrated the effects of the other parameters on the growth and development of the varieties, and these were either positive or negative (Table 4 and 5). The high correlations that were observed between the physiology, growth and metabolism of the seed-derived cocoa varieties fall in line with the findings of Li et al. (2022) and Ofori et al. (2015). These strong correlation among the compounds, physiology and growth found is an indication of a huge dependence of physiology, starch and soluble sugars accumulation on the concentration of non-structural carbohydrates, carbon and nitrogen for growth of the plants under stressful environment (Table 4 and 5).
Materials and methods
Planting materials and experimental design
The experiment was established in 2019 on the experimental plot (J9 B) of the Cocoa Research Institute of Ghana (CRIG), New-Tafo. A total of 15 varieties of seed-derived cocoa seedlings, six-month after nursing were transplanted into the field with a planting distance of 3 m x 3 m. The experiment was conducted during wet and dry growing seasons and the results compared. The period between May to September with optimum temperature, high rainfall and soil moisture content was classified as wet growing season whilst October to February with high temperature, low rainfall and soil moisture content classified as dry growing season (Fig. 1). The texture of the soil detected at the experimental site was classified as sandy loam with high percentage of sand. Apart from total nitrogen and exchangeable magnesium, all the chemical properties of the soil obtained in the experimental plot were below the critical value for optimum soil conditions of establishing cocoa plantation (Supplementary Table 1).
Ghana, Guiana, Trinidad and Upper Amazon Basin were the main parental sources of the varieties (Table 1). See Lockwood and Gyamfi, (1979); Motamayor et al. (2008); Padi et al (2015) and Padi and Ofori, (2016) for detail description of the varieties. The seed pods of the varieties were generated by artificial or manual pollination. The flower buds designated as female parents (first parents) were covered by specialized designed caps to prevent insect and air pollination. Fresh pollen collected from male parents (second parents) was brushed onto the stigma of flowers of the female trees covered at the previous day. This process was conducted between 07:00 to 10:00 hours. The specialized caps were removed after 24 hours and the position of each manually pollinated pod on the tree marked. The natural pollinated flowers were removed at the cherelle (immature pod) stage at weekly intervals. The resulted seed-derived cocoa varieties were then tested for combining abilities for vigour and yield (Padi et al., 2017). The best vigorous and yielding varieties were evaluated for their physiology, growth and metabolism resilience in response to field stress conditions.
A single tree randomization procedure in a randomized complete block design (RCBD) with 4 replications was followed. A total number of cocoa trees in a replicate was 8-16 trees per variety. The experimental period covered 2022/2023 growing cycle at the vegetative stage of the plants between 29 to 39 months after planting (MAP). Data were taken in June 2022 and January 2023 representing wet and dry growing seasons.
Ambient temperature and soil moisture content
Mean monthly temperatures were obtained from the meteorological station located in New-Tafo, Cocoa Institute of Ghana, Eastern Region. Soil moisture content was determined using three Delta T probes in each replicate. These probes were inserted 1.5 m away from the cocoa plants. Monthly records were taking during the experimental period.
Morphological assessment
Morphological data such as plant height and stem diameter growth rate were determined at six months interval using a meter ruler and electronic digital vernier calipers (Factory IP67, China), respectively. Plant height was measured from the ground level to the canopy top, while stem diameter was measured at 15 cm of the varieties from the ground surface.
Physiological studies
Chlorophyll content, photochemical efficiency measured as Fv/Fm, stomatal conductance, leaf functional traits, relative water content and membrane stability measured as electrolyte leakage were assessed at two time points (June 2022 and January 2023 as wet and dry growing season, respectively). Four trees per plot per variety were tagged. Two fully new developed leaves (the third leaf from the flushes) from each of the tagged trees were selected and the parameters measured using SPAD 502 plus leaf chlorophyll meter (Spectrum Technologies, 3600 Thayer Ct, IL, USA) for relative chlorophyll content. Fluorescence meter (Fluorpen FP 110, Drásov, Czech Republic) was used for measuring Fv/Fm and leaf porometer (Decagon Devices, Inc. 2365 NE Hopkins Court, Pullman WA 99163) for stomatal conductance. For the destructive sampling again two fully new developed leaves on the tagged plants per varieties per plot were harvested. Leaf area estimation was done using WinDIAS 2.0 Image Analysis System (Delta-T Devices Limited, UK). The leaves were then oven dried at 65 °C for three days. Leaf dry mass (g) was then measured using analytical scale (Scitek Global Co., Ltd. Jinan City, Shandong Province, China).
