

Aust J Crop Sci. 19(03):246-257 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.03.p231
No-tillage effects in bean cultivation and the application of fomesafen on soil dynamics
Heytor Lemos Martins1*, Vanesca Korasaki2, Pedro Gomes Peixoto1, Eduardo da Silva Martins2, Vitor Adriano Benedito1, Arthur Nardi Campalle1, Gabriela de Sousa Barbosa1, Pedro Luís da Costa Aguiar Alves1
1School of Agricultural and Veterinary Sciences, São Paulo State University (UNESP), Jaboticabal, São Paulo, 14884-900, Brazil.
2 University of the State of Minas Gerais (UEMG), Frutal, 38202-436, Brazil
*Corresponding author: Heytor Lemos Martins 
Abstract: Soil quality is defined as the measure of its capacity to perform vital ecological functions for agricultural development. With the expansion of cultivated areas and even their exposure to long periods without plant presence, issues such as erosion and soil nutrient depletion can arise. Therefore, practices like crop rotation or the incorporation of green manures have gained traction in soil preparation for large-scale cultivation. The common bean plant, being a member of the legume family, possesses such capabilities, being a potential nitrogen fixer and contributing to soil maintenance. Thus, this study aimed to assess the effects of cultivating common beans in fallow areas with sugarcane straw and fomesafen application. The experiment was conducted in an area with Eutrophic-Dystrophic Red Latosol. The experimental design was a randomized block design in a 4x2 factorial system, with four replicates, considering leaves straw presence (0, 1, 5, and 10 t ha-1) and two types of herbicide application (with and without application), totaling 8 experimental treatments and 32 plots. Soil chemistry, soil enzymatic activity, leaf area, plant dry matter, and productivity were evaluated. Higher straw quantities in the system improved soil enzymatic activity dynamics and soil chemistry. Plant development was correlated with higher soil enzymatic activity. Thus, it is concluded that in fallow areas: 1) the straw enhances soil quality and common bean productivity; 2) The herbicide (fomesafen) application has no impact on soil dynamics; 3) chemical, physical, and biological soil variables show worse conditions in treatments without straw, regardless of herbicide application; 4) enzymatic activity (β-glucosidase) is higher in the interaction between straw and plants, responding better to soil dynamics compared to physical and chemical variables, resulting in higher common bean productivity. It is recommended to maintain or increase straw coverage to improve soil quality and common bean productivity, with the safe use of the herbicide fomesafen, as it does not negatively affect soil dynamics.
Keywords: Soil health. Common bean. Fallow. Soil quality.
Abbreviations: N (nitrogen), P (phosphorus), S (sulfur), CTC (cation exchange capacity), and OM (organic matter), K⁺ (potassium) showed a strong positive correlation with the variables Ca²⁺ (calcium), SB (sum of bases), Mg²⁺ (magnesium), V% (base saturation), Al (aluminum), m% (aluminum saturation), H+Al (hydrogen and aluminum), Beta (β-glucosidase), LA (leaf area), LAI (leaf area index), NP (number of pods), DMP (dry mass of pods), DML (dry mass of leaves), DMS (dry mass of stems), PM (pod mass), NP (number of pods), IAF (leaf area index), DML (dry mass of the leaf), DMF (mass of leaves), Prod. (productivity), C (With), S (Without), 0 (without residue), 1 (one t ha⁻¹), 5 (five t ha⁻¹), 10 (ten t ha⁻¹), pH (hydrogen potential), MUB (modified universal buffer), PNG (p-nitrophenyl-β-D-glucopyranoside), AF (leaf area), MSV (dry pod weight), MSF (Dry Leaf Mass), MSC (Dry Stem Mass), NV (number of pods), MF (leaf dry weight), MC (stem dry weight), NT (with no-tillage), CT (conventional tillage), LDM (leaf dry mass), SDM (stem dry mass), CEC (cation exchange capacity).
Introduction
Soil quality is defined by its capacity to perform vital ecological functions, such as supporting plant growth, purifying water, and recycling nutrients and organic waste (Bünemann et al., 2018). Soil degradation, often caused by deforestation, removal of organic residues, and fallow periods, leads to erosion, and depletion of organic matter and nutrients (Karlen & Rice, 2015; Lal, 2012; Li et al., 2016). Sustainable soil management techniques, such as increasing soil organic carbon (SOC) stocks, can mitigate these issues by enhancing soil's physical, chemical, and biological properties (Karlen & Rice, 2015; Bayer et al., 2004).
Legumes, such as common beans, are effective cover crops that improve soil quality and sustainability through nitrogen fixation and organic matter addition (Reckling et al., 2016; Jensen et al., 2020). In sugarcane field renovations, combining crop residue management with legume sowing protects the soil, intensifies nutrient cycling, reduces erosion, and improves soil fertility (Chen & Weil, 2011; Thorburn et al., 2017).
Thus, bean cultivation in fallow areas with the insertion of straw can be a viable and advantageous alternative, both for the bean crop, from an economic standpoint, and for subsequent crops, such as sugarcane in succession, especially considering its quick cycle (EMBRAPA, 2020).
Common beans (Phaseolus vulgaris L.) play a crucial role in the diet of Brazilians, being widely recognized as an excellent source of protein, as well as containing significant amounts of carbohydrates, vitamins, minerals, and fibers (Mingotte et al., 2013; Amaral et al., 2016). This crop holds a prominent position both economically and nutritionally in the country, being cultivable in various regions with the possibility of up to three annual harvests (CONAB, 2024).
However, the common bean plant, like other crops, is subject to interference from weeds (Parreira et al., 2012, 2014; Mielle et al., 2019). Given this interference, weed control is essential, with chemical control being the most common method due to its efficiency. Among the herbicides registered for the crop, fomesafen stands out. It is an inhibitor of the enzyme protoporphyrin oxidase (PROTOX), indicated for post-emergence control of broadleaf weeds. Its absorption primarily occurs through the leaves, with low absorption by the roots. After foliar absorption, translocation via xylem occurs only over short distances, thus characterized as a contact herbicide (Barroso & Murata, 2021), with good control efficiency (e.g., Mancuso et al., 2016; Marchioretto & Magro, 2017).
Thus, this study aimed to investigate the effects of carioca bean cultivation in fallow areas with sugarcane straw and fomesafen application. The hypotheses are: 1) A greater amount of straw will improve soil quality and influence higher bean productivity; 2) Fomesafen application will negatively influence soil and plant response; 3) Soil chemical, physical, and biological variables showed variable conditions when subjected to fomesafen application with and without straw; 4) The enzymatic activity of β-glucosidase will be higher in the interaction of straw and plant, leading to better crop productivity.
Results
Physical and chemical soil alterations
The results showed both positive and negative correlations, ranging from weak to strong. The variables N (nitrogen), P (phosphorus), S (sulfur), CEC (cation exchange capacity), and OM (organic matter) did not show any correlation with the other variables subjected to Pearson correlation analysis (r). K+ (potassium) showed a strong positive correlation with the variables Ca²+ (calcium), SB (sum of bases), Mg²+ (magnesium), and V% (base saturation). Ca²+ exhibited a very strong positive correlation with SB, a strong positive correlation with Mg²+ and V%, and a moderate correlation with CEC. SB had a very strong positive correlation with Mg²+ and V%, and a moderate correlation with CEC. Additionally, a moderate negative correlation between SB and m% was observed (Figure 1).
Mg²+ (magnesium) showed a very strong positive correlation with V% (base saturation) and a moderate correlation with pH.
On the other hand, a moderate negative correlation was observed with Al (aluminum), m% (aluminum saturation), and H+Al (hydrogen and aluminum). For V%, the correlation was moderately positive with pH and moderately negative for Al and m%. When correlated with H+Al, a strong negative correlation was observed. Aluminum showed a very strong positive correlation with m% and a moderate positive correlation with Al+H. m% exhibited a moderate correlation with H+Al, and H+Al correlated strongly positively with CTC (Figure 1).
