

Aust J Crop Sci. 19(01):89-98 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.01.p237
Impact of environmental factors on the antibacterial activity of leaf extracts from Moroccan strawberry trees (Arbutus unedo L.)
Ilias Oussif, Nora Salim, Mohamed El Habty, Nadya Wahid *
Environmental, Ecological and Agro-Industrial Engineering Laboratory, Department of Life Sciences, Faculty of Science and Techniques, Sultan Moulay Slimane University, Beni-Mellal, Morocco
*Corresponding author: WAHID Nadya 
Abstract: The present work determines the environmental factors and evaluates origin effect of leaf extracts from natural populations of the Moroccan strawberry tree (Arbutus unedo L.) on the potential antibacterial activity of human pathogenic strains. Samples of strawberry tree from different geographically distinct areas were collected for methanolic dried leaf extraction by tree/population. Each leaf methanolic extract were analysed for antioxidant compounds and antibacterial activity test. The results show a variation in antibacterial activity depending on the concentration of the extract and on the origin of the plant material used for the preparation of the methanolic extracts. The bacterial strains tested were susceptible to the antibacterial effects of the methanolic leaf extract even at a lower concentration of 25 mg/ml, indicating that the extract is potent enough to affect the bacteria at this lower dosage. The analysis of variance shows a significant provenance effect of Moroccan Arbutus extracts on the inhibition of the antibacterial activity zone of the strains at low and medium concentrations. The results of the geographical structure of Arbutus leaf extracts classified populations into three groups. The first group is composed of (OUL, IKA, DAR) populations which can synthesize phenolic active compounds against the Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae bacteria. The second group (ZRI, BT) is characterized by high precipitation and high antibacterial activity only against Citrobacter freundii. The third group (TIM, ELK, BS, OUM, BRI, IZA) is represented by the presents a negative correlation for all the antioxydante componends and antibacterial activity. This population structure of Arbutus leaf extracts results shows that the antioxidant compounds from populations of high altitude and more humid origins have weak antibacterial effects against the four tested strains. Leaf extracts from populations originating from low altitudes with moderate precipitation record high antibacterial effects. The determination of the effect of the environment on the phytochemical composition of Arbutus leaf extracts, and indirectly on the inhibition activity of the antibacterial zone, is necessary to select elite natural population products for applications in the food, therapeutic and pharmaceutical fields.
Keywords: Arbutus Unedo, leaf extract yield, total polyphenols, antioxidant activity, antibacterial activity and environmental factors.
Abbreviations: MIC_Minimum inhibitory concentration; MBC_minimum bactericidal concentration.
Introduction
Medicinal plants have always had an important place in humanity's therapeutic arsenal. According to the World Health Organization (WHO), around 80% of the world's population in developing countries, rely essentially on traditional medicinal plants for their primary health care (Guaouguaou et al., 2019). Despite remarkable progress in synthetic organic chemistry in the twentieth century, more than 25% of drugs prescribed in industrialized countries derive directly or indirectly from plants (Süntar, 2020).
The strawberry tree (Arbutus unedo L.). is among the important phytogenetic resources in the region. This shrub belongs to the Ericaceae family and can reach a height of approximately 1.5 to 3.0 meters. The arbutus is endemic to the Mediterranean flora and is found mainly in southern Europe, northern Africa, parts of the UK and the Macaronesian islands (Martins et al., 2016; Faida et al., 2019). This small perennial Ericaceae is well adapted to the environmental conditions of its Mediterranean range, making it an ecologically interesting species. In Morocco, it grows in three different biogeographical regions, tolerating the most varied edaphic and climatic conditions, ranging from subhumid to semi-arid regions (Faida et al., 2019).
Several phytochemical studies have revealed that arbutus leaves and fruit contain various classes of phytochemicals: phenolics (e.g. epicatechin, catechin and catechin gallate), terpenoids, anthocyanins, flavonoids (e.g. genins and heterosides), quinones, tannins, vitamins C and E, carotenoids and organic acids (Bebek Markovinović et al., 2022; Bouyahya et al., 2016; Žlabur et al., 2020). This diversity of secondary metabolites gives arbutus interesting biological activities for the pharmaceutical, food and cosmetics industries. Among these biological properties are antibacterial, antioxidant, anti-inflammatory, antidiabetic, antihypertensive and antitumor activity (Morgado et al., 2018; Rhattas et al., 2016). The adaptation of Arbutus to its environment may impact its secondary metabolism, which may affect some of its biological activities. Furthermore, according to previous studies, plants manage climate change by varying their secondary metabolism that involves several genes and pathways (Di Ferdinando et al., 2014; Nenadis et al., 2015; Martins et al., 2022). The secondary metabolites such as polyphenols play an essential role in the antimicrobial activity of plant extracts (Cushnie et al., 2003). These extracts are used as an alternative to antimicrobial agents to treat serious human infections caused by bacterial organisms (Nathan, 2004; Cornaglia, 2009). In response to climate change, particularly drought, some plants increase their phenolic and active principe content and decrease their protein and carbon metabolites (Khan et al., 2015: Martins et al., 2022). However, other plants increase their sugar and carbon metabolites as well as certain phenolic acids (Hare et al., 1998; De Simón et al., 2017). Generally, plant species synthesize a wide range of active ingredients, classified as primary and secondary metabolites, which help them to adapt to the environment, in which they originate.
