

Aust J Crop Sci. 18(11):715-722 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.11.p97
Canonical correlations of maize yield components with biological and chemical soil indicators in a subtropical climate
Guilherme Bergeijer da Rosa1*, Diego Nicolau Follmann1, Felipe Tascheto Bolzan1, Henrique Eggers1, Alessandro Dal Col Lúcio1, Rodrigo Josemar Seminoti Jacques2, Valéria Ortaça Portela2, Volmir Sergio Marchioro3, Luís Antônio Klein3, Ivan Carlos Maldaner4
1Department of Plant Sciences, Federal University of Santa Maria, Santa Maria (RS), Brazil
2Department of Soil Sciences, Federal University of Santa Maria, Santa Maria (RS), Brazil
3Department of Agricultural and Environmental Sciences, Federal University of Santa Maria, Frederico Westphalen (RS), Brazil
4Polytechnic College of the Federal University of Santa Maria (RS), Brazil
*Corresponding author: Guilherme Bergeijer da Rosa

ORCID: 0000-0002-1121-6574)
Abstract: Understanding the interaction between chemical and biological soil variables and their impact on the agronomic performance of agricultural crops is critical for enhancing management practices and promoting sustainability in agriculture. Thus, this study aimed to identify canonical correlations between chemical and biological soil variables and the yield components of maize under a subtropical climate in Brazil. Six experiments were conducted, with planting carried out in two different seasons across three locations. Four experiments occurred in rainfed areas, while two utilized pivot irrigation during the 2020/2021 growing season. Three hybrids were sown with three replications in each experiment. All 54 experimental units were evaluated for chemical and biological soil variables, as well as maize yield components. A canonical correlation analysis was conducted between the groups of variables, along with a test for comparing means for the yield components. The planting in two different seasons was exclusively employed to increase the variability of the interaction among the evaluated variables and was not considered in the analyses and interpretations. The results underscore the profound influence of soil and climatic conditions on the interaction between variables, as observed through the canonical correlation. In the irrigated location, a significant correlation between chemical and biological soil variables was observed, accompanied by the highest means in yield components. This emphasizes the need for balanced fertilization to stimulate soil biological activity and enhance grain production. Conversely, in non-irrigated locations, a significant correlation between biological soil variables and yield components was found. Strategies aimed at mitigating the effects of water deficit, such as the addition of plant materials and crop rotation, emerge as viable alternatives. These practices promote the activity of soil microbiota, expanding benefits such as nutrient cycling, with consequential impacts on grain productivity.
Keywords: Linear correlation; soil chemical properties; soil quality bioindicators; subtropical environment; Zea mays L.
Abbreviations: CN_Condition number; BSR_Basal soil respiration; CEC_Cation exchange capacity; NGR_Number of grains per row; TGW_Thousand-grain weight; GY_grain yield; NR_Number of rows; SM_Santa Maria; SVS_São Vincente do Sul; FW_Frederico Westphalen; VIF_Variance inflation factor
Introduction
Maize (Zea mays L.) occupies a central role in both Brazilian and global agriculture due to its significant social and economic impacts. Its widespread cultivation necessitates extensive understanding of the relationship between management practices, environmental such as soil conditions—and crop performance. Soil quality is evaluated through indicators categorized into chemical, physical, and biological metrics, which are responsive to environmental conditions and management methods (Panico et al., 2020; Cai et al., 2021). Importantly, soil quality—and consequently, crop yields—can be enhanced through the implementation of optimal management practices within the no-till system.
Essential practices such as crop diversification and rotation, as well as the preservation of soil cover with living plants or plant residues, play critical roles in preventing erosion and the resultant loss of soil, nutrients, and organic matter (OM). These practices significantly affect microbial activity and the soil's potential fertility (Moitinho et al., 2015; Panico et al., 2020; Lopes et al., 2021). The no-tillage system is recognized for its ability to improve soil quality, thereby positively affecting maize crop traits directly related to grain yield. Among the biological indicators, the activity of soil organisms and the rate of plant residue decomposition stand out due to their substantial impact on nutrient availability for plants. Generally, a positive correlation exists between the decomposition rate, of residues soil organism activity, CO2 emissions, and nutrient mineralization. However, the decomposition rate of organic residues also depends on additional factors such as the chemical composition of the plant material, temperature, and rainfall (Cai et al. (2021).
Organic matter and pH are two chemical indicators considered highly relevant for assessing soil quality (Bünemann et al., 2018). Organic matter contributes to the biological, chemical, and physical modification of soil, serving as a reservoir of organic carbon for soil organisms, retaining and supplying nutrients and water, and acting as a binding agent in soil aggregation (Vidal et al., 2021). Moreover, pH levels are closely linked with nutrient availability, particularly phosphorus in tropical and subtropical conditions (Cardoso et al., 2013). They also affect microbial activity and the availability of aluminum, which is toxic to both plants and soil organisms (Jones et al., 2019).
Biological and chemical indicators may have interrelated aspects, as some studies have found a strong relationship between CO2 emission and OM and a significant correlation between pH and BSR (Moitinho et al., 2015; Lopes et al., 2021). Determining the correlations between variables is crucial; however, relying solely on Pearson’s correlation analysis can introduce distortions in the obtained results. Therefore, exploring multivariate relationships, such as canonical correlations, allows for the estimation of relationships between groups of variables and the identification of dependencies among them (Mingoti, 2005).
Given this context, identifying the linear correlations between soil quality indicators and maize grain yield components will provide insights that can enhance soil management practices and achieve higher grain productivity in a more sustainable manner. Thus, this study hypothesizes that the attributes indicative of soil quality are directly related to maize grain yield components and that grain yield positively responds to improvements in soil quality. Consequently, the objective of this study was to identify canonical correlations between biological and chemical attributes of the soil and the components of maize grain yield in a subtropical climate in Brazil.