Soil variables such as K+ and CEC did not show significant differences among the treatments. However, pH, P, S, and OM exhibited higher values at 60 days, likely due to the influence of the bean crop. Nitrogen showed significant differences between the treatments and in the interaction between mulch and herbicide, with higher values observed 60 days after emergence (DAE) (Table 1).
The breakdown of the interaction between herbicide and the amount of mulch on N concentration, without herbicide application, showed no differences among the mulch quantities. However, where herbicide was applied, the amount of 10 t ha⁻¹ resulted in higher N concentrations (Table 2).
When comparing herbicide application within each mulch quantity, the treatments without mulch and with 5 t ha⁻¹ did not show significant differences. In contrast, the treatments with 1 and 10 t ha⁻¹ exhibited higher N values when the herbicide was applied (Table 2).
Enzymatic activity: β-glucosidase
The activity of the β-glucosidase enzyme, assessed at 30 and 60 days, showed a significant difference between herbicide application and the presence of mulch in the system (Table 3). Regarding herbicide application, both periods exhibited higher enzymatic activity in the plots treated with fomesafen. At the mulch densities, during the 30 DAE period with 10 t ha⁻¹, there was a significant increase in β-glucosidase activity. By 60 DAE, the equivalent of 10 t ha⁻¹ of mulch provided better conditions for the enzyme to exhibit greater activity, followed by 1 and 5 t ha⁻¹, while the treatments without mulch showed low activity.
The interaction of the residue left in the system with the application of the herbicide on soil enzymatic activity shows that, at 30 days, in treatments with and without herbicide application, the amount of 10 t ha⁻¹ provided better conditions for enzymatic activity in the soil (Table 4). The application of the herbicide at amounts of 1 and 10 t ha⁻¹, within each residue quantity, influenced the increase of enzymatic activity in the herbicide application treatments.
At 60 days, the treatment without herbicide application at the amount of 10 t ha⁻¹ provided better conditions for greater enzymatic activity (Table 4). When the herbicide was applied at the highest amount of residue, there was an increase in soil enzymatic activity. Evaluating the herbicide application within each residue quantity, a greater enzyme activity was observed in the treatments with application.
Table 1. Effect of straw amounts, herbicide, and time on soil physical and chemical variables: pH, potassium (K+), nitrogen (N), phosphorus (P), sulfur (S), cation exchange capacity (CTC), and organic matter (OM).
| Factors | pH | K | N | P | S | CTC | M.O. |
| - | mmolc dm-3 | mg dm-3 | mmolc dm-3 | ||||
| Herbicide | |||||||
| With | 4.68 | 2.62 | 65.45a | 15.44 | 9.11 | 42.97 | 17.00 |
| No | 4.62 | 2.55 | 54.86b | 14.65 | 8.41 | 45.02 | 15.95 |
| Straw (t ha-1) | |||||||
| 0 | 4.65 | 2.74 | 53.34b | 16.55 | 8.92 | 44.86 | 15.24b |
| 1 | 4.67 | 2.49 | 59.76ab | 15.41 | 9.33 | 42.77 | 15.88b |
| 5 | 4.71 | 2.60 | 57.18b | 14.08 | 8.33 | 44.32 | 18.08a |
| 10 | 4.78 | 2.51 | 70.33a | 14.55 | 8.46 | 44.04 | 15.88a |
| Causes of variation | |||||||
| Fherbicide | 1,04ns | 0.66ns | 4.29* | 5.04* | 4.68* | 1.91ns | 0.90ns |
| Fstraw | 1,77ns | 0.17ns | 14.80** | 0.22ns | 1.37ns | 2.71ns | 1.23** |
| Finteractions | 1,97ns | 0.45ns | 6.99** | 0.74ns | 0.6ns | 0.51ns | 0.88ns |
| CV (%) | 3.83 | 22.27 | 15.85 | 28.81 | 23.49 | 9.78 | 16.41 |
Means followed by the same letter do not differ by Tukey's test at the 5% probability level. * and ** = Significant at the 5% and 1% probability level by the F test. NS = Not significant by the F test. CV(%) = Coefficient of variation.
Table 2. Analysis of the breakdown of the interaction between herbicide and straw amount on soil nitrogen content.
| Herbicide | Straw Amount (t ha-1) | |||
| 0 | 1 | 5 | 10 | |
| With | 50.73Ba | 65.47Ba | 62.38Ba | 83.22Aa |
| No | 55.95Aa | 54.05Ab | 51.98Aa | 57.45Ab |
Means followed by different letter, uppercase in the row and lowercase in the column, differ by Tukey's test at a 5% probability level.
Plant response
For the variables leaf area (AF), dry pod weight (MSV), number of pods (NV), leaf dry weight (MF), and stem dry weight (MC), no significant difference was observed between plants with and without application of fomesafen (Table 5). Regarding the straw factor, only for MSV no significant difference was observed. For AF, treatments with 5 and 10 tons showed higher values than those found in treatments without straw and with 1 ton. For NV, MF, and MC, it was observed that the effect of straw influences a significant increase in the variables (Table 5).
In terms of bean productivity, no significant effect of the interaction between the herbicide and straw factors was observed. However, regarding the herbicide factor, plants from treatments without herbicide application showed higher yields compared to those receiving fomesafen application. Concerning the amount of straw, plants from the treatment with 10 t ha-1 showed higher yields compared to the treatment with 1 t ha-1 and without straw (Table 5).
Multivariate analysis
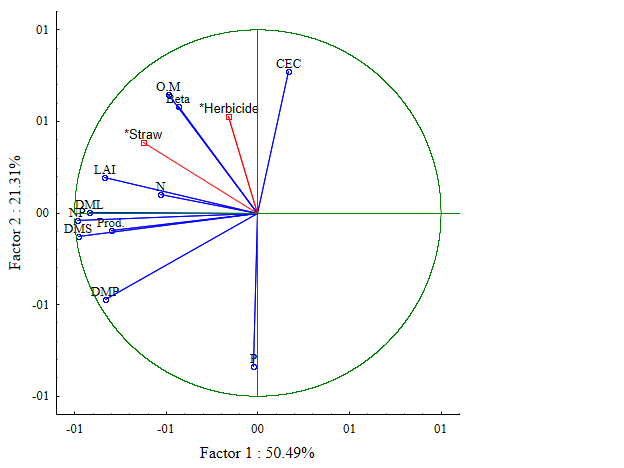
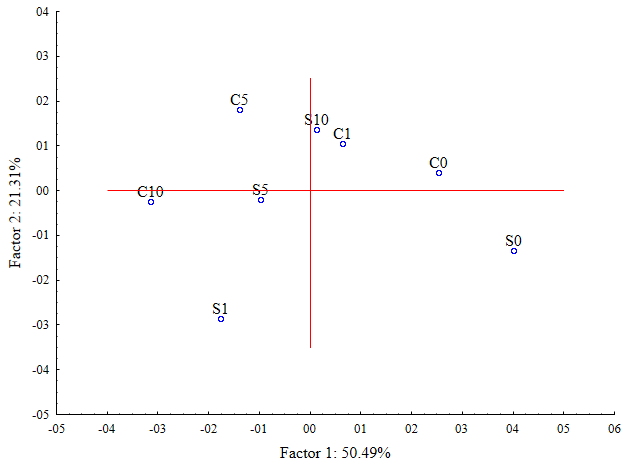
In the principal component analysis (PCA), PC1 explains 50.49% and PC2 explains 21.31%, totaling 71.8% of the data variation. The variables PM (pod mass), NP (number of pods), LAI (leaf area index), LDM (leaf dry mass), SDM (stem dry mass), and Prod. (productivity) are associated with axis 1, while O.M. (organic matter), P (phosphorus), N (nitrogen), CEC (cation exchange capacity), and Beta (β-glucosidase) are on axis 2 (Figure 2). The herbicide factor did not show any difference among the factors. However, the higher amounts of residue influence the better responses of soil variables, enzymatic activity, and bean
productivity at quantities of 5 and 10 t ha⁻¹. The treatment without residue, regardless of herbicide application, is positioned opposite the quadrant of the variables, indicating a lower influence on the evaluated parameters.