The objective of this study is (i) to evaluate the effect of the provenance of leaf extracts from natural populations of Moroccan arbutus on the antibacterial activity of potentially pathogenic strains and (ii) to determine the action mechanisms of leaf extracts on antibacterial activity in relation to total polyphenol content, antioxidant activity, and in relation to environmental factors (Altitude, Temperature and Precipitation).
Results
Antibacterial activity and the effect of provenance of A. unedo leaf extracts
Disk diffusion method
Figures 1, 2, 3, and 4 present the diameters of inhibition of bacterial activity zone as a function of the concentrations of methanolic leaf extracts per population of strawberry tree. The studied extracts exhibit antibacterial activity against the four targeted pathogenic strains tested (Escherichia coli CIP 54127, Klebsiella pneumoniae ATCC 13883, Citrobacter freundii ATCC 8090, and Enterococcus faecalis ATCC 19433). This antibacterial activity varies according to the extract concentration, and according to the plant material origin used for the preparation of the methanolic extracts (Table 2).
In terms of concentration, the results prove that the diameters of the zone inhibition of bacteria tested for the majority of extracts from the populations studied are greater than 20 mm for the concentration 100 mg/ml (Figures 1, 2, 3 and 4). We can say that the studied bacteria are extremely sensitive to the concentration of 100 mg/ml. For the two concentrations 50 mg/ml and 25 mg/ml, the diameters of the inhibition zones for most populations are greater than 9 mm (Figures 1, 2, 3 and 4). It could be inferred that the sensitivity of the bacteria tested at low concentrations of 25 mg/ml is reduced to half compared to that at the high concentration of 100 mg/ml.
A significant variation (P= 0.0001; p ≤ 0.05) was observed in the inhibitory effect of extracts from the studied populations against all the bacterial strains. This reveals that the environmental origine of natural populationof Moroccan strawberry is highly significant on the antibacterial activity of the strains, apart from Citrobacter freundii and Klebsiella pneumoniae at high concentrations, which are not sensitive to the environmental origine of natural population (Table 2).
Moreover, for the Escherichia coli bacterium, for the first concentration (100 mg/ml) the diameter of the zones of inhibition varies from 25.13 ±0.25 mm (DAR) to 18.97 ±2.21 mm (OUM). The DAR (Rif Occidental), ZRI (Pre-rif) and BE (Moyen Atlas) populations showed the highest antibacterial activity againest Escherichia coli bacteria (25.13 ±0.25 mm; 23.13 ±2.84 mm and 24.98 ±1.62 mm, respectively) (Figure 1). The mean inhibition diameters were recorded in the OUL (Central Plateau, 22.51 ±1.60 mm), ELK (Middle Atlas, 22.02 ±1.39 mm) and BT (Western Rif, 22.75 ±0.96 mm) populations. While the lowest inhibition diameters are registered in the BRI (Rif Occidental), TIM and OUM (Middle Atlas) populations (19.13 ±1.67 mm; 19.69 ±2.23 mm and 18.97 ±2.21 mm respectively). Concerning Citrobacter freundii bacteria, the analysis of variance (Table 2) shows that the diameters of the Citrobacter freundii inhibition zones do not reveal any significant difference between the populations studied for the two high concentrations (100 mg/ml and 50 mg/ml). Thus, the diameter of the zones of inhibition for all populations studied varied from 24.23 ±1.03 mm (BT) to 18.75 ±3.09 mm (TIM) for the 100 mg/ml concentration. The provenance effect is highly significant during the application of low concentrations of the order of 25 mg/l and 12.5 mg/l. Thus, the best inhibition effects are recorded in the BT (Rif Occidental, 24.23 ±1.03 mm), DAR (Rif Occidental, 23.25 ±2.53 mm) and ZRI (Rif, 22.75 ±0.96 mm) populations (Figure 2). For Enterococcus faecalis, analysis of variance showed that there is significant population variation in the expression of inhibition zone diameters for all concentrations. However, the OUL population showed the highest antibacterial activity against Enterococcus faecalis with an inhibition zone diameter of 25.12 ±1.40 mm for the 100 mg/ml concentration, followed by the ZRI (22. 25 ±1.5 mm), IKA (22.22± 0.69 mm), BT (22.88± 1.18 mm) and TIM (22.01 ±1.87 mm) populations (Figure 3). In contrast, the OUM (Middle Atlas) and BS (Central Plateau) populations show the weakest inhibitory effect against Enterococcus faecalis bacteria.