Results
The meteorological conditions during the experiments are provided in Fig 2. The mean total rainfall volumes recorded were 352 mm in Frederico Westphalen (FW), 325 mm in Santa Maria (SM), and 547 mm in São Vincente do Sul (SVS). In the specific period of 45 days of litter bag exposure in the field, which began at the VT phenological stage of the crop, the accumulated volumes were 139 mm in FW, 193 mm in SM, and 291 mm in SVS (including irrigation).
The chemical characterization of the six experiments can be observed in Table 1 and reveals marked differences among them. There was a substantial variation in the mean clay content, with values higher than 600 g kg-1 in FW, between 200 and 300 g kg-1 in SM, and less than 150 g kg-1 in SVS. Another significant difference was observed in the mean OM: FW was classified as a medium class, with values above 30 g kg-1, while SM and SVS had low levels, equal to or less than 25 g kg-1. Regarding phosphorus, the levels were classified as low in FW and SM but high in SVS. The potassium levels were considered high in SM and SVS, and very high in FW, according to the Fertilization and Liming Manual (CQFS, 2016). The soil pH values were similar across the six experiments, ranging between 5.1 and 5.4. Despite the low pH values, the aluminum content was also low at all sites.
To meet the criteria for weak multicollinearity, defined as CN < 100 and the absence of variance inflation factor (VIF) values ≥ 10 within the same variable group, we opted to exclude the variables Ca, Mg, Al, H+Al, base saturation, and clay, as they were inflating multicollinearity in group 1 (chemical soil variables). Conversely, in Groups 2 (biological soil variables) and 3 (maize grain yield components), it was not necessary to exclude any variables for the canonical correlation analysis.
In SVS, a significant canonical correlation (r = 0.941, p < 0.05) between the group of chemical soil variables and the group of biological soil variables was observed through the first canonical pair (Table 2). It is noteworthy that the basal soil respiration (BSR) and the decomposition rate, biological soil variables, showed direct correlations with OM and phosphorus, while they were inversely correlated with soil pH (chemical soil variables). The other chemical soil variables—effective CEC, aluminum saturation, potassium, and the calcium-magnesium ratio—presented magnitudes lower or close to 0.300, indicating minimal interference in the linear correlation between the groups (Cohen, 1977). No significant canonical correlation was observed between the group of chemical soil variables and the group of maize grain yield components in any of the three study sites.
The analysis of canonical correlations between the group of biological soil variables and the grain yield component group showed significant effects at FW and SM. In FW, there was a significant linear correlation (r = 0.858, p < 0.05) in the first canonical pair. The decomposition rate was directly related to number of grains per row (NGR), thousand-grain weight (TGW), and grain yield (GY); BSR and number of rows (NR) showed a low magnitude, indicating the independence of these variables from the groups.
In SM, a significant correlation (r = 0.813, p < 0.05) was also observed between the groups in the first canonical pair; BSR had a high magnitude, along with the decomposition rate, was related to NGR and GY. The NR and TGW variables proved to be independent of the others, showing low magnitudes.
The mean values of GY, TGW, NGR, and NR at each site are shown in Fig 3. There was no significant difference between the sites for NR (Fig 3D). For NGR, SVS had a mean higher than 32 grains per row, statistically different from FW and SM, which did not differ from each other, with means below 30 grains (Fig 3C). There was a significant difference for TGW among the three environments, with SVS recording the highest mean (396 g), SM showing an intermediate value (330 g), and FW the lowest value (283 g) (Fig 3B). Finally, GY differed significantly among the three environments, with means of 9987 kg ha-1 in SVS, 8667 kg ha-1 in SM, and 5724 kg ha-1 in FW (Fig 3A).
Discussion
The best water distribution during the crop development in the SVS environment is evidenced by the arrangement of columns in the graph (Fig 2C), resulting from the central pivot irrigation system. In contrast, in the rainfed environments of FW and SM (Figs 2A and 2B), periods of low precipitation or with reduced volumes, often below 10 mm, are noticeable, especially in the second half of November and December. These weather conditions can have adverse impacts on the full development of plants, as the maximum agronomic performance of a hybrid is determined by solar radiation and temperature, being limited by water availability (Grassini et al., 2009).
It is important to highlight that, besides uniformity in the distribution, we observed that the irrigated environment had a total volume superior to the rainfed environments. These discrepancies in meteorological variables have a direct impact on the dynamics of soil fauna, significantly influencing the rate of decomposition of plant residues (Cai et al., 2021) and consequently on plant development and the GY of maize (Grassini et al., 2009).
The GY of maize is significantly correlated with soil fertility (Zhang et al., 2018), which is also associated with the activity of organisms that decompose plant material and utilize
Table 1. Soil properties in two sowing seasons in the municipalities of Frederico Westphalen, Santa Maria, and São Vicente do Sul in Rio Grande do Sul State (southern Brazil).