Discussion
Soil quality responses
The presence of sugarcane straw can play a crucial role in modifying soil dynamics (Valim et al., 2016), resulting in significant increases in key parameter values (Trivelin et al., 2013; Varanda et al., 2019). The presence of straw can have an indirect effect on soil pH, but it does not necessarily improve pH, as observed in this work. The decomposition of sugarcane straw can release organic acids that may slightly influence soil pH, making it slightly more acidic. However, the overall effect of sugarcane straw on modifying soil pH is considered minimal, showing little difference between treatments (Cherubin et al., 2021).
Furthermore, straw is a rich source of nitrogen (N), phosphorus (P), potassium (K+), and sulfur (S), among various other essential nutrients for plant development (Cherubin et al., 2018). The decomposition of straw gradually releases these nutrients into the soil, increasing their availability to plants and microorganisms; additionally, through degradation, its presence can promote an increase in soil organic matter (Junior et al., 2018). The decomposition of straw adds organic matter to the soil, enhancing its water retention capacity, improving its structure, and promoting beneficial microbial activities while providing a greater amount of in the soil (Morais et al., 2019; Mazetto Junior et al., 2019).
Figure 1. Pearson correlation matrix of physical and chemical soil variables of bean cultivation under different amounts of coverage and herbicide application. Legend: pH, organic matter (OM), nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), aluminum (Al), potential acidity (H+Al), sum of bases (SB), cation exchange capacity (CTC), base saturation (V%).
Table 3. β-glucosidase activity in soil from bean cultivation subjected to different amounts of straw with and without fomesafen application.
| Factors | 30 DAE | 60 DAE |
| Herbicide | ||
| With | 30.88a | 185.39a |
| No | 19.82b | 111.36b |
| Straw (t ha-1) | ||
| 0 | 18.61b | 79.17c |
| 1 | 16.04b | 152.02b |
| 5 | 19.21b | 128.83b |
| 10 | 47.54a | 203.49a |
| Initial sample | 8,98 | |
| Causes of variation | ||
| Fherbicide | 33.15** | 165.76** |
| Fpalha | 59.73** | 50.12** |
| Finteraction | 4.97* | 4.92* |
| CV (%) | 18.56 | 9.49 |
Means followed by different letters differ according to Tukey's test at a 5% probability level. * and ** = Significant at the 5% and 1% probability levels by the F test. ns = Not significant by the F test. CV(%) = Coefficient of variation.
Table 4. Analysis of the breakdown of the interaction of herbicide with the amount of straw on β-glucosidase activity in the soil.
| 30 days after emergence | |||||
| Herbicide | Straw Amount (t ha-1) | ||||
| 1 | 5 | 10 | F | ||
| With | 19.27Ba | 23.86Ba | 22.67Ba | 57.74Aa | 20.81** |
| No | 17.95Ba | 18.23Bb | 15.75Ba | 37.34Ab | 43.89** |
| F | 0.12ns | 16.52** | 3.25ns | 28.16** | |
| 60 days after emergence | |||||
| Herbicide | Straw Amount (t ha-1) | ||||
| 0 | 1 | 5 | 10 | F | |
| With | 137.67Ca | 127.80Ca | 163.02Ba | 233.07Aa | 27.04** |
| No | 80.67Bb | 96.24Bb | 94.63Bb | 173.92Ab | 28.00** |
| F | 24.57** | 94.11** | 35.37** | 26.46** | |
Means followed by different letters, uppercase in the row and lowercase in the column, differ according to Tukey's test at the 5% probability level.
 |
 |
|---|
Figure 2. Multivariate analysis of soil data, enzymatic activity, and bean variables. M.O. = Organic Matter, CTC = Cation Exchange Capacity, Beta = Beta-Glucosidase, IAF = Leaf Area, MSF = Dry Leaf Mass, N = Nitrogen, NV = Number of Pods, MSC = Dry Stem Mass, MSV = Dry Pod Mass, P = Phosphorus, C = With, S = Without, 0 = without mulch, 1 = one t ha-1, 5 = five t ha-1, and 10 = ten t ha-1.
Table 6 demonstrates the plant responses regarding soil nutrient availability. Thus, the significant correlations observed in Figure 1 were compiled according to the positive or negative relationships observed in the plant.
The increase in nitrogen (N) observed in the soil highlights the primary importance of using beans as a nitrogen-fixing plant, particularly in terms of crop rotation or the structuring of fallow areas (Nascente et al., 2017; Karavidas et al., 2022). Furthermore, its usage is extended when considering that besides the ecosystem service of nitrogen cycling, there's the possibility of harvesting the beans, adding economic value to the crop (approximately $330.00 or $65.66 per 1 US Dollar equal to 5.03 Brazilian Real according to CONAB (2024)).
Additionally, other effects of the crop were observed, such as an increase in pH, availability of P, S, and organic matter (OM). This can be attributed to plant activity through the soil-plant interaction with the production of exudates such as 4-coumarate, 4-hydroxybenzoate, Citrate, Glucosaminato, Histidinol, Lactate, Sinapate, Deoxyadenosine, Trimethylamine oxide + isopropanolamine, Asparagine, Deoxycytidine, Deoxyguanosine, Hippurate, Mucate, Riboflavin by the bean plant (Tawaraya et al., 2014). Among these, Citrate, Glucosaminato, Asparagine, and Deoxycytidine are responsible for mediating interactions with microorganisms, especially in nutritive relationships (source of carbon and energy), as well as in the availability and solubilization of metallic ions such as iron and aluminum (Siqueira et al., 1994; Donato et al., 2019).
Relationships between cultural management and enzymatic activity
It is noteworthy that, in general, the physical and chemical factors of the soil did not show significant variations among the treatments, except for OM and N, unlike what was observed for β-glucosidase activity. This result agrees with the studies by Mendes et al. (2021), Santos et al. (2022), and Soares et al. (2023), which suggest that enzymatic activity can be a more sensitive
indicator for analysis in this context. This is due to the complexity of soil responses to herbicide use, especially when considering the rhizosphere environment, which hosts an abundant microbial community that is both influenced by and influences the soil characteristics and interactions with plants (Zobioli et al., 2011 Tejada et al., 2017; Jiang et al., 2017).
The presence of herbicides in agricultural systems can either provide benefits or antagonisms to microorganisms, influencing the production of enzymes to a greater or lesser extent. Hu et al. (2019) identified significant effects of fomesafen on soybeans in pots, with a reduction in soil microbial activity and alteration of its composition and functional diversity. Additionally, there was a long-term negative effect on the abundance of nitrogen-fixing bacteria.
In the same study, Hu et al. (2019) identified a decrease in urease and invertase enzyme activity at 30 days, although microbial activity did not cease but likely experienced selection pressure. Fomesafen and other agrochemicals (e.g., insecticides, fungicides, and fertilizers) likely exert selection pressure on the established microbial community, followed by rapid subsequent growth (15 days after aplication) and recovery of microbial and enzymatic activities. This is a process of ecological succession that allows the recolonization of microorganisms with the degradation of agrochemicals into less toxic chemical species, even serving as substrates for other microorganisms (Li et al., 2023). Herbicides can be degraded by abiotic factors (usually chemistry or light action - photodegradation) and microbial activity (or biodegradation). Regardless of the origin, chemical reactions occur (hydrolysis, oxidation, reduction, etc.) resulting in non-toxic forms of the original molecule, or even in its complete mineralization, with final products such as carbon dioxide (CO2), water (H2O), ammonia (NH3), and inorganic ions (Correia, 2018).
