

Aust J Crop Sci. 18(11):707-714 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.11.p3697
Antimicrobial, acetylcholinesterase and antioxidant activities of essential oils from Allium sativum, Coriandrum sativum and Anethum graveolens
Ianca Carneiro Ferreira1, Luís Roberto Batista1, Alex Rodrigues Silva Caetano2, Gabriela Aguiar Campolina1, Vanuzia Rodrigues Fernandes Ferreira2, Suzana Reis Evangelista1, Cassia Duarte Oliveira1, Carolina Salles Freire2, Marcus Vinicius Prado Alves2, David Lee Nelson3, Maria das Graças Cardoso2*
1Food Sciences Department, Federal University of Lavras (UFLA), Lavras, MG, Brazil
2Chemistry Department, Federal University of Lavras (UFLA), Lavras, MG, Brazil
3Postgraduate Program in Biofuels, Federal University of The Jequitinhonha and Mucuri Valleys (UFVJM), Diamantina, MG, Brazil
*Corresponding author: Maria das Graças Cardoso 
Abstract: Essential oils have received attention because they contain a variety of terpene and phenylpropanoid compounds that are responsible for their biological activities. Some plants, such as spices, are rich in these substances, in addition to being widely used in gastronomy. Therefore, the chemical composition and the antioxidant, anticholinesterase and biological activities of the essential oils from Allium sativum, Coriandrum sativum and Anethum graveolens against the bacteria Escherichia coli and Staphylococcus aureus and the fungi Aspergillus carbonarius and Aspergillus ochraceus were evaluated. The essential oils were extracted using the hydrodistillation technique and characterized by GC-MS and GC-FID. The principal constituents found were linalool, carvone and diallyl trisulfide in the essential oils from C. sativum, A. graveolens and A. sativum, respectively. C. sativum and A. graveolens were observed to be the most efficient in controlling bacterial growth, and the growth of both fungi was completely inhibited by the essential oil from A. sativum at all the concentrations tested. For C. sativum and A. graveolens, a dose-dependent relationship with the concentrations was observed for the antifungal activity. A decrease in acetylcholinesterase activity in the presence of the A. sativum oil was observed, and the IC50 was 9.67 µg mL-1. Satisfactory results in the antioxidant assay using thiobarbituric acid and in the reduction of the phosphomolybdenum complex were only observed with the oil from C. sativum. A. sativum was found to be the most promising species for the development of sanitizers, drugs and agrochemicals.
Keywords: Acetylcholinesterase activity; antibacterial; antifungal; antioxidant; microorganisms.
Abbreviations: AChE_acetylcholinesterase; ATP_adenosine triphosphate; BHT_butylated hydroxytoluene; CFU_colony forming unit.
Introduction
Interest in natural sources as alternatives to synthetic chemical products has increased significantly, especially with respect to the essential oils (EOs). The constituents present in EOs include mainly the terpene and phenylpropanoid classes, whose bioactivity depends on the structural configurations of the molecules (Asbahani et al. 2015).
Since ancient times, garlic (Allium sativum) has been used in gastronomy and traditional medicine (Rouf et al. 2020). Its biological properties are mainly attributed to organosulfur compounds that belong to the thiosulfinate class. However, because of their high instabilities, new compounds rearrange to give rise to a wide variety of sulfur-derived substances, diallyl sulfide, diallyl disulfide, and diallyl tetrasulfide (Tsai et al. 2013; Llana-Ruiz-Cabello et al. 2015). The antimicrobial and antioxidant activities of garlic EO has been reported by some authors (Teixeira et al. 2014; Garcia-Diez et al. 2016).
In addition to garlic, two spices from the Apiaceae family are known for their biological properties, namely coriander (Coriandrum sativum) and dill (Anethum graveolens). Linalool is the main constituent of the EO extracted from the coriander seeds, and it is responsible for the biological effects (Ilc et al. 2016). Duarte et al. (2016) found that coriander EO and linalool were active against Campylobacter bacteria and also interfered with quorum sensing and biofilm formation. Furthermore, it was proven by Das et al. (2019) that the nanoencapsulated EO possessed antifungal, antiaflatoxigenic and antioxidant activity, as well as being able to inhibit an afflatoxin precursor. The main constituents of dill EO reported in the literature are carvone, limonene, apiole, thymol and α-pinene (Kazemi et al. 2015; Karimi et al. 2016).
The application of EOs, which are generally considered safe, is an alternative for the development of food additives and agrochemicals because some of those products contain compounds that are toxic for humans, animals and the environment. The use of EOs also reduces the risks of selecting insects and microorganisms resistant to synthetic products. Therefore, the application of EOs can lead to a more sustainable agricultural practice as well as guaranteeing food security (Ribeiro et al. 2015; Nguyen and Jang 2021; Teneva et al. 2021). Therefore, the EOs from A. sativum, C. sativum and A. graveolens were characterized, and their antimicrobial activities against pathogenic bacteria and mycotoxigenic fungi, their antioxidant capacities and their effects on acetylcholinesterase activity were determined.
Results and discussion
Chemical characterization of the essential oils
The results obtained from the chemical characterization of the EOs from coriander (CEO), dill (EEO) and garlic (AEO) are shown in Table 1. The principal constituent identified in the CEO was linalool (93.277%). In the EEO, five constituents were identified. Carvone was present in the highest concentration (83.038%). In the case of AEO, five major constituents were also quantified, with diallyl trisulfide (75.126%) being the principal constituent.
Investigations by El-sayed et al. (2017) indicated that diallyl trisulfide was the principal constituent of the AEO obtained from different cultivars, ranging from 45.76 to 58.53%. Esmaeili (2020), found diallyl trisulfide (33.47%) to be the principal compound in the AEO, followed by diallyl tetrasulfide (19.77%). The results obtained in this study are in agreement with those of the aforementioned authors with respect to chemical composition; however, the concentrations differ from those reported in the literature.