| Attributes | Frederico Westphalen | Santa Maria | São Vicente do Sul | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | ||
| Clay | 661.0 | 615.0 | 286.0 | 235.0 | 131.0 | 125.0 | |
| pH | 5.3 | 5.1 | 5.2 | 5.4 | 5.3 | 5.1 | |
| OM | 37.0 | 34.0 | 23.0 | 25.0 | 16.0 | 23.0 | |
| P | 10.4 | 6.7 | 11.0 | 11.4 | 52.3 | 88.3 | |
| K | 352.0 | 256.0 | 100.4 | 136.4 | 104.0 | 100.4 | |
| Ca | 6.2 | 6.2 | 5.2 | 5.9 | 4.7 | 4.7 | |
| Mg | 3.0 | 3.2 | 2.9 | 3.3 | 1.6 | 1.5 | |
| Al | 0.1 | 0.1 | 0.3 | 0.0 | 0.3 | 0.2 | |
| H+Al | 5.4 | 6.6 | 7.9 | 5.4 | 5.5 | 5.8 | |
| Effective CEC | 10.3 | 10.2 | 8.8 | 9.6 | 6.9 | 6.7 | |
| Al saturation | 1.3 | 0.9 | 4.2 | 0.7 | 5.0 | 3.9 | |
| Base saturation | 64.9 | 60.6 | 52.6 | 63.9 | 54.4 | 53.4 | |
| Ca:Mg | 2.0 | 1.9 | 1.8 | 1.7 | 2.9 | 3.0 | |
Clay (g kg-1, densimeter), pH (in water 1:1), organic matter (OM; g kg-1, Walkley-Black carbon), phosphorus (P; mg dm-3, Mehlich-1), potassium (K; mg dm-3, Mehlich-1), calcium (Ca; cmolc dm-3, KCl), magnesium (Mg; cmolc dm-3, KCl), aluminum (Al; cmolc dm-3, KCl), hydrogen + aluminum (H + Al; cmolc dm-3, titration), effective cation exchange capacity (effective CEC; cmolc dm-3), aluminum saturation (%), base saturation (%), and calcium-magnesium ratio (Ca:Mg). Nine soil samples were taken for each environment.
nutrients at some point in their life cycles (Liu et al., 2006). The low pH values observed in the three sites are common in soils in the state of Rio Grande do Sul (Althaus et al., 2018) (Table 1). According to Li et al. (2019), soil pH directly influences the availability of nutrients, affecting fertilizer use efficiency and playing a crucial role in achieving higher GY. Acidic soils are often associated with the presence of Al3+, which can be toxic to both plants and the soil microbiota; however, the values of Al3+ were low in the three studied sites. Variations in the content of OM observed among the soils are related to the clay content and its physical protection (Sarkar et al., 2018). Notably, the OM content is an important variable, as it is used to determine the nitrogen fertilization rate and has a strong relationship with the microbial population (CQFS, 2016; Da Rosa et al., 2017).
The only site where a significant correlation was observed between soil biological variables and chemical variables was in SVS (Table 2). We observed a direct relationship between BSR and the decomposition rate, indicating faster vegetal residue decomposition where microbial activity was more intense. This decomposition rate, besides being influenced by microbial activity, is affected by various factors, such as the residence time of plant residue in the soil, as longer-residing residues allow for more extensive microbial colonization, potentially increasing the decomposition rate over time.
The composition of the plant residue itself is also an important factor, as residues with a higher content of lignin and cellulose tend to decompose more slowly due to the difficulty in breaking down these complex compounds (Cai et al., 2021; Zhao et al., 2021). This composition, in turn, is influenced by climatic and soil properties, as observed by Cai et al. (2021), where the soil pH negatively affected the properties of the residue and the sand content correlated positively with lignin content and negatively with the decomposition rate, due to lignin's resistance to degradation by organisms.
Climatic characteristics, such as temperature and humidity, also affect microbial activity and, consequently, the decomposition rate. Warmer and more humid environments generally favor greater microbial activity, accelerating the decomposition process (Liu et al., 2006; Zhao et al., 2021). This positive association between microbial activity and the decomposition rate was observed in our study, specifically in the only irrigated environment, where there was no water deficit, and the humidity was high. In a study conducted by Cai et al. (2021), 85% of the variation in decomposition among the studied environments was explained by the
Fig 1. Locations of the three experimental sites where the six experiments were conducted at two different sowing times in the 2020/2021 growing season in the state of Rio Grande do Sul, southern Brazil.
annual mean temperature, rainfall, sand content, soil pH, as well as nitrogen and lignin content of the plant material.
In addition to influencing the composition of plant material, physical and chemical properties of the soil, such as pH, texture, and nutrient content like phosphorus and OM, affect decomposition by influencing both microbial activity and the availability of nutrients necessary for the process. Vain et al. (2021) assert that soil microorganism activity, measured by CO2 emission, is limited by nutrient availability. Among soil properties, OM and phosphorus content are directly related to the microbial population and, consequently, to soil respiration (Paz-Ferreiro and Fu, 2013; Spohn and Schleuss, 2019). In the present study, BSR correlated positively and directly with soil OM and phosphorus content. The activity of the microbiota, measured by CO2 emission, demonstrates that the higher the emission, i.e., the higher the BSR, the greater the activity of the microbial community. This justifies the direct correlation between the decomposition rate,
Table 2. Correlations and cross-canonical charges estimated between (A) soil biological variables and soil chemical variables, (B) maize grain yield components and soil chemical variables and (C) soil biological variables and maize grain yield components variables at three sites. Frederico Westphalen, Santa Maria, and São Vicente do Sul, Rio Grande do Sul, Brazil.