Similarly, Hu et al. (2019) identified negative effects on invertase activity, an enzyme active in the final stages of
Table 4. Analysis of the breakdown of the interaction of herbicide with the amount of straw on β-glucosidase activity in the soil.
| 30 days after emergence | |||||
| Herbicide | Straw Amount (t ha-1) | ||||
| 1 | 5 | 10 | F | ||
| With | 19.27Ba | 23.86Ba | 22.67Ba | 57.74Aa | 20.81** |
| No | 17.95Ba | 18.23Bb | 15.75Ba | 37.34Ab | 43.89** |
| F | 0.12ns | 16.52** | 3.25ns | 28.16** | |
| 60 days after emergence | |||||
| Herbicide | Straw Amount (t ha-1) | ||||
| 0 | 1 | 5 | 10 | F | |
| With | 137.67Ca | 127.80Ca | 163.02Ba | 233.07Aa | 27.04** |
| No | 80.67Bb | 96.24Bb | 94.63Bb | 173.92Ab | 28.00** |
| F | 24.57** | 94.11** | 35.37** | 26.46** | |
Means followed by different letters, uppercase in the row and lowercase in the column, differ according to Tukey's test at the 5% probability level.
the carbon cycle, acting on low-complexity molecules. In contrast, this study observed that the presence of the herbicide led to a 74.16 µg p-nitrophenol g−1 soil h−1 increase in β-glucosidase activity compared to herbicide-free treatments. The ability of an enzyme to degrade specific molecules is related to the complexity of the substrate. For example, the invertase enzyme acts to break the glycosidic bond between sugars in sucrose, releasing glucose and fructose, which are simpler forms of sugars (Sardans et al., 2008; Manoochehri et al., 2020; Sun et al., 2023). This means that invertase requires the action of precursor enzymes like β-glucosidase, which acts in the process of breaking down more complex substrates (cellulose, flavonoids, lactoses), making them available for invertase action.
The increase in β-glucosidase activity in this experiment can be justified by the ability of selected microorganisms by herbicides to degrade complex molecules, which are then used as substrates (Huang et al., 2017). Therefore, it enhances soil enzymatic activity, specifically for β-glucosidase, which acts on the breakdown of oligosaccharides, structures of greater complexity. Thus, the assessment of this enzyme has become promising for diagnosing changes in management in agricultural systems. Studies conducted by Mendes et al. (2017, 2018) in soybean cultivation areas with no-tillage (NT) and conventional tillage (CT) systems presented the same dynamics described, with an impact on yield reduction of approximately 30 sc ha-1 from NT to CT management (Mendes et al., 2018).
The traditional soil assessment involving physical and chemical parameters may become insufficient to verify soil health (Mendes et al., 2017, 2018), where less discrepant variations (Tables 2 and 3) are determined and significantly affect soil enzymatic activity, making it a
more sensitive and determinant proxy for productivity. In this study, enzymatic activity presents values indicative of healthy soil when crop residues are incorporated into the system (values above 110 µg p-nitrophenol g−1 soil h−1), while soils without residues and with the crop show moderate values according to the indices of Mendes et al. (2017; 2021).
The enzymes found in soil, such as β-glucosidase, are important for indicating changes in the environment (Santos et al., 2022; Carneiro et al., 2024). They serve as soil indicators because they are associated with the non-living fraction of the soil, being adsorbed onto clay particles and organic matter (Dick; Burns, 2011; Wallenstein; Burns, 2011). Therefore, soil capable of storing and stabilizing organic material has better conditions to protect the enzyme, shielding it from protease actions and denaturation (Hojjati & Nourbakhsh, 2007; Lin et al., 2014; Zhao et al., 2019, Khosrozadeh & Nourbakhsh, 2022). The reflection of this can be observed in the biometric variables of the beans and in productivity, where increased straw amount and herbicide application provided significant enhancement (Table 6).
Common-bean variables response
The presence of crop residues in bean cultivation plays a crucial role in its development and optimization of agricultural production, as demonstrated in various studies (Aires et al., 2019; D’Amico-Damião et al., 2020; de Oliveira-Araújo et al., 2024). Crop residues act as a protective layer on the soil, helping to maintain adequate moisture and reducing excessive evaporation, especially in hot and dry climates (Thorburn et al., 2017). This is essential for beans, as water is crucial for healthy plant growth, especially during flowering and pod formation periods (Mingotte et al., 2018). Additionally, the presence of crop residues in the soil helps suppress weed growth, providing a more favorable environment for bean plant development without competition for essential resources such as nutrients and water (Concenço et al., 2017; Mingotte et al., 2018).
In this study, crop residues directly influenced the development of the aboveground part of the bean plant (leaf number, stem dry matter, shoot dry matter, and leaf area), suggesting that the presence of crop residues in maintaining the microclimate results in a benefit for plant development and greater leaf area, allowing for a larger area for photosynthesis. This translates into a greater production of carbohydrates, which in turn sustain pod growth and leaf development (Galdos et al., 2009 and Trivelin et al., 2013).
Furthermore, crop residues contribute to improving soil structure over time by promoting biological activity and increasing nutrient availability for plants. This is
Table 5. Leaf area (LA), number of pods (NP), dry mass of pods (DMP), dry mass of leaves (DML), dry mass of stems (DMS), at 60 DAE and productivity of the BRS-FC104 bean subjected to different amounts of residues with and without the application of fomesafen.
| Factors | LA | NP | DMP | DML | DMS | Yield |
| Herbicide | cm2 | - | g plant-1 | kg ha-1 | ||
| With | 854.37a | 37.31a | 19.55a | 5.23a | 6.77a | 1108.07a |
| No | 709.12a | 32.87a | 20.81a | 5.07a | 6.25a | 936.63b |
| Straw (t ha-1) | ||||||
| 0 | 562.68b | 22.87b | 16.16a | 3.22b | 3.48b | 683.81c |
| 1 | 673.87b | 36.75a | 21.85a | 5.24a | 7.15a | 1009.24b |
| 5 | 838.75a | 39.87a | 21.20a | 5.27a | 7.18a | 1104.04a |
| 10 | 1051.67a | 40.87a | 21.09a | 6.88a | 8.22a | 1292.30a |
| Causes of variation | ||||||
| Fherbicide | 3.92ns | 1.49ns | 0.42ns | 0.07ns | 0.44ns | 5.87* |
| Fstraw | 8.40** | 5.26** | 1.53ns | 5.82** | 6.78** | 12.94** |
| Finteraction | 1.60ns | 1.09ns | 2.83ns | 1.08ns | 3.08ns | 0.85ns |
| CV (%) | 26.55 | 29.29 | 27.20 | 34.11 | 34.69 | 19.57 |
Means followed by the same letter do not differ by Tukey's test at a 5% probability level. * and ** = Significant at the 5% and 1% probability levels by the F test. ns= Not significant by the F test. CV(%) = Coefficient of variation.
particularly important for the efficient absorption of essential nutrients such as nitrogen, phosphorus, and potassium, which play a fundamental role in bean plant growth and productivity (Simarmata et al., 2023; Silva et al., 2022). The gradual decomposition of crop residues also releases nutrients into the soil, creating a continuous nutrient cycle that benefits plants throughout the entire growing season (Cherubin et al., 2018; Junior et al., 2018).
In addition to the influence on the structures, productivity was significantly influenced by increased crop residues, with an approximate increase of 326, 421, and 608 kg ha-1 (1, 5, and 10 t ha-1, respectively) compared to the treatment without crop residues. Similar results were observed by some authors when beans were
cultivated under maize + sunn hemp residue (D’Amico-Damião et al., 2020), sunn hemp, velvet bean, and pigeon pea residues (Araújo et al., 2024), rice straw (Ahmed et al., 2020), brachiaria (Urochloa ruziziensis), and maize residues (Cunha-Chiamolera et al., 2022), which influenced a better crop development and consequently higher grain productivity.