In the present study, five compounds were identified in CEO, namely linalool (93.28%), camphor (2.93%), γ-terpinene (2.22%), α-tujene (1.00%) and ρ-cymene (0.57%). The first three compounds were present in the studies of other authors, and, although linalool was the principal constituent, the concentration was different (Bazargani and Rohloff 2016; Lasram et al. 2019; Micić et al. 2019). Weisany et al. (2019) identified fifteen constituents in the EO from dill seeds. Carvone was the principal (87.91%) constituent, followed by limonene (3.13%). These compounds are similar to those found in the present study, but with different concentrations (83.04 and 12.63%, respectively). The differences in these results can be explained by the differences in geographic and climatic conditions of the growing region. The interaction between the plant and the environment affects the synthesis of secondary metabolites because environmental factors influence the metabolic route and, consequently, the production of different phytochemicals (Gobbo-Neto and Lopes 2007).
Antimicrobial activity
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) by the disk diffusion method
Significant antibacterial activities were observed for the CEO and EEO, whose MIC and MBC values ranged from 6.25 to 12.50 µL mL-1, respectively (Table 2). For the AEO, the results ranged from 25.00 to 50.00 µL mL-1 (E. coli) and from 12.50 to 25 µL mL-1 (S. aureus). The MIC and MBC values for AEO were higher for E. coli bacteria. This observation can be explained because Gram-negative bacteria are naturally more resistant to the action of EOs because of the presence of an outer membrane in the cell wall, unlike Gram-positive bacteria, which have a single thick layer of peptidoglycan (Nazzaro et al. 2013). EOs are lipophilic substances and, therefore, have the ability to interact with cell membrane lipids to increase the permeability of the bacterial cell. This effect causes a reduction in the Proton Motive Force, in the synthesis of ATP and in the release of intracellular constituents (Bhavaniramya et al. 2019). Rao, Chen and Mcclements (2019) mention that the antimicrobial activity of an EO is related to its chemical constituents, mainly those present in highest concentration, and interactions with other components. Terpenoids with polar functional groups are known to have such activity. Bhavaniramya et al. (2019) mentioned that carvone is capable of disrupting the structures of the cell's outer membranes. Park et al. (2012) inferred that linalool causes damage to the cell wall, inhibits enzymatic activity and interrupts the translation of certain regulatory genes. In the case of sulfur compounds, Rouf et al. (2020) mentioned that there is an interaction of thiol groups with pathogen proteins, which causes structural change.
No formation of an inhibition halo was observed for the E. coli bacteria in the presence of the three EOs tested (Table 2). On the contrary, the AEO completely inhibited the growth of S.aureus. The diameters of the inhibition halos for the CEO and EEO were 8.1±1.78 mm and 2.0±1.0 mm, respectively. For Rao, Chen and Mcclements (2019), the antimicrobial activity is classified according to the zone of inhibition, which can be defined as strong (≥20 mm), moderate (>12 mm <20 mm) and weak (<12 mm). Thus, weak inhibitory activity was observed for the CEO and EEO. It is important to emphasize that no inhibition was observed for the EOs diluted in Tween 80.
Inhibitory effect of EOs on mycelial growth of mycotoxigenic fungi
Mycelial growth of mycotoxigenic Aspergillus fungi was inhibited by CEO and EEO in a dose-dependent manner (Table 3). The growth of the fungi A. carbonarius and A. ochraceus was completely inhibited by the AEO at all the tested concentrations. This EO was considered to be the most effective in the control of the two fungi. It was found that A. ochraceus was the fungus most resistant to CEO and EEO (Figure 1). There was no inhibition of fungal growth by the CEO at 1000 µL mL-1, and a statistical difference was observed at higher concentrations. Nevertheless, the inhibition of growth of A. carbonarius was greater than that of A. ochraceus. Greater inhibition by EEO than by CEO was observed for both fungi. The inhibition of growth of A. carbonarius was observed up to a concentration of 500 µL mL-1, whereas a 75.79% decrease in growth rate at the same concentration was observed for A. ochraceus (Table 3). The activity of coriander essential oil against fungal species Aspergillus genus were reported by Das et al. (2019), but no tests were performed with A. carbonarius and A. ochraceus.
The resistance of A. ochraceus was greater than that of A. carbonarius because of the difference in the chemical constituents of the EOs. Lasram et al. (2019) showed that carvone had greater antifungal activity than linalool; this fact explains why EEO was more effective in controlling fungal growth than CEO.
As mentioned, the antimicrobial activity of EOs can be attributed to their fat-soluble character. It is suggested that the antifungal effect of EOs is based on membrane rupture by inhibiting ergosterol biosynthesis, which causes the leakage of cytoplasmic content and generates an internal cellular imbalance with a change in the functioning of the organelles (Das et al. 2019; Kujur, Kumar and Prakash 2021). In the study by Brandão et al. (2020), the authors confirmed the antifungal and antimycotoxigenic effect of the essential oil from Eremanthus erythropappus against three different Aspergillus species, including A. carbonarius and A. ochraceus. They demonstrated that the inclusion of the EO inhibited ergosterol biosynthesis and damaged the integrity of the fungal cell membrane.