The analysis of variance results indicates that the diameters of the Klebsiella pneumoniae inhibition zones do not show any significant difference between the extracts of the populations studied for the high concentration (100 mg/ml), while for the other concentrations (50, 25 and 12.5 mg/ml) it revealed a significant effect of provenance (Table 2). The DAR, IKA, BE and TIM populations showed higher activity, with inhibition zone diameters of 23.35 ±2.07 mm; 23.82 ±0.85 mm; 23.89± 1.26 mm and 23.34 ±2.52 mm, respectively. Whereas the two populations OUM (Middle Atlas) and ZRI (Pre-Rif) show low activity vis-à-vis Klebsiella pneumoniae (20.08 ±1.58 mm and 20.75 ±1.94 mm, respectively).
It can be concluded that the Western Rif DAR population has the best inhibitory effect against the four tested strains, as it records high inhibition zone diameters for all bacteria, and all concentrations used show an inhibitory effect against all bacteria except for Enterococcus faecalis, for which the fourth concentration showed no effect.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) method for methanolic extracts in different populations
Table 3. shows the descriptive analysis and analysis of variance of the mean MICs and MBCs of methanolic extracts from the leaves of natural populations of Moroccan arbutus. The results of the analysis of variance indicate that the means of MIC and MBC do not reveal significant differences among the extracts from the studied populations, for the four bacteria Escherichia coli, Citrobacter freundii, Enterococcus faecalis, and Klebsiella pneumoniae. However, the means of MIC for the Klebsiella pneumoniae strain reveal a significant difference among the extracts from the studied populations (P = 0.000 ≤ 0.05) (Table 3). In general, we can conclude that the origin of the plant material has no significant effect on the MIC and MBC values of the extracts. This was generally true for individual metabolites.
These results show that MIC values for Escherichia coli range from 12.5 ±0.00 mg/ml (ZRI, DAR, BT, OUM and OUL) to 18.75 ±7. 22 mg/ml (BRI, IZA, ELK, BE, TIM and BS), and MBC values range from 25.00 ±0.00 mg/ml (ZRI, BT, OUM and OUL) to 37.5 ±14.43 mg/ml (BRI, ELK, TIM and BS). For Citrobacter freundii, MIC values range from 12.5 ±0.00 mg/ml (DAR and BT) to 21.88 ±6.25 mg/ml (ELK and OUM), while MBC values vary from 25.00 ±0.00 mg/ml (ZRI, DAR and BT) to 43.75±12.05 (OUM). Enterococcus faecalis MIC values range from 12.5 ±0.00 mg/ml to 18.75 mg/ml, and MBC values from 25.00 ±0.00 mg/ml to 43.75 ±12.5 mg/ml. For Klebsiella pneumoniae, recorded MIC values range from 12.5 ±0.00 mg/ml to 37.5 ±14.43 mg/ml, while MBC values vary from 25.00 ±0.00 mg/ml to 43.75 ±12.5 mg/ml.
Gegraphical structure of A. unedo populations through total polyphenols, antioxidant activity, antibacterial activity of leaf extracts and ecological conditions
Principal component analysis (PCA) was carried out using the average values of total polyphenols, antioxidant activity (IC50), activity of antibacterial inhibition zones (AZI at 100 mg/ml (C1) and AZI 25 mg/ml (C3)), and ecological factors (Figure 5). The PCA analysis shows that 51.22% of the total variation between the populations studied was explained by the first two components. The first component (PC1) explained 28.41% of the total variation linked to the diameters of the inhibition zones of
Table 1. Geographical and ecological characteristics of the sampled natural populations of unedo Strawberry tree in Morocco.