| Cross canonical charges | ||||
|---|---|---|---|---|
| Variables | Frederico Westphalen | Santa Maria | São Vicente do Sul | |
| Soil biological variables | ||||
| BSR | 0.420 | -0.877 | 0.808 | |
| Decomposition rate | -0.838 | -0.294 | 0.857 | |
| Soil chemical variables | ||||
| pH | -0.448 | -0.299 | -0.545 | |
| Effective CEC | -0.162 | -0.554 | -0.265 | |
| Al saturation | -0.133 | 0.454 | -0.068 | |
| OM | -0.458 | -0.796 | 0.798 | |
| P | -0.230 | -0.124 | 0.654 | |
| K | -0.562 | -0.579 | -0.108 | |
| Ca/Mg | -0.520 | -0.003 | 0.339 | |
| Canonical correlation (r) | 0.857 | 0.877 | 0.941 | |
| p | 0.150ns | 0.189ns | 0.008* | |
| Maize grain yield components | ||||
| NR | 0.401 | 0.486 | 0.252 | |
| NGR | 0.877 | -0.730 | 0.413 | |
| TGW | 0.603 | -0.493 | -0.330 | |
| GY | 0.814 | -0.752 | 0.567 | |
|
||||
| pH | 0.561 | 0.086 | 0.076 | |
| Effective CEC | 0.313 | 0.002 | 0.251 | |
| Al saturation | 0.036 | -0.025 | 0.193 | |
| OM | 0.642 | 0.658 | -0.361 | |
| P | 0.490 | 0.055 | -0.374 | |
| K | 0.817 | 0.350 | -0.018 | |
| Ca/Mg | 0.691 | -0.158 | -0.087 | |
| Canonical correlation (r) | 0.970 | 0.845 | 0.793 | |
| p | 0.059ns | 0.519ns | 0.954ns | |
| Soil biological variables | ||||
| BSR | 0.315 | -0.702 | -0.734 | |
| Decomposition rate | -0.856 | -0.613 | -0.458 | |
| Maize grain yield components | ||||
| NR | -0.300 | -0.012 | 0.260 | |
| NGR | -0.633 | -0.685 | 0.292 | |
| TGW | -0.641 | -0.217 | -0.239 | |
| GY | -0.838 | -0.424 | 0.589 | |
| Canonical correlation (r) | 0.858 | 0.813 | 0.736 | |
| p | 0.015* | 0.047* | 0.069ns | |
Biological variables - BSR: soil basal respiration (mg) of C-CO2 kg-1 soil hour-1; decomposition rate (g g-1 day-1). Chemical variables - pH; effective cation exchange capacity (cmolcdm3); Al saturation (%); OM: organic matter (%); P: phosphorus (mg dm-3); K: potassium (mg dm-3); Ca/Mg. Maize grain yield components - NR: number of rows; NGR: number of grains per row; TGW: thousand-grain weight (g); GY: grain yield (kg ha-1). *Significant according to the χ² test at 5% probability of error.
influenced by microbial activity, and the contents of OM and phosphorus in the soil.
For some soil properties, such as pH, there are disagreements about their influence on the activity of organisms and, consequently, on BSR. Mitra et al. (2019) did not find a significant correlation between these two variables. In the present study, we observed that BSR and the decomposition rate correlated inversely with the soil pH, indicating that microbial activity and residue decomposition decreased as the soil pH increased. This contradicts the findings of Jones et al. (2019), who claim that a pH above 5.5 promotes greater microbial energy efficiency.
The soil microbiota responds directly to soil pH and to the presence of available aluminum, which is toxic. At pH levels below 5.5, aluminum is available in the soil solution; however, in the present study, no significant quantities of this element were found, as indicated by the low magnitude of aluminum saturation (-0.068). Furthermore, pH values varied little, being similar across the three studied environments. Thus, we can infer that other factors influence microbial activity. For instance, Huang et al. (2021) observed that BSR correlates
positively with temperature. Mitra et al. (2019) corroborates this result and includes rainfall as a determining factor for activity in the soil.