Effect of EOs on acetylcholinesterase enzyme activity
The results obtained regarding the effect of the EOs on the enzymatic activity are shown in Figure 2. No significant decrease in acetylcholinesterase (AChE) activity was observed with the application of the CEO and EEO, even at the highest concentrations utilized (IC50>100 µg mL-1). There are differences in the chemical structures of the constituents of EOs that could influence the interactions and enzyme inhibition. Barbosa et al. (2020), reported that EOs composed mainly of sesquiterpenes have a greater inhibitory effect
Table 1. Chemical compositions of EOs from C. sativum, A. graveolens and A. sativum by GC-MS
| Coriandrum sativum | ||||
|---|---|---|---|---|
| Constituents | RT (min) | RI tab | RI cal | N. Area (%) |
| Linalool | 12.246 | 1095 | 1100 | 93.277 |
| Camphor | 14.267 | 1141 | 1147 | 2.931 |
| γ- Terpinene | 10.600 | 1054 | 1057 | 2.218 |
| α-Tujene | 6.457 | 924 | 933 | 1.001 |
| p-Cymene | 9.335 | 1020 | 1023 | 0.573 |
| Total | 100.000 | |||
| Anethum graveolens | ||||
| Constituents | RT (min) | RI tab | RI cal | N. Area (%) |
| Carvone | 18.451 | 1239 | 1243 | 83.038 |
| Limonene | 9.526 | 1024 | 1028 | 12.626 |
| Apiole (NI) | 33.918 | 1677 | 1616 | 4.337* |
| Total | 100.000 | |||
| Allium sativum | ||||
| Constituents | RT (min) | RI tab | RI cal | N. Area (%) |
| Diallyl. trisulfide | 20.947 | - | 1300 | 75.126 |
| Diallyl. tetrasulfide | 11.431 | - | 1078 | 19.506 |
| Allyl methyl trisulfide | 13.872 | - | 1138 | 5.299 |
| Diallyl sulphide | 4.647 | - | 856 | 0.034 |
| Methyl 2-propenyl Disulfide | 5.971 | - | 916 | 0.019 |
| Total | 99.984 | |||
RT: Retention time; RItab: Literature retention index; RIcal: Calculated retention index; N. Area: Normalization of the area; *Confirmed by the Kovats index; -: Quantified but not identified.
Table 2. Minimum Inhibitory Concentration (µL mL-1) and Mínimum Bactericidal Concentration (µL mL-1) of the essential oils from C. sativum, A. graveolens and A. sativum against the E. coli and S. aureus bacteria.
| Bacteria | C. sativum | A. graveolens | A. sativum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difusion in disk (mm) | MIC | MBC | Difusion in disk (mm) | MIC | MBC | Difusion in disk (mm) | MIC | MBC | |
| E. coli | NI | 6.25 | 12.50 | NI | 6.25 | 12.50 | NI | 25.00 | 50.00 |
| S. aureus | 8.1 ± 1.78 | 6.25 | 12.50 | 2.0 ± 1.0 | 6.25 | 12.50 | ** | 12.50 | 25.00 |
NI (No inhibition); ** Inhibition
Table 3. Percent inhibition of the mycelial growth of Aspergillus carbonarius e Aspergillus ochraceus by the EOs from C. sativum, A. graveolens and A. sativum
Concentration (µL mL-1) |
Percent of inhibition of mycelial growth | |||
|---|---|---|---|---|
| C. sativum | A. graveolens | A. sativum | ||
| Aspergillus carbonarius | 3000 | 100.00 Aa | 100.00 Aa | 100.00 Aa |
| 2000 | 100.00 Aa | 100.00 Aa | 100.00 Aa | |
| 1000 | 76.90 Bb | 100.00 Aa | 100.00 Aa | |
| 500 | 25.30 Bc | 100.00 Aa | 100.00 Aa | |
| 250 | 9.63 Cd | 79.51 Bb | 100.00 Aa | |
| Aspergillus ochraceus | 3000 | 100.00 Aa | 100.00 Aa | 100.00 Aa |
| 2000 | 28.92 Bb | 100.00 Aa | 100.00 Aa | |
| 1000 | NI Cc | 73.49 Bb | 100.00 Aa | |
| 500 | NI Cc | 75.79 Bb | 100.00 Aa | |
| 250 | NI Cc | 38.98 Cb | 100.00 Aa | |
Means followed by the same lower case (column) and uppercase (row) letters do not differ by the Tukey test (5% probability). NI: No inhibition
than those composed of monoterpenes. This fact can be explained by the synergistic interactions that occur in these compounds. High concentrations of linalool and carvone are necessary for strong inhibition of AChE to occur, and greater inhibition by carvone is observed than by linalool because of its conjugated double bond (Lopez and Pascual-Villalobos 2010).
Barbosa et al. (2020) inferred that the main constituents of EOs interact with AChE through Van der Waals forces and hydrophobic interactions. Such binding was considered exergonic, reversible and competitive, and because of these characteristics; the action of other substances is possible. The inhibitory activity itself occurs through the bonds between the compounds of the EOs with the enzyme's amino acids. In the case of those EOs that contain organosulfur compounds, such as AEO, the interaction can occur between sulfur and
oxygen atoms, forming π-sulfur interactions and hydrogen bonds, respectively (Zilbeyaz, Oztekin and Kutluana 2021).
AEO was efficient in decreasing AChE activity. The IC50 (9.67 µg mL-1) did not differ statistically from that of the carvacrol standard (IC50 12.53 µg mL-1). Rocchetti et al. (2022) reported that extracts obtained from different species of the genus Allium have the ability to inhibit AChE activity. In cases of poisoning caused by agrochemicals or toxic metals, activation of the AChE enzyme is necessary. Previous studies by Pari and Murugavel (2007) obtained promising results in the activation of AChE by diallyl tetrasulfide. The authors suggested that this compound was able to reduce the oxidative stress induced by cadmium. There must be a balance between enzyme and neurotransmitter because AChE is a key enzyme in behavioral processes.