Relative water content was measured and calculated using the estimated fresh mass, turgor mass and dry mass of 4 sample leaves harvested from 2 trees per varieties per plot as described by Pieczynski et al. (2013). Then percentage RWC calculated as:
\(RWC \leq \frac{Fm - Dm}{Tm - Dm}\ x\ 100\) Eq. 2
Where RWC is relative water content, FM is fresh mass, TM is turgor mass and DM is dry mass of sampled leaf. Membrane stability was determined using electrolyte leakage of 4 sample leaves harvested from 4 trees per variety per plot following Kim et al. (2007) protocol. The percentage of membrane stability or index of electrolyte leakage was expressed as:
MS \(\leq \frac{Ci}{Cm}\ x\ 100\) Eq. 3
Where MS is membrane stability, Ci is initial leakage, Cm is maximum electrolyte leakage.
Sampling and carbohydrates analyses
Sampling procedure and non-structural carbohydrate were determined as described by Landhäusser et al. (2018). The enzymatic activity of the sampled leaves (new fully developed leaves - third after the flushes) were immediately stopped. This was done by cutting the leaves into smaller pieces and 0.5 g boiled in 80% ethanol under reflux for 30 minutes. The supernatant separated into a new flask after boiling. The ethanol was then evaporated under reduced pressure using rotary evaporator. Two milliliters of water were added to dissolve all sugars in the solution. The solution was then filtered into clean flasks using Whatman No. 41 filter paper. The filtered solution was clarified by removal of non-sugar components using a solution of 0.3 N Ba(OH)2 and 5% ZnSO4 solution. The resulting solution was shaken with cation and anion exchange and resins to remove inorganic ions and acids present in the solution though Whatman No. 41 filter paper. The volume of the filtrate was poured into vials and labelled as soluble extract (SE) for further analysis. The residue that remained after the removal of 80% ethanol was digested under reflux for one-hour using 25 ml of 1.5 N sulphuric acid. The solution was filtered and the residue washed with water several times. The acid in the solution was neutralized by adding solid BaCO3. The solution was centrifuged at high speed (26,000 x g) to remove precipitate, filtered and shaken with ion exchange, resins to deionize the solution. The filtered solution was poured into vials and labelled as acid extract (AE). In the analysis, 1 ml of solutions (SE and AE) was added to 1 ml 10% phenol reagent in a test tube and mixed. To the solution in the test tube, 5 ml of concentrated sulphuric acid was added straight and mixed thoroughly and left to cool. The sugar and starch were analyzed by reading absorbance on the Jenway 6405 UV/Vis spectrophotometer (American Laboratory Trading, USA) at 490 nm. Non-structural carbohydrate was then estimated from the analysis of soluble sugars and starch.
Determination of leaf nitrogen and carbon contents
Oven dried leaf samples at the temperature of 65 °C for three days were ground using TissueLyzer (RetschGmbh and Co.). The ground samples were analyzed for nitrogen and carbon contents using Kjeldahl (Bremner, 1965) and wet oxidation (Walkley and Black, 1934) methods, respectively.
Soil properties and analysis
Physical and chemical soil analysis were conducted on soils sampled from 0 to 20 cm depth on each of the plots before planting (Supplementary Table1). This assessment established the levels of soil fertility in the plot and compared to the critical levels for cocoa growth and development. The soil texture was sandy loam which was determined using Hydrometer method (Supplementary Table 1). Soil pH was measured potentiometrically in a slurry using an electronic pH meter (SevenCompact Duo pH/conductivity, Mettler Toledo, US). Ammonium acetate and Bray 1 methods were used to extract exchangeable bases and Mehlich III methods for available P while organic carbon was determined using Walkley and Black method (Adusei et al., 2021).
Data analysis
Two-way ANOVA and contrast analysis were performed for each trait using GENSTAT 9th edition. The means were separated by a Tukey–Kramer, Fisher pair wise comparison and student t-test at P ≤ 0.05 to identify differences between the means of the individual varieties and the sampling time points (growing cycles). The varietal statistically significant differences were not observed within the varieties in some of the parameters either in a season or both seasons (Supplementary Table 3 and 4).
Conclusion
The varieties exhibited diverse levels of tolerance to the changing field temperature, rainfall and soil moisture content during dry growing season. There were insignificant changes of the parameters among the varieties in wet or in dry growing seasons except for soluble sugars, non-structural carbohydrates and soluble sugar to starch ratio. The varieties exhibited tightly stomatal regulations and reduced photochemical efficiency at various magnitudes in response to dry season compared to wet season conditions. The leaves of the varieties turned hefty, increased chlorophyll contents, thickness and retained relative water content culminating in the reduced electrolyte leakage. Strong relationships were observed between the physiology, growth and central carbon metabolism parameters. The accumulation of carbohydrate, carbon and nitrogen under high field temperature, low rainfall and soil moisture content could therefore been used by the varieties for physiological resilience, survival and growth in response to field stress. The varieties AMAZ 15-15 x EQX 78, CRG 2029 x AMAZ 3-2, CRG 2029 x CRG 0314/102, CRG 9006 x AMAZ 3-2 and PA 150 x CRG 0314/102 showed much prominence in response to high temperature, low rainfall and soil moisture content stress in the field.