Our findings also revealed the absence of significant canonical correlation between soil chemical variables and maize productivity components in the three study sites (Table 2), indicating that the variables from the two groups are independent. This independence may be attributed to the fact that both sites are areas with a consolidated no-till system, with the same preceding crop and similar nutritional managements, based on technical recommendations specific for each site, aiming for an expected grain production equivalent. Additionally, within each site, meteorological conditions were uniform among the experimental units, influencing the productivity components of maize in each plot equally.
The analysis of canonical correlations between soil biological variables and maize productivity components was significant only in the rainfed environments (FW and SM). This result emphasizes the direct relationship between the activity of soil organisms and the development of maize plants in these
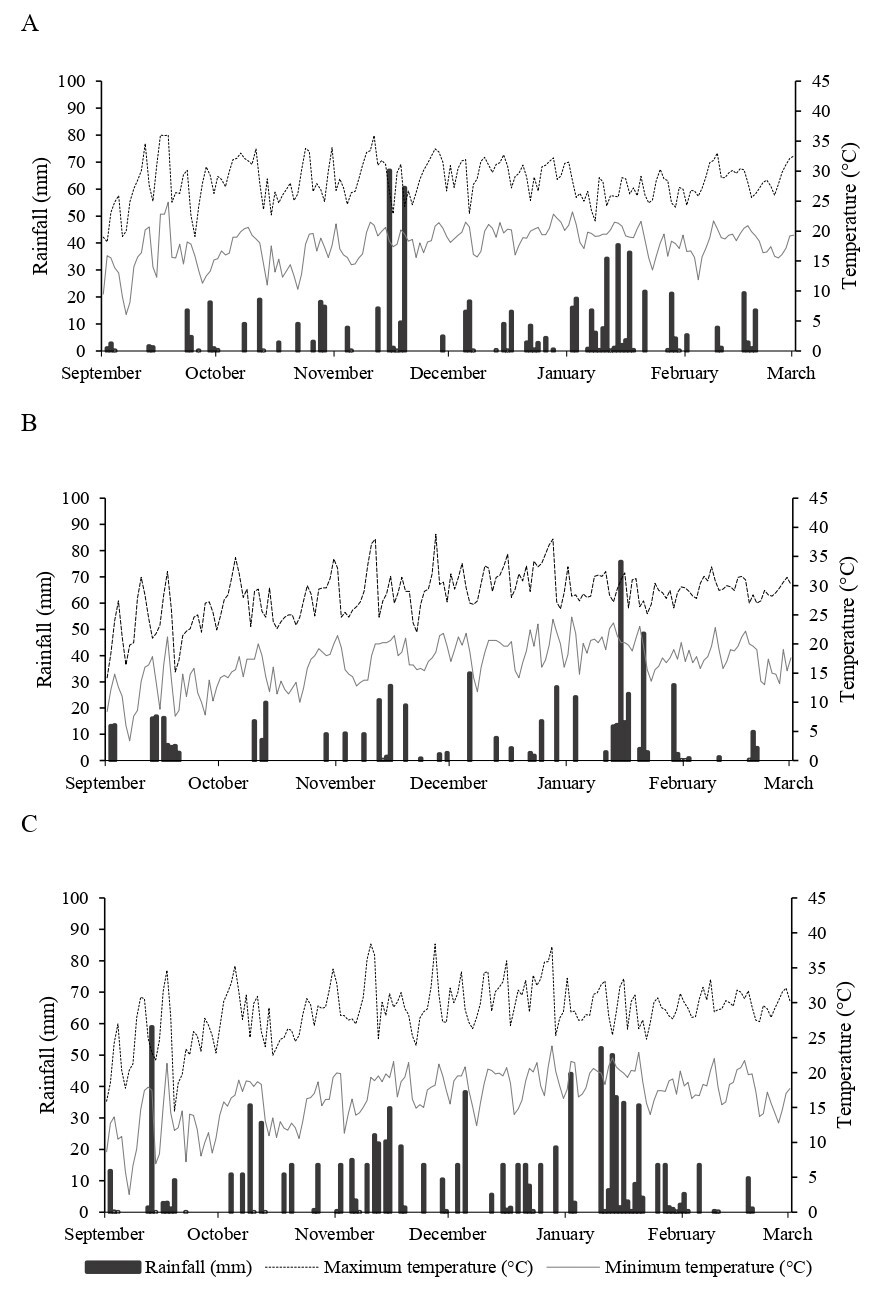
Fig 2. Rainfall (columns), maximum and minimum temperature (rows) during the experiments in Frederico Westphalen (A), Santa Maria (B) and São Vicente do Sul (C), in the state of Rio Grande do Sul from September 2020 to March 2021
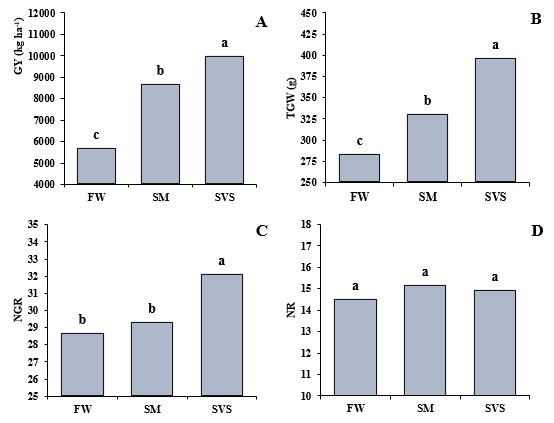
Fig 3. Mean values for: GY, grain yield (A), TGW, thousand-grain weight (B), NGR, number of grains per row (C), and NR, number of rows per cob (D) in FW: Frederico Westphalen, SM: Santa Maria, and SVS: São Vicente do Sul, in the state of Rio Grande do Sul, southern Brazil. Columns with the same letter did not differ statistically at 5% probability of error using Tukey’s test.
environments. In FW, the decomposition rate showed a direct correlation with NGR, TGW, and GY. On the other hand, in SM, both the decomposition rate and BSR correlated with NGR and GY. This suggests that microbial activity and decomposition are crucial for nutrient availability and the development of maize plants in these rainfed environments. In both environments, NR was found to be independent of the other studied variables. This suggests that the NR characteristic is predominantly influenced by other factors, such as the genetic factors of maize hybrids (Liu et al., 2015).
Furthermore, among the maize yield components, NGR has a direct correlation with GY in both environments, and with TGW in SM. The mean values and differences between the means of the maize productivity components in the three environments can be viewed in Fig 3. For the NR component, there was no statistical difference among the three environments, highlighting that this variable is strongly determined by the genetic characteristics of the maize hybrids (Liu et al., 2015). The irrigated environment, SVS, showed the highest values for NGR, TGW and, consequently, GY, exceeding 10,000 kg ha-1. This is due to the better water distribution provided by irrigation, which avoided periods of water deficit throughout the crop cycle, as this is one of the main limiting factors for crop yield (Grassini et al., 2009). On the contrary, the FW environment, with a GY below 6,000 kg ha-1, faced periods of water deficit at critical moments of crop development, such as the flowering and grain filling stages, stages of high water demand by the plant, compromising the formation and filling of the grains and, therefore, significantly reducing yield (Bergamaschi and Matzenauer, 2014).
In this study, we observed a strong correlation between OM content and soil microbial activity, suggesting that an increase in OM can stimulate greater activity of soil organisms, resulting in more efficient decomposition of plant residues. Higher decomposition rates are directly associated with better maize productivity, due to the symbiosis between plants and soil microorganisms and efficient nutrient cycling, thus reflected in higher NGR, TGW, and GY.