| Populations | Code | Ecological zones | Latitude (N) |
Longitude (W) |
Altitude (m) | T (°C) | Pr (mm) | Bioclimatic zone | Density of species |
|---|---|---|---|---|---|---|---|---|---|
| Zrizer | ZRI | Rif | 34°36′ 31.39″ | 04°35′ 16.47″ | 625 | 17.24 | 706.21 | Sub-humid | More dense |
| Ikawen | IKA | 34°43′ 57.09″ | 04°36′ 58.56″ | 608 | 17.24 | 706.21 | Sub-humid | More dense | |
| Izarane | IZA | Rif Occidental/North | 34°48’ 13.81″ | 05°30’ 06.29″ | 436 | 18.75 | 600.93 | Sub-humid | More dense |
| Brikcha | BRI | 34°55’ 27.37″ | 05°31’ 45.52″ | 252 | 18.75 | 600.93 | Sub-humid | More dense | |
| Dardara | DAR | 35°05’ 57.52″ | 05°16’ 22.68 | 402 | 16.7 | 753.01 | Sub-humid | Medium dense | |
| Bab Taza | BT | 35°01’ 48″ | 05°09’ 43″ | 705 | 16.7 | 753.01 | Humid | Medium dense | |
| Elkasiba | ELK | Middle Atlas | 32°31’ 36.41″ | 06°01’ 23.56″ | 1343 | 18.37 | 429.36 | Sub-humid | Medium dense |
| Bin El Ouidane | BE | 32°05’42.86″ | 06°29’ 20.92″ | 1135 | 14.43 | 388.67 | Semi-arid | Less dense | |
| Timoulilt | Tim | 32°12’ 03.98″ | 06°24’ 58.39″ | 1084 | 14.43 | 388.67 | Semi-arid | Less dense | |
| Oum Errabia | OUM | 33°02’ 08.91″ | 05°26’ 46.56″ | 1458 | 15.98 | 618.96 | Sub-humid | Medium dense | |
| Benslimane | BS | Central Plateau | 33°39’ 28″ | 07°02’ 59″ | 277 | 18.2 | 489.5 | Semi-arid | Less dense |
| Ouelmès | OUL | 33°28’41.15″ | 06°06’ 44.22″ | 929 | 18.23 | 474.7 | Sub-humid | More dense |
T (°C): Average annual temperature in °C (Faida el al., 2019); Pr (mm): Average annual precipitation in mm (Faida et al., 2019).
Table 2. Analysis of variance (one-way ANOVA) of the diameters of inhibition zones of the bacteria tested.
| Bacteria | Concentration (mg/ml) |
F Statistics | P level |
| Escherichia coli | 100.00 | 4.65 | 0.0001*** |
| 50.00 | 5.07 | 0.0001*** | |
| 25.00 | 4.75 | 0.0001*** | |
| 12.50 | 164.740 | 0.0001*** | |
| Citrobacter freundii | 100.00 | 1.52 | 0.166 |
| 50.00 | 1.64 | 0.129 | |
| 25.00 | 3.78 | 0.001*** | |
| 12.50 | 168.7 | 0.000*** | |
| Enterococcus faecalis | 100.00 | 2.58 | 0.016** |
| 50.00 | 2.7 | 0.012** | |
| 25.00 | 3.63 | 0.002*** | |
| 12.50 | 136.53 | 0.000*** | |
| Klebsiella pneumoniae | 100.00 | 1.02 | 0.448 |
| 50.00 | 2.59 | 0.015** | |
| 25.00 | 3.17 | 0.004*** | |
| 12.50 | 80.060 | 0.0001*** |
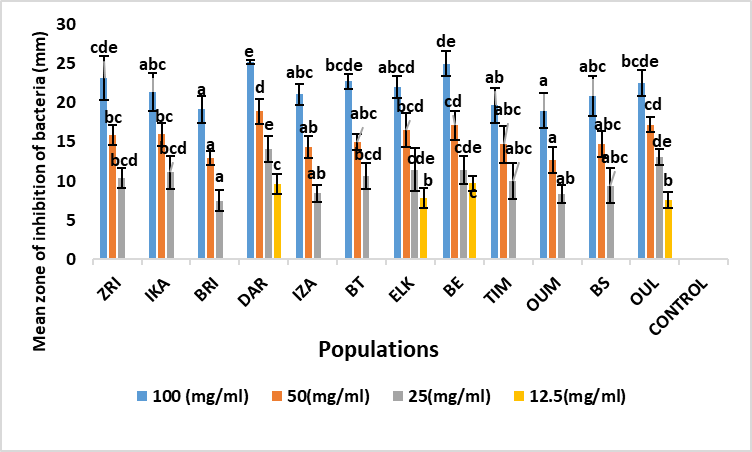
Fig .1. Mean zone of inhibition of A. unedo leaf extracts against Escherichia coli (mm). Values marked by the same letter, are not significantly different (p <0.05) using Duncan post hoc test (p <0.05).
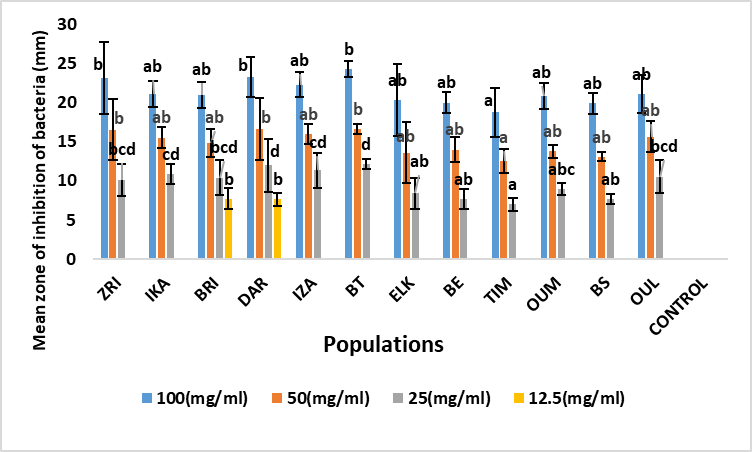
Fig. 2. Mean zone of inhibition of A. unedo leaf extracts against Citrobacter freundii (mm). Values marked by the same letter, are not significantly different (p <0.05) using Duncan post hoc test (p <0.05).