Acknowledgements
We appreciate the role played by all those who were involved in the various levels of the project for their contribution, especially Daniel Tetteh Djangmah, Eric Adjei Adjetey, Nicholas Kwadjo Narh, Moses Ahenkan, Rafiatu Kotei, Emmanuel Akpi, George Danchie, Linda Yeboah and Opoku Acheamfour. We are grateful to Cocoa Research Institute of Ghana for financing this work and the permission to publish as manuscript number CRIG/01/2022/060/ 001.
Statement of contributions
MKA organized and analyzed the data and wrote the manuscript. MTA methodology. EA, AO, AA and FKP supervised the work. FKP and AO oversaw the research project. All authors read and approved the manuscript.
References
Acheampong K, Hadley P, Daymond AJ, Adu-Yeboah P (2015) The influence of shade and organic fertilizer treatments on the physiology and establishment of Theobroma cacao clones. Am J Exp Agric. 6:347-360.
Adusei G, Aidoo MK, Srivastava AK, Asibuo JY, Gaiser T (2021) The variability of grain yield of some cowpea genotypes in response to phosphorus and water stress under field conditions. Agron. 11: 28.
Ai Z, Hed L, Xin Q, Ting Y, Liu G, Xue S (2017) Slope aspect affects the non-structural carbohydrates and C:N:P stoichiometry of Artemisia sacrorum on the Loess Plateau in China. Catena 152: 9-17.
Aidoo MK, Sherman T, Lazarovitch N, Fait A, Rachmilevitch S (2017) A bell pepper cultivar tolerant to chilling enhanced nitrogen allocation and stress-related metabolite accumulation in the roots in response to low root-zone temperature. Physiol Plant. 161: 196-210,
Aidoo MK, Sherman T, Lazarovitch N, Fait A, Rachmilevitch S (2019) Physiology and metabolism of grafted bell pepper in response to low root-zone temperature. Funct Plant Biol. 46: 339-349.
Alban MKA, Apshara SE, Hebbar KB, Mathias TG, Severin A (2016) Morpho-physiological criteria for assessment of two-month-old cacao (Theobroma cacao L.) genotypes for drought tolerance. Indian J Plant Physiol. 21: 23-30.
Aoyama S, Huarancca Reyes T, Guglielminetti L, Lu Y, Morita Y, Sato T, Yamaguchi J (2014) Ubiquitin ligase ATL31 functions in leaf senescence in response to the balance between atmospheric CO2 and nitrogen availability in Arabidopsis. Plant Cell Physiol. 55: 293-305.
Bremner JM (1965) Total nitrogen. In methods of soil analysis, part 2 end. 1149-1178 (Ed Black CA). Madison: American Society of Agronomy.
Chowdhury JA, Karim MA, Khaliq QA, Ahmed AU, Mondol ATMAI (2017) Effect of drought stress on water relation traits of four soybean genotypes. SAARC J of Agric. 15: 163-175.
De Almeida Dos Santos J, Tezara W, Herrera A (2016) Physiological responses to drought and experimental water deficit and waterlogging of four clones of cacao (Theobroma cacao L.) selected for cultivation in Venezuela. Agric Water Manag. 171: 80-88.
Edwin J, Master WA (2005) Genetic improvement and cocoa yields in Ghana. Exp Agric. 41: 491-503.
Galvez DA, Landhausser SM, Tyree MT (2011) Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 31: 250-257.
Hailemichael G, Catalina A, Gonzalez MR, Martin P (2016) Relationships between water status, leaf chlorophyll content and photosynthetic performance in Tempranillo vineyards. S Afr J Enol Vitic. 37: 149-156.
Huang B, Fu J (2000) Photosynthesis, respiration, and carbon allocation of two cool season perennial grasses in response to surface soil drying. Plant and Soil 227: 17-26.
Joshi J, Stocker BD, Hofhansl F, Zhou S, Dieckmann U, Prentice IC (2022) Towards a unified theory of plant photosynthesis and hydraulics. Nat Plants. 11: 1304-1316,
Kim JY, Park SJ, Jang BS, Jung CH, Ahn SJ, Goh CH, Cho K, Han O, Kang HS (2007) Functional characterization of a glycine-rich RNA-binding protein 2 in Arabidopsis thaliana under abiotic stress conditions. Plant J. 50: 439-451.
Läderach P, Martinez-Valle A, Schroth G, Castro N (2013) Predicting the future climatic suitability for cocoa farming of the world’s leading producer countries, Ghana and Côte d’Ivoire. Clim Change. 119: 841-854.