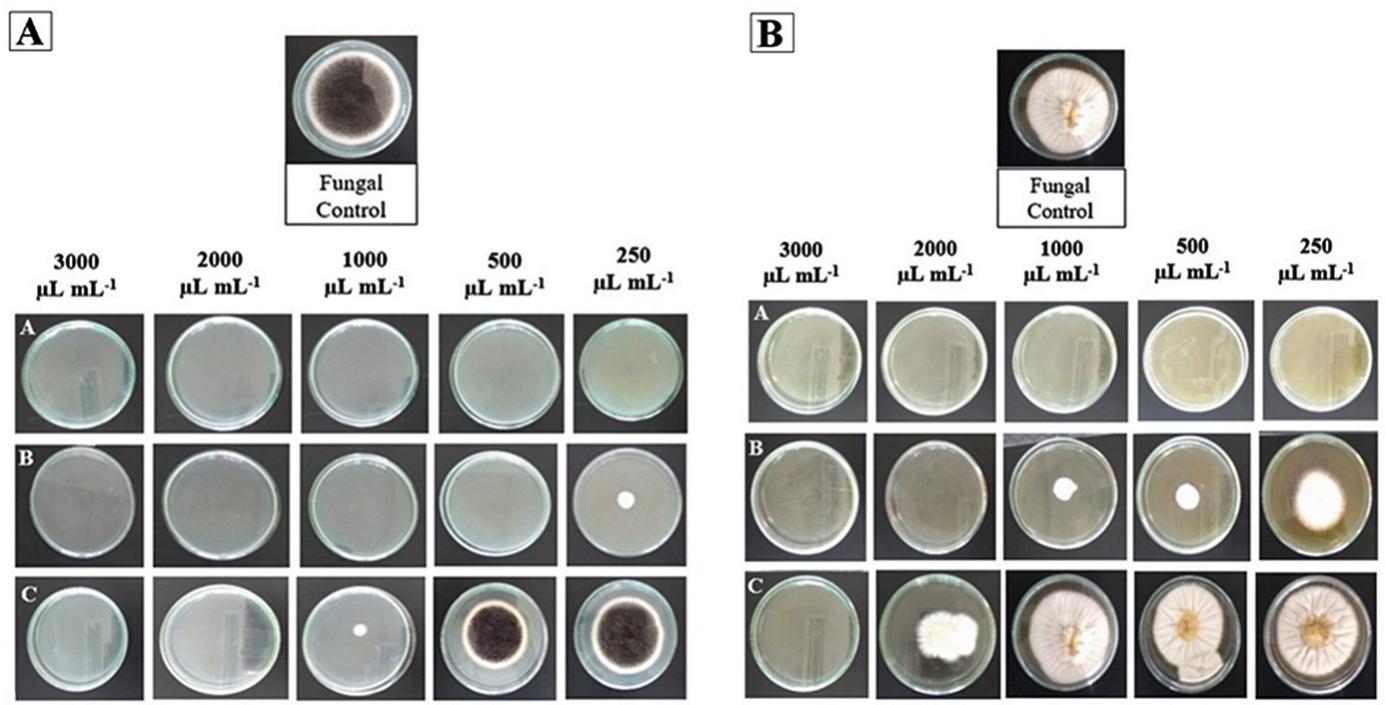
Figure 1. Total inhibition of the mycelial growth of Aspergillus carbonarius (A) and Aspergillus ochraceus (B) by the EO from Allium sativum (A) and parcial inhibition by EOs from Anethum graveolens (B) and Coriandrum sativum (C).
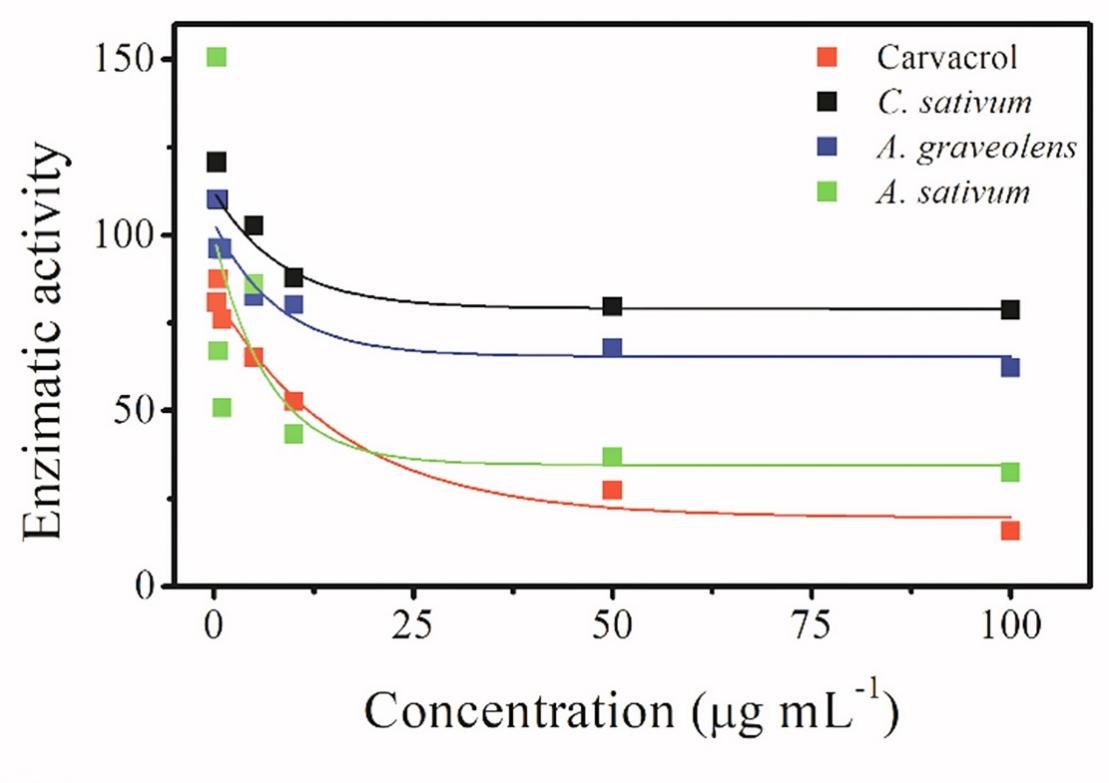
Figure 2. Effect EOs from Allium sativum, Coriandrum sativum and Anethum graveolens concentration on acetylcholinesterase enzyme activity
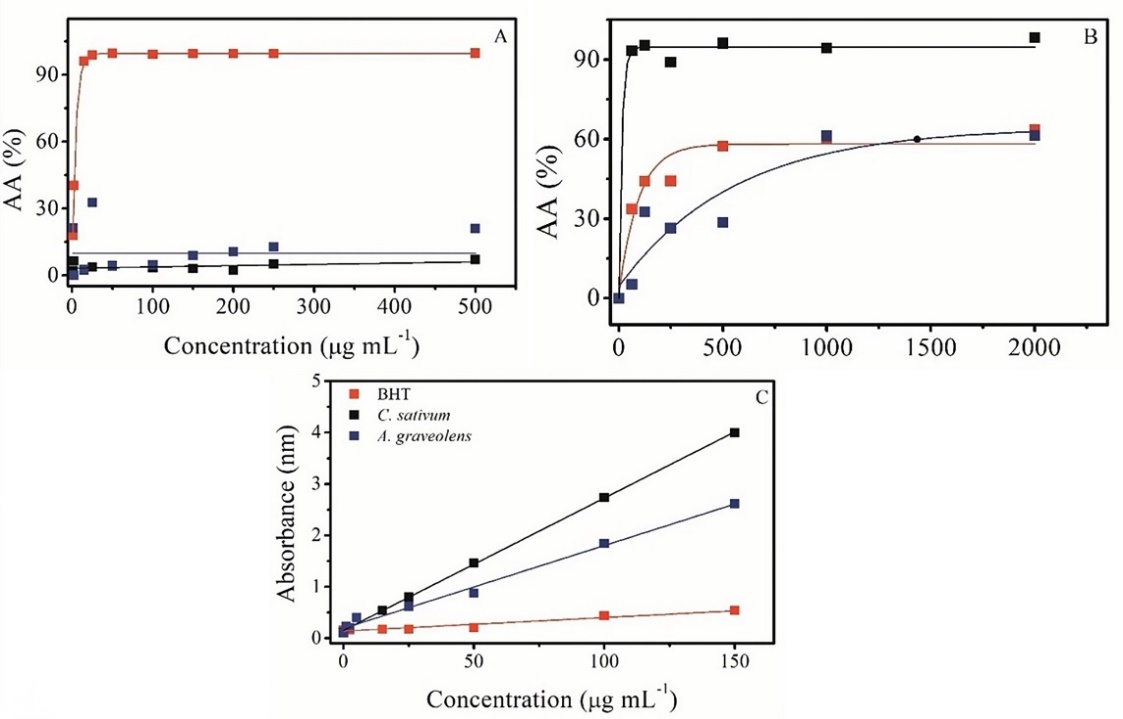
Figure 3. Antioxidant capacity of EOs from Coriandrum sativum and Anethum graveolens in three colorimetric assays. A: ABTS (µg mL-1), no activity was observed for EOs; B: TBARS (µg mL-1) and C: Phosphomolybdenum (Abs nm), activity was observed for both EOs;
In vitro antioxidant activity of EOs (AOX)
No AOX was observed in the colorimetric assays with AEO. Garlic is considered to be a potent antioxidant, but its action is indirect. The constituents of garlic are responsible for activating factor 2, which is related to the activation of antioxidant and detoxifying enzymes, such as glutathione, superoxide dismutase, catalase, glutathione peroxidase and heme 1-oxygenase. Therefore, the levels of mitochondrial damage caused by reactive oxygen species decreased (Ribeiro et al. 2021). In the case of allicin, which is a reactive sulfur-containing substance, the redox reaction occurs through disulfide bonds between the thiol groups and glutathione or proteins that contain cysteine (Borlinghaus et al. 2014). Thus, no results were observed for the AEO in conventional assays because such compounds act on living systems by activating antioxidant enzymes. In the study by Mallet et al. (2013), the authors also found that the AEO did not affect the presence of AOX by the DPPH radical capture method.
A dose-dependent relationship was observed in the ABTS assay (Figure 3A) for the BHT standard; that is, the antioxidant activity was proportional to the sample concentration (IC50 3.30 µg mL-1). On the other hand, no activity was observed for EEO and CEO, whose IC50 were >500 and 7387 µg mL-1, respectively. This behavior was also verified in the DPPH assay, and it is in agreement with the study by Alves-Silva et al. (2013). The principal compounds in EOs that have high AOX values usually possess phenolic characteristics. These substances can stabilize free radicals through the transfer of hydrogen atoms or electrons (Ferreira et al. 2019). The chemical structure of carvone (principal constituent) does not allow it to react by such a mechanism. In addition to the radical-scavenging assays, no AOX was observed for EEO in the other tests, except for phosphomolybdenum.