Escherichia coli (C1), Citrobacter freundii (C1 and C3) and Enterococcus faecalis (C1 and C3). The second component (PC2) explained 22.81% of the total variation associated with the diameters of the inhibition zones of Escherichia coli (C3) and Klebsiella pneumoniae (C1 and C3). Precipitation is negatively correlated with the second component. The rest of the variables are linked to the other components.
The analysis of the PCA results shows the grouping of populations into three groups (Figure 5). Group 1 (G1) is represented by a mixture of Rif populations (DAR) characterized by a subhumid bioclimate and high precipitation (753.01mm), a Rif population (IKA, subhumid, 706.21mm), and Plateau Central (OUL, semi-arid, 489.5mm). These populations developed under moderate humid climatic conditions are characterized by a high extract yield with an activity of the zones of high inhibition of the bacterial activity studied of Escherichia coli, Enterococcus faecalis and Klebsiella pneumoniae. The second group (G2) brings together the BT populations of the Rif, ZRI of Rif. This group is characterized by high precipitation, and high antibacterial activity against Citrobacter freundii. The third group (G3) is represented by three populations from the Middle Atlas (TIM, ELK and OUM), an OUL population from the Central Plateau and two populations from the Rif (IZA and BRI). This group 3 generally brings together populations collected from high altitudes with a weak antibacterial effect of leaf extracts. This suggests that the antioxidant compounds from populations of high altitude and more humid origins have small diameters of the inhibition zones against the four strains tested (Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Citrobacter freundii). Extracts from populations originating from low altitudes with moderate precipitation record high antibacterial effects.
Discussion
The present work determines environmental factors and evaluates the effect of the origin of leaf extracts from natural populations of the Moroccan arbutus tree on the antibacterial activity of potentially pathogenic strains (Escherichia coli CIP 54127, Klebsiella pneumoniae ATCC 13883, Citrobacter freundii ATCC 8090, Enterococcus faecalis ATCC 19433). The results show a variation in antibacterial activity depending on the concentration of the extract and depending on the origin of the plant material used for the preparation of the methanolic extracts.
The evaluation of the antibacterial activity shows that the methanolic extracts of the strawberry tree are effective against the four tested pathogenic strains (Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Citrobacter freundii). This notable antibacterial activity of Arbutus leaf extracts is due to their