Sustainable management practices that increase soil OM content can play a fundamental role in boosting the GY of maize. Soil chemical correction, including liming to neutralize toxic aluminum, along with crop rotation, the cultivation of cover crops, and reducing soil tillage, are key strategies to increase OM content and promote an active soil biota, essential for efficient nutrient cycling (Schmidt et al., 2019; Lal, 2020). Moreover, adjusting the sowing time, taking into account the cycle length of the hybrids, is extremely important to avoid potential productivity losses due to periods of water deficit at critical moments of the crop. In irrigated areas, where water availability is not a major problem, microbial activity is strongly impacted by soil fertility. Therefore, the importance of performing soil correction and balanced fertilization is emphasized. Even in rainfed areas, where water availability issues exist, we observed a strong correlation between microbial activity and agronomic performance of maize (Lal, 2020).
It is important to emphasize the need to use a variety of soil biological parameters in assessing its quality, given the sensitivity of these parameters to environmental variations (Fierer, 2017). Investing in conservation practices and closely monitoring soil health are essential steps to ensure sustainable agricultural productivity and the preservation of natural resources in the long term (Six and Paustian, 2014).
Materials and Methods
Experiment site
The study was conducted at three sites in Rio Grande do Sul State (southern Brazil): Frederico Westphalen (FW; 27°23’50”S, 53°25’35”W), Santa Maria (SM; 29°43’27”S, 53°43’39”W), and São Vicente do Sul (SVS; 29°42’27”S, 54°41’28”W) (Fig 1). The climate at all three sites is classified as humid subtropical with hot summers (Cfa) according to the Köppen classification (Alvares et al., 2013). Meteorological data for each site were obtained from automatic weather stations located up to 600 m from the experimental areas (Fig 2). The soils at FW are classified as typical Oxisol (or dystrophic Red Latosol in the Brazilian Soil Classification System), while the soils at SM and SVS are classified as Ultisol (or dystrophic Red Argisol).
Experiment characterization
During the 2020/2021 growing season, the experiment was conducted in two sowing seasons: the second half of September (season 1) and the second half of October (season 2). The sowing dates were determined based on agricultural zoning for each location in areas suitable for maize cultivation. Six experiments were conducted (three locations multiplied by two sowing seasons). The experiments in FW and SM were carried out under rainfed conditions, while the experiment in SVS utilized center pivot irrigation. The decision to irrigate two experiments and to sow in two different segments aimed to increase the variability of the edaphoclimatic conditions. This allowed observation of the interaction between chemical and biological soil variables and maize plants under various moments and conditions.
The winter crop preceding the maize was a cover crop consortium of black oat (Avena strigosa L.) and forage turnips (Raphanus sativus L.). After the desiccation of the cover crops, fertilization was carried out using a seeder to achieve a target grain yield of 12 t ha-1, after which the maize was sown manually at a density of 70,000 plants ha-1. The experiments were set up in randomized blocks, with three representative maize hybrids commonly grown in the regions (AG 9025 PRO3, MG 300 PW, and DKB 230 PRO3), and each experiment was conducted in three replications. Each experimental unit consisted of six rows spaced 0.50 m apart and five meters long, resulting in a total area of 15 m2. Cultural treatments such as nitrogen fertilization and fungicide, herbicide, and insecticide applications followed the recommended practices for maize cultivation in Rio Grande do Sul State (Da Rosa et al., 2017).
Evaluations
Soil chemical variables
Soil chemical characterization was performed for each of the 54 experimental units. Samples were collected with an auger at a depth of 0–10 cm when the plants were in the vegetative phenological stages V4 to V6, the period that defines the productive potential of the crop. The evaluated attributes included clay content (g kg-1, densimeter), pH (in water 1:1), OM (g kg-1, Walkley-Black carbon), phosphorus (P; mg dm-3, Mehlich-1), potassium (K; mg dm-3, Mehlich-1), calcium (Ca; cmol_c dm-3, KCl), magnesium (Mg; cmol_c dm-3, KCl), aluminum (Al; cmol_c dm-3, KCl), hydrogen + aluminum (H + Al; cmol_c dm-3, titration), effective cation exchange capacity (cmol_c dm-3), aluminum saturation (%), base saturation (%), and calcium-magnesium ratio (Ca:Mg).
Soil biological variables
To determine the decomposition rate of plant residue by soil organisms, we used the litter bag method, which was adapted from Bocock and Gilbert (1957). We created nylon bags measuring 20 × 15 cm with a mesh size of 0.5 × 0.5 cm. These bags allowed decomposer organisms from the soil fauna to enter. We filled the bags with green leaves from the middle third of maize plants, which had a dry mass of 20 g (obtained by drying the leaves in a forced air oven at 60 °C for 72 h). In each experimental unit, we evenly distributed four bags, placing them in direct contact with the soil between the phenological stages of VT and R1. After 45 days, we removed the bags and dried them to determine the remaining dry mass. We calculated the decomposition constant (k) using Equation 1 (Thomas and Asakawa, 1993):
\(X_{t}\ = \ X0.e^{- kt}\) (1)
where Xt is the dry mass of the remaining material after t days and X0 is the initial dry mass at t is 0. Basal soil respiration was determined according to Alef and Nannipieri (1995). Soil samples were collected from a 0-10 cm depth using an auger between the phenological stages of VT and R1 in the 54 experimental units. Samples were kept in a thermal box with ice (4–6 ºC) during collection and subsequently refrigerated. Gravimetric moisture content was determined by weighing the samples after drying at 105 ºC. Each sample was divided into three subsamples of 50 g of soil, which were incubated in respirometry jars for seven days (168 h), in the dark, at temperatures between 25 and 28 ºC, with 0.1 M NaOH to capture CO2. After incubation, titration with 0.1 M HCl was conducted to determine C-CO2 using Equation 2:
\(BSR\ (mg\ of\ C - {CO}_{2}\ {kg}^{- 1}soil\ {hour}^{- 1} = \lbrack(V_{b} - V_{a}).\ M.6.1000\rbrack/Ps/T\) (2)
Where BSR is carbon from basal soil respiration, Vb (mL) is the volume of hydrochloric acid spent on white titration (without soil), Va (mL) is the volume spent on titration of the sample with soil, M is the exact molarity of the HCl (determined with Tris), Ps (g) is the dry soil mass, and T is sample incubation time in hours.