An IC50 of 8.02 µg mL-1 for the inhibition of the formation of species reactive to thiobarbituric acid was observed with CEO (Figure 3B). This value was lower than that found for BHT, for which an IC50 of 229.31 µg mL-1 was observed. Lipid oxidation is a chain reaction in which free radicals interact with unsaturated fatty acids to form a multitude of compounds, including malonaldehyde, which is responsible for imparting strange flavors and odors to foods, in addition to being a marker of oxidative damage in physiological systems. Malonaldehyde reacts with thiobarbituric acid to form a pink colored complex, so it is possible to determine whether an antioxidant is able to protect lipids against oxidation in the TBARS test (Ghani et al. 2017). In the case of CEO, whose principal compound identified was linalool, this activity can be attributed to the chemical structure of the monoterpene, which has double bonds and reduced functional groups that are susceptible to oxidation (Noacco et al. 2018), unlike carvone, the principal constituent identified in the EEO. In the study of Devasagayam, Boloor and Ramasarma (2003), the authors inferred that functional groups such as ketones can interfere in the assay because they can react with thiobarbituric acid.
Another test used to assess the ability of EOs to inhibit lipid peroxidation was that of β-carotene/linoleic acid. The capacity of the EO to protect the β-carotene system was evaluated (Duarte et al. 2016). However, the results were not as significant as in the TBARS method, where an IC50 of 446.23 µg mL-1 was observed for the CEO. Regarding BHT, the IC50 values were 0.69 µg mL-1. In the liposome assay, no activity was observed for either EO, with IC50>2000 µg mL-1. An IC50 of 258.30 µg mL-1 was observed for the BHT standard. Liposomes are strongly affected by the incorporation of EOs, as was observed in the study by Allaw et al. (2021), and this incorporation can influence the test result.
Metal complexation is also one of the mechanisms by which oxidation can be controlled because the metal ions catalyze this reaction and also participate in the formation of reactive oxygen species. The phosphomolybdenum assay is often used to determine the total antioxidant activity by evaluating the chelating capacity of a substance (Pavlić et al. 2021). In this study, it was observed that CEO and EEO were efficient in reducing the phosphomolybdenum complex. The absorbance values were higher than those of the BHT standard. The greater the slope of the straight line, the greater the reducing effect of the molecule (Figure 3C). Anthocyanins and some constituents of EOs are considered to be potent antioxidants. They are able to donate electrons or hydrogen atoms to free radicals or transition metals because they are stabilized by resonance structures that provide a certain stability to the radical formed (Qian et al. 2017).