Table 3. Mean and standard deviation of MIC and MBC of methanolic extracts from leaves of natural populations of Moroccan strawberry tree.
| Population | Escherichia coli | Citrobacter freundii | Enterococcus faecalis | Klebsiella pneumoniae | ||||
|---|---|---|---|---|---|---|---|---|
MIC (mg/ml) |
MBC (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | MIC (mg/ml) | MBC (mg/ml) | |
| ZRI | 12.5±0.00a | 25.00±0.00ab | 15.63±6.25a | 25.00±0.00a | 12.5±0.00a | 25.00±0.00a | 37.5±14.43b | 37.5±14.43ab |
| IKA | 15.63±6.25a | 31.25±12.5ab | 15.63±6.25a | 31.25±12.5a | 12.5±0.00a | 25.00±0.00a | 12.5±0.00a | 25.00±0.00a |
| BRI | 18.75±7.22a | 37.5±14.43b | 18.75±7.22a | 37.5±14.43a | 18.75±7.22a | 37.5±14.43ab | 15.63±6.25a | 31.25±12.5ab |
| DAR | 12.5±0.00a | 15.63±6.25a | 12.5±0.00a | 25.00±0.00a | 12.5±0.00a | 25.00±0.00a | 15.63±6.25a | 25.00±0.00a |
| IZA | 18.75±7.22a | 31.25±12.5ab | 18.75±7.22a | 31.25±12.5a | 18.75±7.22a | 37.5±14.43ab | 12.5±0.00a | 25.00±0.00a |
| BT | 12.5±0.00a | 25.00±0.00ab | 12.5±0.00a | 25.00±0.00a | 12.5±0.00a | 25.00±0.00a | 12.5±0.00a | 25.00±0.00a |
| ELK | 18.75±7.22a | 37.5±14.43b | 21.88±6.25a | 37.5±14.43a | 18.75±7.22a | 37.5±14.43ab | 15.63±6.25a | 31.25±12.5ab |
| BE | 18.75±7.22a | 31.25±12.5ab | 18.75±7.22a | 31.25±12.5a | 15.63±6.25a | 43.75±12.5b | 12.5±0.00a | 25.00±0.00a |
| TIM | 18.75±7.22a | 37.5±14.43b | 18.75±7.22a | 37.5±14.43a | 18.75±7.22a | 25.00±0.00a | 18.75±7.22a | 31.25±12.5ab |
| OUM | 12.5±0.00a | 25.00±0.00ab | 21.88±6.25a | 43.75±12.5a | 18.75±7.22a | 37.5±14.43ab | 21.88±6.25a | 43.75±12.5b |
| BS | 18.75±7.22a | 37.5±14.43b | 18.75±7.22a | 37.5±14.43a | 12.5±0.00a | 25.00±0.00a | 18.75±7.22a | 37.5±14.43ab |
| OUL | 12.5±0.00a | 25.00±0.00ab | 18.75±7.22a | 31.25±12.5a | 15.63±6.25a | 31.25±12.5ab | 15.5±6.34a | 31.25±12.5ab |
| F statistique (P level) | 1.323 (0.252) |
1.745 (0.102) |
0.970 (0.490) |
1.082 (0.402) |
1.259 (0.287) |
2.083 (0.048) |
4.602 (0.000***) |
1.553 (0.156) |
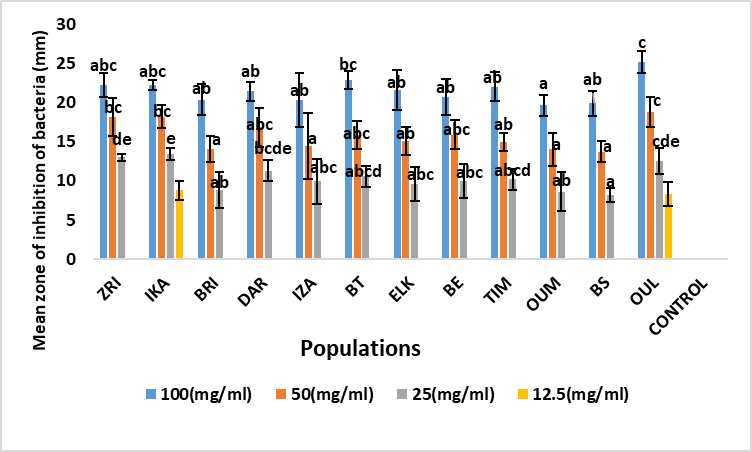
Fig. 3. Mean zone of inhibition of A. unedo leaf extracts against Enterococcus faecalis (mm). Values marked by the same letter, are not significantly different (p <0.05) using Duncan post hoc test (p <0.05).
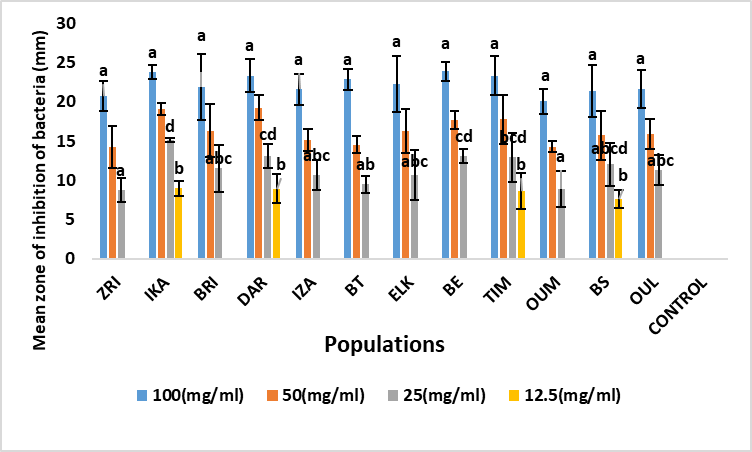
Fig. 4. Mean zone of inhibition of A. unedo leaf extracts against Klebsiella pneumoniae (mm). Values marked by the same letter, are not significantly different (p <0.05) using Duncan post hoc test (p <0.05).