Maize grain yield components
The maize grain yield components were determined in 432 plants. The mean of eight plants per plot represented the value for the experimental unit and was evaluated after the harvest: number of rows per cob (NR), number of grains per row (NGR), and mass of one thousand grains (TGW, in g). Grain yield (GY, in kg ha-1) was estimated over an area of 8 m² in each plot, consisting of the four central rows by four meters in length. The harvest was conducted when the grains reached 20% moisture content, which was then adjusted to 13%, and the grain yield was determined.
Statistical analyses
For the analysis of canonical correlations, each experimental unit represented a value. Descriptive statistics were performed by analyzing the assumptions for the execution of canonical correlations for the 19 variables evaluated. Three groups of variables were formed, and multicollinearity within each group was diagnosed (Group 1 with soil chemical elements variables; Group 2 with soil biological variables; and Group 3 with plant variables). The magnitude of multicollinearity within each group was verified by the condition number (CN) CN \(= \frac{\lambda max}{\lambda min}\), which represents the ratio between the largest and smallest eigenvalue of the correlation matrix and the VIF, where Rj2 is the coefficient of determination, classified according to the criteria of Montgomery and Peck (1982), described in Cruz et al. (2014).
The maximum correlation between linear combinations of characters of Groups 1 and 2 for the canonical correlation analyses was estimated, for example, being X1 e Y1 the linear combinations of the characters of Groups 1 and 2, respectively, having: \(X_{1} = a_{1}x_{1} + a_{2}x_{2} + ... + a_{p}x_{p}\) and \(Y_{1} = b_{1}y_{1} + b_{2}y_{2} + ... + b_{q}y_{q}\)in which: \(a' = \lbrack a_{1}a_{2}...a_{p}\rbrack\) = 1xp vector of weights of the character of Group 1 and \(b' = \lbrack b_{1}b_{2}...b_{q}\rbrack\) = 1xq vector of weights of the characters of Group 2. The first canonical correlation will be the one that maximizes the relationship between X1 and Y1. Functions X1 and Y1 form the first canonical pair associated with that canonical correlation expressed by: \(r_{1} = \frac{C\widehat{o}v(X_{1},Y_{1})}{\sqrt{\widehat{V}(X_{1}).\widehat{V}(Y_{1})}}\) , with \(C\widehat{o}v(X_{1},Y_{1}) = a'S_{12}b\), \(\widehat{V}(X_{1}) = a'S_{11}a\), and \(\widehat{V}(Y_{1}) = b'S_{22}b\) where: S11= pxq matrix of covariances between the characters of Group 1; S22= qxq matrix of covariances between the characters of Group 2; S12 = pxq matrix of covariances between the characters of Group 1 and 2 (Cruz et al., 2014). The canonical correlations between the groups of characters were presented through cross-canonical charges. The significance of canonical correlations was tested using the chi-square test, at a 5% error probability. For the comparison of means among the three sites, the sowing season was disregarded. The Tukey mean comparison test was performed at a 5% error probability. The statistical analyses were conducted using the R statistical software and Microsoft Excel.
Conclusions
Based on the canonical correlation analysis, in maize cultivation within a subtropical environment in Brazil, we found that for sites with irrigation, there exists a linear dependence between the biological and chemical indicators of the soil. The levels of organic matter and phosphorus in the soil directly influence the activity of soil microorganisms, which play a crucial role in the decomposition of plant residues and, consequently, in nutrient cycling. In the case of maize cultivation without irrigation, a linear dependence was found between the rate of decomposition of plant residues and key productivity indicators, such as the number of grains per row, the mass of a thousand grains, and overall maize grain yield. This highlights the significant relationship between the evaluated variables and meteorological conditions. Lastly, our findings demonstrated that maize grain yield components do not correlate with soil chemical indicators when soil nutrient levels are deemed adequate and there is no or reduced toxicity caused by chemical elements.
Acknowledgments
The authors would like to thank the Federal University of Santa Maria for their technical support during the research.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. London: Academic Press, 215-216.
Althaus D, Gianello C, Tedesco MJ, Da Silva KJ, Bissani CA, Felisberto R (2018) Natural Fertility and Metals Contents in Soils of Rio Grande do Sul (Brazil). Revista Brasileira de Ciência do Solo, 42, 1-15.
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Koppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22(6), 711–728.
Bergamaschi H, Matzenauer R (2014) Corn and the climate. Porto Alegre, Brazil: Emater/RS-Ascar, 85p
Bocock KL, Gilbert OJW (1957) The disappearance of leaf litter under different woodland conditions. Plant and Soil, 9, 179-185.
Bünemann EK, Bongiorno G, Bai Z, Creamer RE, De Deyn G, de Goede R, Fleskens L, Geissen V, Kuyper TW, Mäder P, Pulleman M, Sukkel W, Groenigen JWV, Brussaard L (2018) Soil quality – A critical review. Soil Biology and Biochemistry, 120, 105-125–125.
Cai A, Liang G, Yang W, Zhu J, Han T, Zhang W, Xu M (2021) Patterns and driving factors of litter decomposition across Chinese terrestrial ecosystems. Journal of Cleaner Production, 278, 123964.