Some studies (Takano et al., 2015; Mancuso et al., 2016; Schmitt et al., 2019) report a reduction in bean productivity when subjected to fomesafen application. However, in this study, the results show the opposite, with higher productivity in treatments where the herbicide was applied. It can be inferred that this result is linked to the presence of crop residues because, when considering the crop residue factor, plants in treatments with crop residue presence show higher values compared to the treatment without crop residues, with gains of 36.7%, 38.06%, and 50.56% for increasing amounts of crop residue (1, 5, and 10 t ha-1), respectively, compared to the control.
Materials and methods
Characterization of the study area
The experiment was conducted in the experimental area of FCAV/UNESP from May to August 2022. This area (-48.301905 -21.246705) is located at an altitude of 594 m in the city of Jaboticabal, SP.
At the time of the experiment installation, soil samples were taken from 0-20 cm for routine chemical and physical analyses (Table 1). According to the analysis, the values for M.O. (organic matter), P (phosphorus), Ca²⁺ (calcium), Mg²⁺ (magnesium), H+Al (hydrogen + aluminum), SB (sum of bases), and CEC (cation exchange capacity) are very good, with only the values for pH, K⁺ (potassium), and V% (base saturation) being below what is prescribed in Bulletin 100 for bean cultivation (Table 7).
The climate of the region, according to Alvarez classification (2014), is type Cwa, subtropical, dry in winter, with summer rains, presenting an average annual temperature of 22.7°C and average precipitation of 1353 mm. Additionally, during the experiment, rainfall data, maximum, minimum, and average temperatures, as well as humidity, were recorded (Figure 3).
Experimental design
The treatments were arranged as follows: 1) No straw, no herbicide application; 2) 1 t ha-1 straw, no herbicide application; 3) 5 t ha-1 straw, no herbicide application; 4) 10 t ha-1 straw, no herbicide application; 5) No straw, with herbicide application; 6) 1 t ha-1 straw, with herbicide application; 7) 5 t ha-1 straw, with herbicide application; 8) 10 t ha-1 straw, with herbicide application.
In all treatments, the deposition of residue was done manually, as the study area was in fallow. Thus, the residue was collected after the sugarcane cutting in nearby regions and brought to the experiment for distribution.
The experimental design was a randomized complete block design, in a 4x2 factorial system, with four replications. The factors were straw presence (0, 1, 5, and 10 t ha-1) and two types of herbicide application (with and without application), totaling 8 experimental treatments and 32 plots.
Planning and structuring of the experiment
The BRS FC104 cultivar chosen for the study is currently the first super-early cultivar on the market. This cultivar
Table 6. Summary of the interactions of the chemical and physical soil variables obtained in this study.
| Variable | Correlation | Interpretation | Overall effect |
| K e Ca | Strong + | A higher potassium content induces greater calcium availability in the soil. | + |
| Ca, SB e Mg | Strong + | It indicates that these elements are interrelated and that a change in the concentration of one of them can affect the others. | + |
| Mg, V% e CTC | Strong + | Mg is an important element for soil base saturation (V%). V% and CEC are indicators of the soil's ability to retain or make nutrients available to the plant. | + |
| pH e M.O: | Strong - | It indicates that soils with lower pH (more acidic) generally have lower organic matter content. Organic matter is important for soil fertility as it provides nutrients and improves soil structure. | + |
| pH e Al | Strong - | It indicates that soils with lower pH (more acidic) generally have higher aluminum (Al) content. Aluminum is a toxic element for plants at high concentrations. | - |
Source: Batista et al. (2018).
Table 7. Physical and chemical characteristics of a composite soil sample extracted from the 0-20 cm depth layer in the experimental area.
pH (CaCl2) |
O.M. g.dm-3 | P resina | S | K | Ca | Mg | H+Al | SB | CTC | V% | |||
| mg.dm-3 | mmolc.dm-3 | ||||||||||||
| 4.2 | 11 | 63 | 10 | 2.2 | 11 | 5 | 31 | 18.9 | 49.3 | 37 | |||
| Clay (%) | Silt (%) | Sand (%) | Textural class | ||||||||||
| 57 | 7 | 46.0 | Mean | ||||||||||
has a cycle of approximately 65 days from sowing to grain production (EMBRAPA, 2018).
Each plot had an area of 11.25 m2, where the 'BRS FC104' beans were sown in 5 rows spaced 0.45 m apart, each row being 5 m long. The useful area was 5.4 m2, with the three central rows (1.35 m) being 4 m long each. Seeding was performed by depositing 13 seeds per meter, with fertilization equivalent to 300 kg ha-1 of the 4-14-8 formulation.
The sugarcane straw collected in the region of Jaboticabal-SP was transported to the study area, where it was deposited in the plots in quantities equivalent to 1, 5, and 10 t ha-1 (1.25; 6.25; and 12.50 kg.m2). An overhead irrigation system was installed in the area, which was activated every two days, running for a duration of 50 minutes, corresponding to 30 mm of water.
Soil chemistry
For soil chemical analysis, samples were collected at 60 days after emergence (DAE) and sent to a specialized laboratory for pH, organic matter (OM), nitrogen (N), phosphorus (P), potassium (K+), sulfur (S), calcium (Ca²+),
magnesium (Mg²+), aluminum (Al), potential acidity (H+Al), sum of bases (SB), cation exchange capacity (CEC), and base saturation (V%) analysis.
Beta-glucosidase activity
Soil samples collected at 30 and 60 DAE were stored in a dark and well-ventilated area until analysis. To determine β-glucosidase activity, the soil sample was sieved using a 4 mm sieve, removing any roots, plant tissues, and other coarse organic materials that could interfere with the analysis. Subsequently, 1 g of each soil sample was weighed and placed in a test tube. To each tube, 4 mL of modified universal buffer (MUB) pH 6.0 and 1 mL of p-nitrophenyl-β-D-glucopyranoside (PNG) (Sigma-Aldrich) were added to all tubes (except the controls). This material was incubated for one hour at 37°C with rubber stoppers. After this period, 1 mL of 0.5 M CaCl2, 4 mL of THAM pH 12.0, and 1 mL of PNG (only in the controls) were added. The samples were then filtered through Whatman No. 2 filter paper, and the absorbance was read at 420 nm using a spectrophotometer (Mendes et al., 2019).
Leaf area and dry matter
Leaf area index (LAI) evaluations of the bean plants were conducted at 60 days. At 60 DAE, four plants were collected and taken to the laboratory, where leaves, stems, and pods were separated. Leaf area (LA) was determined using a leaf area meter (LI 3000A, LiCor), and the pods were counted (NP). After measurements, plant parts were dried in a forced-air oven at 60°C until constant weight to determine the dry mass of leaves (DMF), pods (DMP), and stems (DMS).
Yield
At harvest (72 DAE), production components were determined. For grain yield estimation, expressed in kg ha-1, plants from the three central rows of each plot were harvested.
Data analysis
To check the normality of the data, a Shapiro-Wilk test was used, and for homogeneity of variances, a Levene test was employed. The corrplot package was utilized for determining the correlation between soil variables. The principal component analysis (PCA) was conducted using the factoextra, devtools, and FactoMineR packages. Statistical differences were considered at a significance level of 95% (α=0.05), using R software (version 4.1.1) (R Core Team, 2023).
To assess the difference between factors in the variables β-glucosidase, AF (leaf area), MSV (dry pod weight), NV (number of pods), MSF (Dry Leaf Mass), MSC (Dry Stem Mass), and productivity, normality and homoscedasticity were tested first, followed by analysis of variance (F-test) at a 5% significance level. When significance was found by the F-test, treatment means were compared using Tukey's test at a 5% significance level.
Conclusion
In conclusion, in fallow areas: 1) The addition of crop residues improves soil quality and increases bean productivity; 2) The application of fomesafen does not impact soil dynamics; 3) Soil chemical, physical, and biological variables show poorer conditions in treatments without crop residues, regardless of herbicide application; 4) Enzymatic activity (β-glucosidase) is higher in the interaction of crop residues and plants, responding better to soil dynamics when compared to physical and chemical variables and resulting in higher bean productivity.