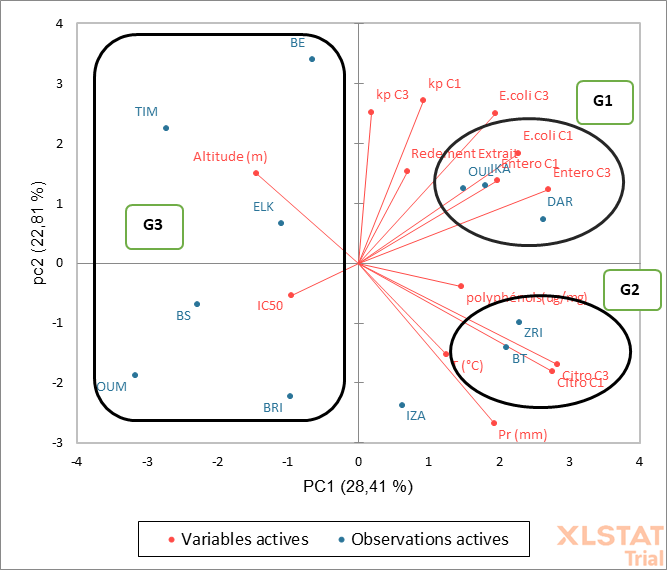
Fig. 5. Spatial projection of study populations in relation to the matrix study variables defined by the first two principal components (axis 1: 28.41%; axis of 2: 22.81%). *E. coli: Escherichia coli – Citro : Citrobacter freundii – Entero: Enterococcus faecalis. Kp: Klebsiella pneumoniae - T (°C) : temperature in degrees Celsius - Pr(mm): precipitation in millimeters.
richness in bioactive phytochemical compounds such as phenolic compounds, flavonoids, etc. (Bouyahya et al., 2016). Furthermore, antimicrobial properties have been correlated with such compounds, notably flavonoids, in various works and for several plant extracts (Hernández et al., 2000; Cushnie and Lamb, 2005; Pereira et al., 2006, 2007; Oliveira et al., 2008). Klebsiella pneumoniae was sensitive to our extracts, in agreement with previous studies on arbutus leaf extracts. These studies showed that the Klebsiella pneumoniae strain responsible for urinary infections is very sensitive to arbutus extracts with very low MIC values, ranging from 0.108 mg/ml to 6.4 mg/ml (Dib et al., 2010; Ferreira et al., 2012 and Jurica et al., 2017). Therefore, we conclude
that the leaves of the strawberry tree could be an important source of active ingredients to treat infections caused by this bacteria. The extracts from our study also revealed greater antibacterial activity against Enterococcus faecalis compared to that observed in previous studies (Ferreira et al., 2012 and Jurica et al., 2017).
The results of the diameters of the inhibition zones of the four bacteria show that the origin has a significant effect (P<0.05) on the antibacterial activity. This variation could be explained by the diversity in quality and quantity of phenolic compounds present in each extract, as well as the interaction between the bacteria and the compound. Indeed, the mechanisms of inhibition of bacteria vary depending on the type and structure of the polyphenols, as well as the targeted bacterial species (Lobiuc et al., 2023; Makarewicz et al., 2021). The results also reveal that at the minimum concentration of 25 mg/ml, the diameters of the inhibition zones for most populations is greater than 9 mm, indicating a significant inhibitory effect at this concentration. Therefore, to reinforce this effect rather than increasing the concentration of the extract, it would be better to select the population, for which the observed diameter is the greatest. Consequently, the DAR population could be considered as the most effective population against the tested bacteria, due to its highest inhibition zone diameters.
It should be noted that the effect of the origin of the extracts studied has no notable effect on the antibacterial activity in the case of high concentrations of 100 mg/ml, and in the case of use of the MIC and MBC methods. The MIC values of Escherichia coli range from 12.5±0.00 to 18.75±7.22 mg/ml and the MIC values range from 25.00±0.00 to 37.5±14.43 mg/ml. These values are lower than that obtained by Doudach et al. , 2023 (MIC=25 mg/ml; MBC=50mg/ml). While it is higher than that obtained by Dib et al., 2010 (MIC=2.2 mg/ml), Malheiro et al., 2012 (MIC=2.5 mg/ml), Jurica et al., 2017 (MIC=6.4 mg/ml; MBC=51.2 mg/ml) and Bouyahya et al., 2016 (MIC=8 mg/ml; MBC> 8mg/ml). On the other hand, there was no antimicrobial activity against E. coli in the studies carried out by Ferreira et al. 2012 and Oral et al. 2011. So we can explain the effect of the origin of population on the activity. antibacterial to the MIC method, the phenolic composition of the extracts, and the types of extraction solvents.
The population structure of natural Moroccan Arbutus is determined by principal component analysis, based on total polyphenols, antioxidant activity, antibacterial activity and environmental conditions of the sites. Three majority groups are defined in the present study. Our results show a separation of populations, which means that the origin of the plant material has an effect on the variables studied. The first group (G1) is positively and significantly correlated with the diameters of the inhibition zones of Escherichia coli and Enterococcus faecalis (for 100 mg/ml and 25 mg/ml). It is also positively and moderately correlated with the diameters of the inhibition zones of Klebsiella pneumoniae (for 100mg/ml and 25mg/ml). This indicates that this group is composed of populations which synthesize phenolic compounds active against the following bacteria: Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae. The second group (G2) group is characterized by high precipitation, and high antibacterial activity only against Citrobacter freundii. This means that there is a positive correlation between precipitation and the inhibitory effect against Citrobacter freundii. The third group (G3) is represented by a negative correlation for all the studied variables. So, we can say that the environmental conditions of the populations grouped in this group are not very favorable for synthesizing the phenolic compounds that are very active against the tested bacteria. The Middle Atlas BE population constitutes a group isolated from the rest of the populations and it is positively correlated with the second component, presenting high antibacterial activity against Klebsiella pneumoniae.