Thus, the EO constituents are responsible for conferring AOX. This effect was isolated, synergistic or antagonistic. The methods used have different mechanisms of action, so the activity of an EO might be observed in a certain method and not seen in other tests, as was the case in this study.
Materials and methods
Plant materials
The dried seeds of the Coriandrum sativum L. (coriander) and Anethum graveolens (dill) and the dehydrated bulbs of Allium sativum (garlic) were purchased from the local market in the city of Lavras, Minas Gerais, Brazil.
Extraction and chemical characterization of EOs
The extraction of EOs was performed by the hydrodistillation technique using a modified Clevenger apparatus, with an extraction period of two hours (Brasil, 2010). The hydrolate was centrifuged at 965.36g for fifteen minutes, separated with a Pasteur micropipette and placed in an amber glass flask under refrigeration at 4 ºC.
The identification of the constituents was performed by gas chromatography using a Shimadzu (Model QP 2010 Plus) chromatograph coupled to a mass spectrometer (GC-MS), and the quantitative analyses were performed using a Shimadzu gas chromatograph (Model GC–2010) equipped with a flame ionization detector (FID). The experimental conditions described by Ferreira et al. (2019) were followed. The constituents were identified by comparing the retention indexes with those in the literature (Adams 2007) using two NIST107 equipment libraries and the NIST21, by which the mass spectra obtained can be compared with those existing in the libraries.
Evaluation of antimicrobial activity
Two species of pathogenic bacteria Escherichia coli (ATCC–EPEP 055) and Staphylococcus aureus (ATCC–13565) and two species of mycotoxigenic fungi, Aspergillus carbonarius (CCDCA 10507) and Aspergillus ochraceus (CCDCA 10490), were used. The microbial species were acquired from the Microorganisms Culture Collection of the Laboratory of Mycotoxins and Food Mycology, Federal University of Lavras, Lavras, MG, Brazil.
Determination of Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and antimicrobial activity by disc diffusion
The CLSI (2015) broth microdilution technique was used to determine the Minimum Inhibitory Concentration (MIC). The concentrations of the essential oil were prepared using 0.5% Tween 80 (w/v). Dilutions were performed to yield concentrations of 100; 50; 25; 12.5; 6.25; 3.125; 1.565; 0.781 and 0.391 µl ml-1. The inoculum was diluted in sterile saline solution (0.9%) until a turbidity corresponding to 0.5 on the McFarland scale (1 to 2 x 108 CFU mL-1) was reached and standardized by spectrophotometry with absorbance values between 0.08 to 0.1. Subsequently, 10 µL of this inoculum was added to the wells of a microplate containing Muller-Hinton broth. For the negative control, the bacterial suspension was not added; a sterile control of the culture medium (without the bacteria and without the EO) was used. For the positive control, the suspension was inoculated into wells containing the culture medium and Tween 80 (0.5%), but without the EO. The microplates were sealed and incubated at 37 ºC for 24 hours. After this period, 20 μL of 0.01% (m/v) resazurin dye was added, and the mixture was observed for 2 hours to detect the change in color of the dye from blue to pink, which indicated that the bacterial metabolism was active.
In the determination of MBC, 10 μL aliquots were removed from each of the wells of the previous analysis and transferred to Petri dishes containing Muller-Hinton agar. Plates were incubated at 37 ºC for 24 hours.
The solid medium disk diffusion test was also performed according to the CLSI (2003) method. The pure EO and that diluted with Tween 80 at concentrations of 500, 250, 125, 62.5, 31.25, 15.62, 7.81 and 3.90 µL mL-1 were used. The plates were incubated in a BOD (Biochemical Oxygen Demand) at 37 ºC for 24 hours and measurements of the diameters of inhibition halos were performed. Analyses were performed in triplicate.
Effect of EOs on mycelial growth of mycotoxigenic fungi
The inhibitory effect of EOs on the mycelial growth of filamentous fungi was evaluated according to the method of Caetano et al. (2020). The concentrations of the essential oil used were 3000, 2000, 1000, 500 e 250 µL L-1. Plates containing only the culture medium and the fungus were also prepared. The plates were incubated at 25 ºC for seven days in a BOD. All the analyses were performed in triplicate.
In vitro antioxidant capacity
The in vitro antioxidant capacity (AOX) was evaluated by the capture of ABTS [2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)] and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals, as well as by the reduction of phosphomolybdenum and the inhibition of lipid peroxidation as measured by the thiobarbituric acid reactive species assays (TBARS), the β-carotene/linoleic acid system and liposomes utilizing the methods described in the literature (Prieto, Pineda and Aguilar, 1999; Kulisic et al. 2004; Dandlen et al. 2010; Teixeira et al. 2012; Boulanouar et al. 2013; Guerreiro et al. 2013). The EO was diluted in ethanol to yield the final concentrations of 500, 250, 200, 150, 100, 50, 25, 15, 2.5 and 1 µg mL-1. The concentrations 2000; 1000; 500; 250; 125; 62.5 and 31.25 µg mL-1 were utilized only for the liposome and TBARS tests. BHT was used as a standard for all the analyses. Absorbance measurements were performed on a UV/Vis spectrophotometer (Shimadzu UV-160 1 PC).
Effect of EOs on acetylcholinesterase enzyme activity
The effect of EOs on the activity of acetylcholinesterase was evaluated using the method of Ellman et al. (1961). The samples were diluted in ethanol at concentrations of 100; 50; 10; 5; 1.0; 0.50 and 0.25 µg mL-1, and carvacrol was used as a standard for comparison.