Acknowledgments
We want to thank the Weed Control Laboratory team for their support in developing this research. The Coordination for the Improvement of Higher Education Personnel (CAPES), for the grant to HLM, and the National Council for Scientific and Technological Development (CNPq) for the research grant to PLCAA.
Conflict of interests
The authors declare that there is no conflict of interest.
Authors' contributions
HLM, and PLCAA contributed to the conception and design of the study. HLM, ANC, and VAB, GSB carried out the methodology. HLM, ESM, and PGP wrote the main text of the manuscript. VK, HLM & VAB performed data processing and statistical analysis. PLCAA and VK conducted the review and editing of the manuscript. ANC, VK, GSB, PGP, VAB, and ESM contributed to the reading and revision of the manuscript. All authors approved the submitted version.
References
Ahmed MS, Moursy FS, Sadek II (2020) Evaluating the validity of rice straw as a suitable agricultural substrate for common bean plants under net house conditions. GSCARR. 2(1): 010–020. doi: 10.30574/gscarr.2020.2.1.0002.
Aires BC, Soratto RP, Guidorizzi FVC (2019) Grain yield and quality of common bean cultivars in response to nitrogen. Científica. 47(2): 231-238.
Amaral CB, Pinto CC, Flôres JA, Mingotte FLC, Lemos LB, Fornasieri Filho D (2016) Produtividade e qualidade do feijoeiro cultivado sobre palhadas de gramíneas e adubado com nitrogênio em plantio direto. Pesqui. Agropecu. Bras. 51(9): 1602–1609. doi: 10.1590/s0100-204x2016000900060.
Araújo ÉO, Freitas DS, Catânio JVF, Viana Filho LAB, Guimarães EG (2024) Performance of Common Bean Cultivated in Succession to Cover Crops. Revista De Gestão Social E Ambiental. 18(4): e04638. doi: 10.24857/rgsa.v18n4-009.
Barroso AAM, Murata AT (2021) Matologia: Estudos sobre plantas daninhas. 1 ed. Jaboticabal: Fábrica da Palavra.
Batista MA, Inoue TT, Esper Neto M, Muniz AS (2018) Princípios de fertilidade do solo, adubação e nutrição mineral. In: J. U. T. Brandão Filho, P. S. L. Freitas, L. O. S. Berian, & R. Goto (eds.), Hortaliças-Fruto., EDUEM. doi: 10.7476/9786586383010.0006.
Bayer C, Martin-Neto L, Mielniczuk J, Pavinato A (2004) Armazenamento de carbono em frações lábeis da matéria orgânica de um Latossolo Vermelho sob plantio direto. Pesqui Agropecu Bras. 39(7): 677-683. doi:10.1590/S0100-204X2004000700009.
Bünemann EK, Bongiorno G, Bai Z, Creamer RE, Deyn G, Goede R, Fleskens L, Geissen V, Kuyper TW, Mäder P, Pulleman M, Sukkel W, Van Groenigen JW, Brussaard L (2018) Soil quality – A critical review. Soil Biol Biochem. 120: 105-125. doi: 10.1016/j.soilbio.2018.01.030.
Carneiro RG, Figueiredo CC, Malaquias JV, Mendes IC (2024) A soil health assessment tool for vegetable cropping systems in tropical soils based on beta-glucosidase, arylsulfatase, and soil organic carbon. Appl Soil Ecol. 198: 105394.
Chen G, Weil RR (2011) Root growth and yield of maize as affected by soil compaction and cover crops. Soil Till Res. 117: 17–27. doi: 10.1016/j.still.2011.08.001.
Cherubin MR, Bordonal RO, Castioni GA, Guimarães EM, Lisboa IP, Moraes LAA, Menandro LMS, Tenelli S, Cerri CEP, Karlen DL, Carvalho JLN (2021) Soil health response to sugarcane straw removal in Brazil. Industrial Crops Products. 163: 113315. https://doi.org/10.1016/j.indcrop.2021.113315.
Cherubin, MR, Oliveira DMDS, Feigl BJ, Pimentel LG, Lisboa IP, Gmach MR, Cerri CC (2018) Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Scientia Agricola. 75: 255-272.
CONAB - Companhia Nacional de Abastecimento (2024) Conjecturas da Agropecuária: Feijão 01 a 05.04.24. Disponível em https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-de-conjunturas-de-feijao Acesso em 08 de abril de 2024.
Concenço G, Aguila LSHD, Vernetti Junior FJ (2017) Produtividade da soja no Rio Grande do Sul: genética ou manejo?. Revista Cultivar Grandes Culturas. 221.
Companhia Nacional de Abastecimento. (2024). Acompanhamento da safra brasileira: Grãos, safra 2023/2024 – Nono levantamento. Recuperado em 22 de
setembro de 2024, de https://www.conab.gov.br/uploads/pdf.
Correia NM (2018) Comportamento dos herbicidas no ambiente. Embrapa Hortaliças. Documentos 160, Brasília, DF.
Cunha-Chiamolera TPLD, Lemos LB, Coelho AP, Mingotte FLC (2022) Do the previous crop and top-dressing nitrogen fertilization change the yield and physiological and sanitary quality of common bean seeds?. Journal of Seed Science. 44: e202244038.
D’Amico-Damião V, Nunes HD, Couto Jr PA, Lemos LB (2020) Straw type and nitrogen fertilization influence winter common bean yield and quality. International Journal of Plant Production. 14(4): 703-712.
De Donato A, Maia TF, Conto TD, Pereira MG, & Fraga ME (2019) Micobiota produtora de fitase isolada de solo e serapilheira do Bioma Cerrado. Ciência Florestal. 29, 1270-1281.
De Oliveira Araújo É, de Souza Freitas D, Catânio JVF, Viana Filho LAB, & Guimarães EG (2024) Performance of Common Bean Cultivated in Succession to Cover Crops. Revista de Gestão Social e Ambiental. 18(4): e04638-e04638.
Dick RP, Burns RG (2011) A brief history of soil enzyme research. In: Dick RP (ed) Methods of soil enzymology. Madison: Soil Science Society of America. p. 1-19. doi:10.2136/sssabookser9.c
EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. Feijão - BRS FC104. 2018. Disponível em: https://www.embrapa.br/busca-de-solucoes-tecnologicas/-/produto-servico/5176/feijao---brs-fc104. Acesso em: 16 junho 2020.
EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária (Embrapa). (2024). Sistema Brasileiro de Classificação de Solos (5ª ed.). Brasília, DF.
Galdos MV, Cerri CC, Cerri CEP (2009) Soil carbon stocks under burned and unburned sugarcane in Brazil. Geoderma. 153(3-4): 347-352
Hojjati S, Nourbakhsh F (2007) Effects of cow manure and sewage sludge on the activity and kinetics of L-glutaminase in soil. Biol Fertil Soils. 43: 491-494. doi:10.1007/s00374-006-0149-7.
Hu H, Zhou H, Zhou S, Li Z, Wei C, Yu Y, Hay AG (2019) Fomesafem impacts bacterial communities and enzyme activities in the rhizosphere. Environmental Pollution. 253: 302-311.
https://doi.org/10.1016/j.envpol.2019.07.018.
Huang X, He J, Yan X, Hong Q, Chen K, He Q, et al. (2017) Microbial catabolism of chemical herbicides: microbial resources, metabolic pathways and catabolic genes. Pesticide Biochem Physiol. 143: 272-297.
Jensen ES, Carlsson G, Hauggaard-Nielsen H (2020) Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agronomy Sustainable Develop. 40(1): 5.
Jiang C, Lu YC, Xu JY, Song Y, Song Y, Zhang SH, et al. (2017) Activity, biomass and composition of microbial communities and their degradation pathways in exposed propazine soil. Ecotoxicology and Environmental Safety. 145: 398-407.
Junior JGAS, Cherubin MR, Oliveira BG, Cerri CEP, Cerri CC, Feigl BJ (2018) Three-year soil carbon and nitrogen responses to sugarcane straw management. Bioenergy Research. 11: 249-261. doi:10.1007/s12155-017-9892-x.