Cardoso EJBN, Vasconcellos RLF, Bini D, Miyauchi MYH, Santos CA, Alves PRL, Paula AM, Nakatani AS, Pereira JM, Nogueira MA (2013) Soil health: looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Scientia Agricola, 70, 274-289.
Cohen J (1977) Statistical Power Analysis for the Behavioral Sciences. Academic Press, 109-143.
CQFS - Comissão de Química and Fertilidade do Solo - RS/SC (2016) Manual de adubação and calagem para os Estados do Rio Grande do Sul and Santa Catarina. Viçosa: Sociedade Brasileira de Ciência do Solo.
Cruz CD, Carneiro PCS, Regazzi AJ (2014) Modelos biométricos aplicados ao melhoramento genético. Viçosa: Editora da UFV, 668.
Da Rosa APSA, Emygdio BM, Bispo NB (2017) Indicações técnicas para o cultivo de milho e de sorgo no Rio Grande do Sul safras 2017/2018 e 2018/2019. Instituto Federal Sul-Rio-Grandense, Sertão/RS. Embrapa, 210.
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nature Reviews Microbiology, 15(10), 579-590.
Grassini P, Yang H, Cassman KG (2009) Limits to maize productivity in Western Corn-Belt: A simulation analysis for fully irrigated and rainfed conditions. Agricultural and Forest Meteorology, 149(8), 1254-1265.
Huang F, Ding X, Li W, Jia H, Wei X, Zhao X (2021) The effect of temperature on the decomposition of different parts of maize residues in a solonchak. Catena, 201, 105207.
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019) pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biology and Biochemistry, 138, 07584.
Lal R (2020) Soil organic matter and water retention. Agronomy Journal, 112(4), 3152-3167.
Li Y, Cui S, Chang SX, Zhang Q (2019) Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: a global meta-analysis. Journal of Soils and Sediments, 19, 1393–1406.
Liu L, Du Y, Huo D, Wang M, Shen X, Yue B, Qiu F, Zheng Y, Yan J, Zhang Z (2015) Genetic architecture of maize kernel row number and whole genome prediction. Theor Appl Genet, 128, 2243–2254.
Liu P, Huang J, Han X, Sun OJ, Zhou Z (2006) Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Applied Soil Ecology, 34, 266-275.
Lopes LD, Junior RCF, Pacheco EP, Fernandes MF (2021) Shifts in microbial and physicochemical parameters associated with increasing soil quality in a tropical Ultisol under high seasonal variation. Soil and Tillage Research, 206, 104819.
Mingoti SA (2005) Análise de dados através de métodos de estatística multivariada: uma abordagem aplicada. Belo Horizonte, Brazil: Editora da UFMG, 297.
Mitra E, Mohammad RS, Sinegani AAS, Ahmadi A, Keesstra S (2019) Estimating the soil respiration under different land uses using artificial neural network and linear regression models. Catena, 174, 371-382.
Moitinho MR, Padovan MP, Panosso AR, Teixeira DB, Ferraudo AS, La Scala Jr N (2015) On the spatial and temporal dependence of CO emission on soil properties in sugarcane (Saccharum spp.) production. Soil and Tillage Research, 148, 127-132.
Montgomery DC, Peck EA (1982) Introduction to linear regression analysis. New York: John Wiley & Sons, 504.
Panico SC, Esposito F, Memoli V, Vitale L, Polimeno F, Magliulo V, Maisto G, De Marco A (2020) Variations of agricultural soil quality during the growth stages of sorghum and sunflower. Applied Soil Ecology, 152, 103569.
Paz-Ferreiro J, Fu S (2013) Biological indices for soil quality evaluation: perspectives and limitations. Land Degradation & Development, 27, 14-25.
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Sarkar B, Singh M, Mandal S, Churchman GJ, Bolan NS (2018) Clay Minerals—Organic Matter Interactions in Relation to Carbon Stabilization in Soils. The Future of Soil Carbon, 1, 71-86.
Schmidt R, Gravuer K, Bossange AV, Mitchell J, Scow K (2019) Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLOS ONE, 14(7), e0218408.
Six J, Paustian K (2014) Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biology and Biochemistry, 68, 4-9.
Spohn M, Schleuss PM (2019) Addition of inorganic phosphorus to soil leads to desorption of organic compounds and thus to increased soil respiration. Soil Biology and Biochemistry, 130, 220-226.
Thomas RJ, Asakawa NM (1993) Decomposition of leaf litter from tropical forage grasses and legumes. Soil Biology and Biochemistry, 25, 1351-1361.
Vain AC, Rakotondrazafy N, Razanamalala K, Trap J, Marsden C, Blanchart E, Bernard L (2021) The fate of primed soil carbon between biomass immobilization and respiration is controlled by nutrient availability. European Journal of Soil Biology, 105, 103332.
Vidal A, Klöffel T, Guigue J, Angst G, Steffens M, Hoeschen C, Mueller CW (2021) Visualizing the transfer of organic matter from decaying plant residues to soil mineral surfaces controlled by microorganisms. Soil Biology and Biochemistry, 160, 108347.
Zhang X, Zhu A, Xin X, Yang W, Zhang J, Ding S (2018) Tillage and residue management for long-term wheat-maize cropping in the North China Plain: I. Crop yield and integrated soil fertility index. Field Crops Research, 221, 157-165.
Zhao S, Ciampitti IA, Qiu S, Xu X, He P (2021) Characteristics of maize residue decomposition and succession in the bacterial community during decomposition in Northeast China. Journal of Integrative Agriculture, 20, 3289-3298.