Lahive F, Hadley P, Daymond AJ (2019) The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron Sustain Dev. 39: 5.
Landhäusser SM, Chow PS, Dickman LT, Furze ME, Kuhlman I, Schmid S, Wiesenbauer J, Wild B, Gleixner G, Hartmann H, Hoch G, McDowell NG, Richardson AD, Richter A, Adams HD (2018) Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 38: 1764-1778.
Li Y, Sun H, de Paula Protásio T, Gherard PR, Du B (2022) The mechanisms and prediction of non-structural carbohydrates accretion and depletion after mechanical wounding in slash pine (Pinus elliottii) using near-infrared reflectance spectroscopy. Plant Methods 18: 107.
Liu W, Su J, Li S, Lang X, Huang X (2018) Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan Province, China. Sci Rep. 8: 1083.
Lockwood G, Gyamfi MMO (1979) The CRIG cocoa germplasm collection with notes on codes used in the breeding programme at Tafo and elsewhere. Tech. Bull. 10. Cocoa Research Institute, Ghana. 62 pp.
Motamayor JC, Lachenaud P, Da Silva e Mota JW, Loor R, Kuhn DN, Brown JS, Schnell RJ (2008). Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L). PloS one. 3, e3311.
Mushtaq N, Wang Y, Fan J, Li Y, Ding J (2022) Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants (Basel) 11: 154.
Njoroge B, Li Y, Otieno D, Liu S, Wei S, Meng Z, Zhang Q, Zhang D, Liu J, Chu G, Haider FU, Tenhunen J (2023) Seasonal droughts drive up carbon gain in a subtropical forest. J Plant Ecol. 16:1.
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by nonstructural carbohydrate levels. Nat Clim Change. 4: 710-71.
Ofori A, Padi FK, Acheampong K, Lowor S (2015) Genetic variation and relationship of traits related to drought tolerance in cocoa (Theobroma cacao L.) under shade and no-shade conditions in Ghana. Euphytica 201: 411-421.
Padi FK, Opoku SY, Assuah MK, Dumfeh O, Cudjoe AR, Owusu-Ansah F (2013) Evaluation of cocoa clones for yield and resistance to black pod and cocoa swollen shoot virus diseases. Cocoa Research Institute Ghana, Annual Report pp 47-51.
Padi FK, Ofori A, Takrama J, Djan E, Opoku SY, Dadzie MA, Bhattacharjee R, Motamayor JC, Zhang D (2015). The impact of SNP fingerprinting and parentage analysis on the effectiveness of variety recommendations in cacao. Tree Genet Genomes. 11: 44.
Padi FK, Ofori A (2016) Cacao seed purity and genotype influence on seedling growth under peasant-farmer conditions in Ghana. J Crop Improv. 30: 493-515.
Pieczynski M, Marczewski W, Hennig J, Dolata J, Bielewicz D, Piontek P, Wyrzykowska A, Krusiewicz D, Strzelczyk-Zyta D, Konopka-Postupolska D, Krzeslowska M, Jarmolowski A, Szweykowska-Kulinska Z (2013) Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol J. 11:459-69.
Querejeta JI, Prieto I, Armas C, Casanoves F, Diémé JS, Diouf M, Yossi H, Pugnaire FI, Rusch GM (2022) Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 235: 1351-1364.
Rachmilevitch S, Lambers H, Huang B (2008) Short-term and long-term root respiratory acclimation to elevated temperatures associated with root thermotolerance for two agrostis grass species. J Exp Bot. 59: 3803-3809.
Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134: 1683-1696.
Schroth G, Läderach P, Matinez-Valle AI, Bunn C, Jassogne L (2016) Vulnerability to climate change of cocoa in West Africa: patterns, opportunities and limitations to adaptation. Sci Total Environ. 556: 231-24.
Sperling O, Shapira O, Tripler E, Schwartz A, Lazarovitch N (2014) A model for computing date palm water requirements as affected by salinity. Irrig Sci. 014: 0433-0435.
Walkley A, Black IA (1934) An examination of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 27: 29-38.
Wang Y, Han X, Ai W, ZhanH, Ma S, Lu X (2023) Non-Structural Carbohydrates and Growth Adaptation Strategies of Quercus mongolica Fisch. ex Ledeb. Seedlings under Drought Stress. Forests 14: 404.
Waraich EA, Ahmad R, Halim A, Aziz T (2012) Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nut. 12: 221-244.
Zambrano MAO, Castillo DA, Pérez LR, Terán W (2021) Cacao (Theobroma cacao L.) response to water stress: physiological characterization and antioxidant gene expression profiling in commercial clones. Front Plant Sci. 12:1924.
Zhuang J, Wang Y, Chi Y, Zhou L, Chen J, Zhou W, Song J, Zhao N, Ding J (2020) Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ. 8:e10046.