Karavidas I, Ntatsi G, Vougeleka V, Karkanis A, Ntanasi T, Saitanis C, Agathokleous E, Ropokis A, Sabatino L., Tran, F, et al. (2022). Agronomic practices to increase the yield and quality of common bean (Phaseolus vulgaris L.): A systematic review. Agronomy. 12: 271. https://doi.org/10.3390/agronomy12020271.
Karlen DL, Rice CW (2015) Soil degradation: Will humankind ever learn?. Sustainability. 7(9): 12490-12501.
Khosrozadeh S, Nourbakhsh F (2022) Deforestation alters protease regulation by amino acids. Geoderma. 426: 116097.
Lal R (2012) Climate change and soil degradation mitigation by sustainable management of soils and other natural resources. Agricultural Research. 1: 199-212.
Li J, Niu X, Wang P, Yang J, Liu J, Wu D, Guan P (2023) Soil degradation regulates the effects of litter decomposition on soil microbial nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Frontiers in Plant Science. 13: 1090954.
Lin CHEN, Zhang JB, Bing-Zi ZHAO, Xiu-Li XIN, Gui-Xiang ZHOU, Jin-Fang TAN, Jin-Hua ZHAO (2014) Carbon mineralization and microbial attributes in straw-amended soils as affected by moisture levels. Pedosphere. 24(2): 167-177.
Li Y, Zou Z, Liu G, He C (2016) Improving soil fertility and crop productivity through intercropping with legumes. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science. 66(4): 345–355. doi: 10.1080/09064710.2015.1132204.
Mancuso MAC, Aires BC, Negrisoli E, Corrêa MR, Soratto RP (2016) Seletividade e eficiência de herbicidas no controle de plantas daninhas na cultura do feijão-caupi. Revista Ceres. 63: 25-32.
Manoochehri H, Hosseini NF, Saidijam M, Taheri M, Rezaee H, Nouri F (2020) A review on invertase: Its potentials and applications. Biocatalysis and Agricultural Biotechnology. 25: 101599.
Marchioretto LDR, Dal Magro T (2017). Weed control and crop selectivity of post-emergence herbicides in common beans. Ciência Rural. 47(3): e20160295.
Mazetto Júnior JC, Torres JLR, Costa DDDA, Souza ZMD, & Lemes EM (2019) Production and decomposition of cover crop residues and associations with soil organic fractions. Journal of Agricultural Science. 11(5): 58.
Mendes IC, Sousa DMG, Dantas OD, Lopes AAdeC, Reis Junior FB, Oliveira MIL, Chaer GM (2021) Soil quality and grain yield: a win-win combination in clayey tropical Oxisols. Geoderma. 388: n. 114880.
Mendes I C, Sousa LM, Sousa DMG, Lopes AAC, Reis Junior FB, Lacerda MPC, Malaquias JV (2019) Critical limits for microbial indicators in tropical Oxisols at post-harvest: The FERTBIO soil sample concept. Applied Soil Ecology. 139: 85-93. Doi: 10.1016/j.apsoil.2019.02.025.
Mendes IC, Sousa DMG, Reis Junior FB, Kappes C, Ono FB, Semler TD, Zancanaro L, Lopes AAC (2017) Qualidade biológica do solo: por que e como avaliar. Boletim de Pesquisa da Fundação MT., v. 1, p. 98-105.
Mielle RF, Zanoni HML, Alves PLCA, Parreira MC, Fernandes JMPE (2020) Antagonistic effects of herbicides mixtures for weed control in common beans (Phaseolus vulgaris L.). Research on Crops. 21(3): 549-555.
Mielle RF, Zanoni, H. M. L.; Alves P.LCA, Parreira MC, Fernander JMPEV (2019) Periods of weed interference on bean crop with cultivars plants different architecture types. Research Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences, v. 5, p. 439-450.
Mingotte FLC, Hanashiro RK, Fornasieri Filho D (2013) Response of rice cultivars to nitrogen in upland conditions. Revista Ceres. 60: 86-95.
Mingotte FLC, Lemos LB, Paes JMV, Andrade AT (2018) Rotação e sucessão de culturas: formação de palha para o sistema plantio direto de qualidade no Cerrado. Informe Agropecuário. 39(302): 28-41.
Mollema PN, Fain SJ, de Blecourt M (2020) Tropical soil erosion and changes in soil organic carbon stocks in deforested areas. Geoderma. 366: 114262.
Montanari R, Madalena SF, Vidal JM, Ulbrich AV (2021) The Use of Herbicides in Brazilian Agriculture: Challenges and Advances. Plants. 10(11): 2327.
Morais MC, Ferrari BM, Borges CD, Cherubin MR, Tsai SM, Cerri CC, Cerri CEP, Feigl BJ, (2019) Does sugarcane straw removal change the abundance of soil microbes?. Bioenergy Research. 12: 901–908. https://doi.org/10.1007/s12155-019-10018-5.
Munyaneza F, Jensen ES, Lüscher A (2019) Effects of long-term mixed cropping of common bean (Phaseolus vulgaris L.) and maize (Zea mays L.) on yield, soil organic carbon, and total nitrogen. Journal of Agronomy and Crop Science. 205(5): 504-514.
Nascente AS, Carvalho MDCS, Melo LC, Rosa PH (2017) Nitrogen management effects on soil mineral nitrogen, plant nutrition and yield of super early cycle common bean genotype. Acta Sci Agron. 39: 369–378.
Negrisoli E, Silva EA, Júnior AFS, Aires BC, Cardoso GP (2018) Efficacy of alternative herbicides in controlling weeds in beans (Phaseolus vulgaris L.) and their selectivity for the crop. Revista Brasileira de Herbicidas. 17(1): 61-69.
Nunes AL, Cunha JPAR, Filho JMF (2015) Efeitos do manejo da palha de cana-de-açúcar sobre atributos microbiológicos do solo. Pesquisa Agropecuária Tropical. 45(3): 325-333.
Oliveira EM, Valadão FCA, Souza JM, Silva MA (2018) Management of sugarcane straw and its effect on soil quality. Soil & Tillage Research. 180: 26-35.
Parreira MC, Barrosoo AAM, Pereira FCM, Alves PLCA (2012) Modeling of weeds interference periods in bean. Planta Daninha. 30: p. 713-720.
Parreira MC, Alve PLCA, Lemos LB, (2014) Portugal, J. Comparação entre métodos para determinar o período anterior à interferência de plantas daninhas em feijoeiros com distintos tipos de hábitos de crescimento. Planta Daninha. v. 32: p. 727-738.
PereiraAA, Pereira AAA, Oliveira DSS, Cardoso EL, Pires CVA (2019) Long-term sugarcane straw management and its effects on soil chemical properties and crop production. Agricultural Systems. 176: 102655.
Pinto PRS, Kunes FD, Barbosa LM (2022) Effect of herbicide application and crop residue management on soil microbiota in sugarcane fields. Sugar Tech. 24(4): 812-821.
Reichert JM, Norton LD (2022) Soil erosion control by crop residue and cover crops: A review. Soil & Tillage Research. 215: 105264.
Reckling M, Bergkvist G, Watson CA, Stoddard FL, Zander PM, Walker RL, Bachinger J (2016) Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Frontiers in Plant Science. 7: 669.
Rodrigues BN, Almeida FS (2018) Guia de herbicidas. Londrina: Ed. dos Autores.
Sampaio RA, Gomes DD, Silva RA, Guedes EM, Oliveira FL (2020) Effect of sugarcane straw and urea application on nitrogen availability in tropical soils. Revista Brasileira de Ciência do Solo. 44, e0200077.
Sato M, Ferreira RP, Ribeiro LA (2020) Decomposition of sugarcane straw and the release of nutrients under different levels of soil cover. Revista de Ciências Agrárias. 43(1): 218-228.