Statistical analysis
The effects of the EOs on fungal growth were expressed as percentage inhibition of the colony growth in each treatment relative to the control. The treatments were arranged in a 5x3 factorial scheme (Concentration x OE) and subjected to analysis of variance (one-way ANOVA). The means were compared by the Tukey's post hoc test with a significance level of 5% (p ≤ 0.05) using the Sisvar software version 5.6 (Ferreira, 2011).
Conclusion
The greatest activity in inhibiting the growth of the fungi Aspergillus carbonarius and Aspergillus ochraceus and in inhibiting the activity of the acetylcholinesterase enzyme was observed for the essential oil from Allium sativum. This oil is being considered as an agent in the formulations of sanitizers, drugs and agrochemicals. Antimicrobial effects were also observed for the essential oils from Coriandrum sativum and Anethum graveolens. A satisfactory result in the antioxidant test of reactive species with thiobarbituric acid was only obtained with C. sativum. Thus, in vivo studies must be performed to demonstrate such biological properties in food systems, as well as by using new technologies such as nanotechnology to preserve and release essential oils in a controlled manner.
Funding
This work was supported by the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG); the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance code 001.
Conflict of interest
The authors declare that no conflict of interests exists.
Acknowledgements
The authors thank funding organs for the scholarships and financial support and the Central of Analysis and Chemical Prospecting of the Federal University of Lavras for supplying the equipment for chromatographic analyzes.
References
Adams RP (2017) Identification of essential oil components by gas chromatography/mass spectrometry. 4th edn. Carol Stream: Allured Publishing Corp.
Allaw M, Manconi M, Caboni P, Bacchetta G, Escribano-Ferrer E, Peris JE, Manca ML (2021) Formulation of liposomes loading lentisk oil to ameliorate topical delivery, attenuate oxidative stress damage and improve cell migration in scratch assay. Biomed Pharmacother. (144):112351.
Alves-Silva JM, dos Santos SMD, Pintado ME, Pérez-Álvarez JA, Fernández-López J, Viuda-Martos M (2013) Chemical composition and in vitro antimicrobial, antifungal and antioxidant properties of essential oils obtained from some herbs widely used in Portugal. Food Control. 32(2):371-378.
Asbahani AE, Miladi K, Badri W, Sala M, Addi EA, Casabianca H, Renaud FNR; Elaissari A (2015) Essential oils: From extraction to encapsulation. Int J Pharm. 483:220-243.
Barbosa DCS, Holanda VN, de Assis CRD, de Oliveira Farias JCR,do Nascimento PH, da Silva WV, dos Santos MTC (2020). Chemical composition and acetylcholinesterase inhibitory potential, in silico, of Myrciaria floribunda (H. West ex Willd.) O. Berg fruit peel essential oil. Ind Crops Prod. 151:112372.
Bazargani MM, Rohloff J (2016) Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food control. 61:156-164.
Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D (2019) Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain & oil science and technology (GOST). 2(2):49-55.
Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ (2014) Allicin: chemistry and biological properties. Molecules. 19(8):12591-12618.
Boulanouar B, Abdelaziz G, Aazza S, Gago C, Miguel MG (2013) Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind Crops Prod. 46:85-96.
Brandão RM, Ferreira VRF, Batista LR, Alves Lira NDA, Bellete BS, Cardoso MG (2020) Antifungal and antimycotoxigenic effect of the essential oil of Eremanthus erythropappus on three different Aspergillus species. Flavour Fragr J. 35(5):524-533.
Brasil (2010) Agencia Nacional de Vigilancia Sanitaria (ANVISA). Farmacopeia Brasileira. https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/farmacopeia_volume-1_2010.pdf (9 August 2021, date last accessed).
Caetano ARS, Chalfoun SM, Resende MLV, Angelico CL, Santiago WD, Magalhães ML, Cardoso MG (2020) Chemical characterization and determination of in vivo and in vitro antifungal activity of essential oils from four eucalyptus species against the 'Hemileia vastatrix' berk and Br fungus, the agent of coffee leaf rust. Aust J Crop Sci. 14(9):1379-1384.
CLSI. Methods for dilution antimicrobial susceptibility tests for bactéria that grow aerobically, Approved standard – Tenth Edition. CLSI (Clinical and Laboratory Standards Institute) document M07 – A10, Volume 35, Number 2, 2015.
CLSI. Padronização dos Testes de Sensibilidade a Antimicrobianos por Disco-difusão: Norma Aprovada – Oitava Edição. CLSI (Clinical and Laboratory Standards Institute) document, Volume 23, Número 1, 2003.
Dandlen SA, Lima AS, Mendes MD, Miguel MG, Faleiro ML, Sousa MJ, Figueiredo AC (2010) Antioxidant activity of six Portuguese thyme species essential oils. Flavour Fragr J. 25(3):150-155.
Das S, Singh VK, Dwivedy AK, Chaudhari AK, Upadhyay N, Singh P, Dubey NK (2019) Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int J Biol Macromol. 133:294-305.
Devasagayam TPA, Boloor KK, Ramasarma T (2003) Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys. 40(5):300-8
Duarte A, Luís Â, Oleastro M, Domingues FC (2016) Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control. 61: 115-122.
Ellman GL, Courtney KD, Andres JrV, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88.
El-Sayed HS, Chizzola R, Ramadan AA, Edris AE (2017) Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water-based delivery systems. Food Chemistry. 221:196-204.
Esmaeili H, Cheraghi N, Khanjari A, Rezaeigolestani M, Basti AA, Kamkar AA, Aghaee EM (2020) Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Science. 166:108135.
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc Agrotec. 35 (6): 1039-1042.
Ferreira VRF, Brandao RM, Freitas MP, Saczk AA, Felix FS, Silla JM, Cardoso MG (2019) Colorimetric, electroanalytical and theoretical evaluation of the antioxidant activity of Syzygium aromaticum L., Origanum vulgare L., Mentha spicata L. and Eremanthus erythropappus M. essential oils, and their major constituents. New J Chem. 43(20):7653-7662.
García-Díez J, Alheiro J, Pinto AL, Soares L, Falco V, Fraqueza MJ, Patarata L (2016) Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control. 59:262-270.
Ghani MA, Barril C, Bedgood Jr DR, Prenzler PD (2017) Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food chemistry. 230:195-207.