Santos JV, Bento LR, Bresolin JD, Mitsuyuki MC, Oliveira PPA, Pezzopane JRM, Martin-Neto L (2022) The long-term effects of intensive grazing and silvopastoral systems on soil physicochemical properties, enzymatic activity, and microbial biomass. Catena. 219: 106619.
Sardans J, Peñuelas J, Estiarte M (2006) Aquecimento e secaalterar a atividade da fosfatase do solo e a disponibilidade de P no solo em umMatagal mediterrânico. Solo Vegetal. 289:227–238.
Silva AP, Franco HC, Morelli JL (2020) The impact of sugarcane straw management on soil microbiota and sugarcane productivity. Applied Soil Ecology. 153: 103616.
Silva VA, Oliveira FA, Santos LF, Costa RS (2019) Growth and productivity of sugarcane under different straw management and nitrogen rates. Pesquisa Agropecuária Brasileira. 54: e01923.
Silva PLF (2022) Compactação e seus efeitos sobre o funcionamento do solo e a absorção de nutrientes pelas plantas: Uma revisão bibliográfica. Meio Ambiente (Brasil), v. 3, n. 2.
Simarmata SM, Lase BG, Murcitro BW, Simanihuruk M, Chozin (2023) Combination of organic soil amendments and weed control to optimize the growth and yield of peanuts in sandy soils in coastal areas. E3S Web of Conferences, 10.1051/e3sconf/202337306006, 373, (06006).
Schmitt J, Jochem W, Mello GR DE, Schiessel JJ, Demartini SC, Fioreze SL, Oliveira Neto AMDE, Guerra N (2019) Piraclostrobina reduzindo toxicidade do fomesafen ao feijão. Comunicações em Ciências Vegetais. 9: 29-35, 2019 (2019005). Doi: 10.26814/cps2019005.
Singh SP, Kumar R (2021) Role of microorganisms in biodegradation of herbicides in agricultural soil: An overview. Environmental Biotechnology Research. 2(1): 1-11.
Siqueira JO, Moreira FDS, Grisi BM, Hungria M, Araujo RS (1994) Microrganismos e processos biológicos do solo: perspectiva ambiental.
Soares DDA, Sekiya BMS, Modesto VC, Nakao AH, Freitas LA, Souza IMDD, Andreotti M (2023) Accumulated Carbon Fractions in Tropical Sandy Soils and Their Effects on Fertility and Grain Yield in an Integrated Crop–Livestock System. Sustainability. 15(18): 13829.
Sousa JB, Fernandes JM, Souza AG (2021) Herbicide leaching and sorption in tropical soils under sugarcane straw management. Journal of Agricultural and Food Chemistry. 69(10): 3046-3056.
Souza RA, Pinheiro EFM, Silva WM, Dias BO (2018) Soil organic matter and aggregation after conversion of pasture into sugarcane under different straw management practices. Soil & Tillage Research. 181: 92-101.
Spokas KA, Reicosky DC (2009) Impacts of six tillage methods on soil greenhouse gas emissions. Climatic Change. 93: 123-138.
Stürmer GR, Tormena CA (2018) Influence of soil compaction and sugarcane straw on soil structure and root growth. Soil & Tillage Research. 180: 87-94.
Sun X, Sun M, Chao Y, Shang X, Wang H, Pan H, Zhuge Y (2023) Effects of lead pollution on soil microbial community diversity and biomass and on invertase activity. Soil Ecology Letters. 5(1): 118-127.
Takano HK, Braz GBP, de Oliveira Junior RS, Constantin J, Rios FA, Gheno EA, Franchini LHM (2015) Redução da fitointoxicação por herbicidas aplicados no feijoeiro com a utilização de fungicidas. Agrarian. 8(27): 12-22.
Tawaraya K, Horie R, Saito S, Wagatsuma T, Saito K, Oikawa A (2014) Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites. 4: 599-611. doi:10.3390/metabo4030599
Tejada M, Morillo E, Gómez I, Madrid F, Undabeytia T (2017) Effect of controlled release formulations of diuron and alachlor herbicides on the biochemical activity of agricultural soils. Journal of hazardous materials. 322: 334-347.
Teixeira LGV, Franco HCJ, Pereira EI (2020) Integrated straw management strategies in sugarcane cultivation: Effects on soil fertility and crop yield. Agricultural Systems. 176: 102667.
Thorburn PJ, Biggs JS, Palmer J, Meier EA, Verburg K, Skocaj DM (2017) Prioritizing crop management to increase nitrogen use efficiency in Australian sugarcane crops. Frontiers in Plant Science. 8. https://doi.org/10.3389/fpls.2017.01504
Tormena CA, Alves SJ (2019) Physical quality of soils under sugarcane straw management in different Brazilian regions. Geoderma Regional. 18, e00205.
Torres GL, Reis RR, Silva CM (2021) Sugarcane straw management: Impacts on soil properties and plant growth. Journal of Agricultural Science. 13(2): 42-55.
Trivelin PCO, Franco HCJ, Otto R, Ferreira DA, Vitti AC, Fortes C, Cantarella H (2013) Impact of sugarcane trash on fertilizer requirements for São Paulo, Brazil. Scientia Agricola. 70: 345-352.
Valentim LS, Tormena CA, Ferreira AO (2018) Straw management in sugarcane fields: Effects on soil moisture, temperature, and CO2 emissions. Journal of Soil Science and Plant Nutrition. 18(3): 662-674.
Valim WC, Panachuki E, Pavei DS, Sobrinho TA, Almeida WS (2016) Effect of sugarcane waste in the control of interrill erosion. Semina Ciências Agrárias. 37: 1155–1164. https://doi.org/10.5433/1679-0359.2016v37n3p1155
Varanda LL, Cherubin MR, Cerri CEP (2019) Decomposition dynamics altered by straw removal management in the sugarcane-expansion regions in Brazil. Soil Research. 57: 41–52. https://doi.org/10.1071/SR17298
Vasconcelos ACM, Franco HCJ, Landell MGA (2018) The impact of straw management and nitrogen fertilization on sugarcane growth and yield. Agricultural Systems. 167: 78-88.
Veloso MG, De Conti LF, Oliveira SP (2022) Effects of different straw management practices on the decomposition rate of organic matter in sugarcane fields. Soil & Tillage Research. 223: 105432.
Vitti AC, Lima E, Otto R (2019) Sugarcane straw and nitrogen fertilization: Impact on soil attributes and sugarcane yield. Journal of Plant Nutrition. 42(16): 1839-1853.
Wallenstein MD, Burns RG (2011) Ecology of extracellular enzyme activities and organic matter degradation in soil: a complex community-driven process. In: Dick RP (ed.). Methods of soil enzymology. Madison: Soil Science Society of America. 2011. p. 35-56. DOI: doi.org/10.2136/sssabookser9.c2.
Wong VNL, Dalal RC, Greene RS (2010) Carbon dynamics in tropical and sub-tropical soils: Effects of residue management, soil temperature, and moisture. Soil & Tillage Research. 111(1): 45-52.
Yang Y, Sun B, Zhang M, Zhang X (2021) The influence of crop residue management and tillage on soil organic carbon storage in sugarcane production. Agricultural Systems. 190: 103078.
Zhao S, Liu S, Wang X (2019) Soil erosion and nitrogen losses under different sugarcane straw management practices. Agricultural Water Management, 222, 44-53.
Zhou P, Wu Z (2020) Effects of straw management and fertilization on soil enzyme activities in sugarcane fields. Journal of Soil Science and Plant Nutrition. 20(3): 1796-1807.
Zhu Q, Jin Y (2019) Sugarcane residue and nitrogen management effects on soil organic carbon and nitrogen mineralization. Agricultural Research. 8(2): 227-234.
Zobiole LHS, Kremer RJ, Oliveira RS, Constantin J (2011) Glyphosate affects micro-organisms in rhizospheres of glyphosate-resistant soybeans. Journal of Applied Microbiology. 110: 118-127. https://doi.org/10.1111/j.1365-2672.2010.04864.x