Gobbo-Neto L, Lopes NP (2007) Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Química nova. 30(2):374-381.
Guerreiro AC, Gago CM, Miguel MG, Antunes MD (2013) The effect of temperature and film covers on the storage ability of Arbutus unedo L. fresh fruit. Scientia Horticulturae. 159:96-102.
Ilc T, Parage C, Boachon B, Navrot N, Werck-Reichhart D (2016) Monoterpenol oxidative metabolism: role in plant adaptation and potential applications. Front Plant Sci. 7:509.
Karimi K, Ahari AB, Weisany W, Pertot I, Vrhovsek U, Arzanlou M (2016) Funneliformis mosseae root colonization affects Anethum graveolens essential oil composition and its efficacy against Colletotrichum nymphaeae. Ind Crops Prod. 90:126-134.
Kazemi M (2015). Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. C. 5(4):217-222.
Kujur A, Kumar A, Prakash B (2021) Elucidation of antifungal and aflatoxin B1 inhibitory mode of action of Eugenia caryophyllata L. essential oil loaded chitosan nanomatrix against Aspergillus flavus. Pestic Biochem Phys. 172:104755.
Kulisic T, Radonic A, Katalinic V, Milos M (2004) Use of different methods for testing antioxidative activity of oregano essential oil. Food chemistry. 85(4):633-640.
Lasram S, Zemni H, Hamdi Z, Chenenaoui S, Houissa H, Tounsi MS, Ghorbel A (2019) Antifungal and antiaflatoxinogenic activities of Carum carvi L., Coriandrum sativum L. seed essential oils and their major terpene component against Aspergillus flavus. Ind Crops Prod. 134:11-18.
Llana-Ruiz-Cabello M, Maisanaba S, Gutiérrez-Praena D, Prieto AI, Pichardo S, Jos Á, Cameán, AM (2015) Cytotoxic and mutagenic in vitro assessment of two organosulfur compounds derived from onion to be used in the food industry. Food chemistry. 166:423-431.
López MD, Pascual-Villalobos MJ (2010) Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crops Prod. 31(2):284-288.
Mallet ACT, Cardoso MG, Andrade MA, Costa LMAS, Machado SMF, Piccoli RH (2013) Composição química de óleos essenciais condimentares e suas atividades antioxidante e antibacteriana. Hig Alimente. 108-112.
Micić D, Ostojić S, Pezo L, Blagojević S, Pavlić B, Zeković Z, Đurović S (2019) Essential oils of coriander and sage: Investigation of chemical profile, thermal properties and QSRR analysis. Ind Crops Prod. 138:111438.
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 6(12):1451-1474.
Nguyen DK, Jang CH (2021) An acetylcholinesterase-based biosensor for the detection of pesticides using liquid crystals confined in microcapillaries. Colloids and Surfaces B: Biointerfaces. 200:111587.
Noacco N, Rodenak-Kladniew B, de Bravo MG, Castro GR, Islan GA (2018) Simple colorimetric method to determine the in vitro antioxidant activity of different monoterpenes. Anal Biochem. 555:59-66.
Pari L, Murugavel P (2007) Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 234(1-2):44-50.
Park SN, Lim YK, Freire MO, Cho E, Jin D, Kook JK (2012) Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 18(3):369-372.
Pavlić B, Teslić N, Zengin G, Đurović S, Rakić D, Cvetanović A, Zeković Z (2021) Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chemistry. 338: 127724.
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem269(2):337-341.
Qian BJ, Wu CF, Lu MM, Xu W, Jing P (2017) Effect of complexes of cyanidin-3-diglucoside-5-glucoside with rutin and metal ions on their antioxidant activities. Food chemistry. 232:545-551.
Rao J, Chen B, McClements DJ (2019) Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu Rev Food Sci Technol. 10:365-387.
Ribeiro M, Alvarenga L, Cardozo LF, Chermut TR, Sequeira J, Moreira LDSG, Mafra D (2021). From the distinctive smell to therapeutic effects: Garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin Nutr. 40(7):4807-4819.
Ribeiro RC, Zanuncio TV, de Sousa RF, da Silva CAD, Serrão JE, Zanuncio JC (2015) Feeding and oviposition of Anticarsia gemmatalis (Lepidoptera: Noctuidae) with sublethal concentrations of ten condiments essential oils. Ind Crops Prod. 74: 139-143.
Rocchetti G, Zhang L, Bocchi S, Giuberti G, Ak G, Elbasan F, Zengin G (2022) The functional potential of nine Allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chemistry. 368: 130782.
Rouf R, Uddin SJ, Sarker DK, Islam MT, Ali ES, Shilpi JA, Sarker SD (2020) Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 104:219-234.
Teixeira B, Marques A, Pires C, Ramos C, Batista I, Saraiva JA, Nunes ML (2014) Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT- Food Sci Technol. 59(1):533-539.
Teixeira ML, Cardoso MG, Souza PE, Machado SMF, Andrade MA, Gomes MS, Andrade J (2012) Citrumelo Swingle: Caracterização química, atividade antioxidante e antifúngica dos óleos essenciais das cascas frescas e secas. Magistra. 24:194-203.
Teneva D, Denkova Z, Denkova-Kostova R, Goranov B, Kostov G, Slavchev A, Degraeve P (2021) Biological preservation of mayonnaise with Lactobacillus plantarum LBRZ12, dill, and basil essential oils. Food Chemistry. 344:128707.
Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH, Liang HY, Kuo WW (2013) Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J Cardiol. 168(2):1286-1297.
Weisany W, Amini J, Samadi S, Hossaini S, Yousefi S, Struik PC (2019) Nano silver-encapsulation of Thymus daenensis and Anethum graveolens essential oils enhances antifungal potential against strawberry anthracnose. Ind Crops Prod. 141:111808.
Zilbeyaz K, Oztekin A, Kutluana EG (2021) Design and synthesis of garlic-related unsymmetrical thiosulfonates as potential Alzheimer’s disease therapeutics: In vitro and in silico study. Bioorg Med Chem. 40:116194.