Genetic factors, edaphic and climatic conditions associated with each site could affect the quantity and quality of phenolic compounds synthesized by the plant (Salim et al., 2024). The variation of these compounds could indirectly affect the antibacterial activity of the plant extracts. Several studies show that antimicrobial activity is correlated with phenolic compounds in particular: arbutin and hydroquinone which are the major compounds present in strawberry tree, phenolic acids and esters, which inhibit the growth of bacteria and fungi (Huang et al., 2010; Silici et al., 2007). In various works and for several plant extracts, various classes of flavonoids present antimicrobial properties (Hernández et al., 2000; Cushnie and Lamb, 2005; Pereira et al., 2006, 2007; Oliveira et al., 2008, Salim et al., 2024).
In conclusion, the determination of the effect of the environment on the phytochemical composition of Arbutus leaf extracts, and indirectly on the inhibition activity of the antibacterial zone of the strains studied, is recomended for the selection of elite natural populations extract for applications in the food, therapeutic and pharmaceutical fields.
Materials and methods
Plant materials
The samples were collected systematically between December 2021 and February 2022. Twelve natural populations of strawberry tree were sampled from different biogeographical regions in Morocco (Pre-Rif, Western Rif, Central Plateau, and Middle Atlas). Table 1 summarizes the geographic and ecological characteristics of the various natural populations studied. For each population, tree leaves, aged almost 60 years (Personal discussion with the managers of the water and forestry agency in Morocco) were collected randomly. A number of thirteen trees was collected per population. The collected plant material (≈ 60 years) was dried away from light and moisture, at room temperature. Subsequently, the dried leaves were carefully stored in paper bags, separated by tree/population/region, in a dry place for use in preparing the extracts. Six replicates of each tree per population were considered for the preparation of methanolic extracts.
Preparation of plant extracts
The extraction was performed following the protocol of Bouyahya et al. (2016). Extraction was carried out through maceration, where 3 grams of dried strawberry tree leaf powder were placed in a flask containing 20 ml of methanol at room temperature for 3 days with daily agitation. The plant extract was filtered through filter paper (Whatman) and the methanolic solution was evaporated on a rotary evaporator to obtain a crude extract. Extracts were stored at 4°C until use.
The preparation of extract concentrations for antibacterial activity evaluation consists in taking 100 mg of each extract and solubilizing it in 1 ml of dimethyl sulfoxide (DMSO). Then dilutions (1/2, 1/4, 1/8) are prepared in DMSO. The final concentration of extracts ranged from 100 mg/ml to 12.5 mg/ml.
Bacterial strains and preparation of their suspension
To test the antibacterial activity of methanolic extracts of Arbutus unedo L., four strains of pathogenic reference were used. Three Gram-negative strains are: Escherichia coli CIP 54127, Klebsiella pneumoniae ATCC 13883, Citrobacter freundii ATCC 8090. One Gram-positive strain is Enterococcus faecalis ATCC 19433. The strains were identified, maintained and preserved in the agro-industrial and medical biotechnologies laboratory, Faculty of Science and Technology, Sultan Moulay Slimane University, Béni-Mellal, Morocco.
The strains are conserved on nutrient agar medium at 4°C. Before use, the bacteria are transferred to the nutrient agar and incubated in an oven at 37°C for 24 hours. In order to reactivate them and obtain young cultures which will be used to prepare the inoculum. The bacterial suspension is prepared from young cultures in physiological water with 0.9% NaCl. It is then adjusted to the Mc Farland 0.5 standard using a spectrophotometer, corresponding to an optical density (OD) between 0.08 and 0.1 read at 620 nm, which corresponds to a suspension containing around 108 CFU/ml.
Evaluation of antibacterial activity
Disk diffusion method
The agar disk diffusion method was used to determine the antibacterial activities of methanolic extracts from arbutus leaves (El Hartiti et al., 2020). Briefly, 0.1 ml of a 108 CFU/ml bacterial suspension was spread on Mueller Hinton (MHA) agar plates (60mm). Whatman paper discs (6 mm in diameter) were impregnated with 15 μl of test extract and placed on the inoculated plates. These plates were then left at room temperature for 1 h to pre-diffuse the extract, before being incubated at 37°C in the oven for 24 h. The diameters of the
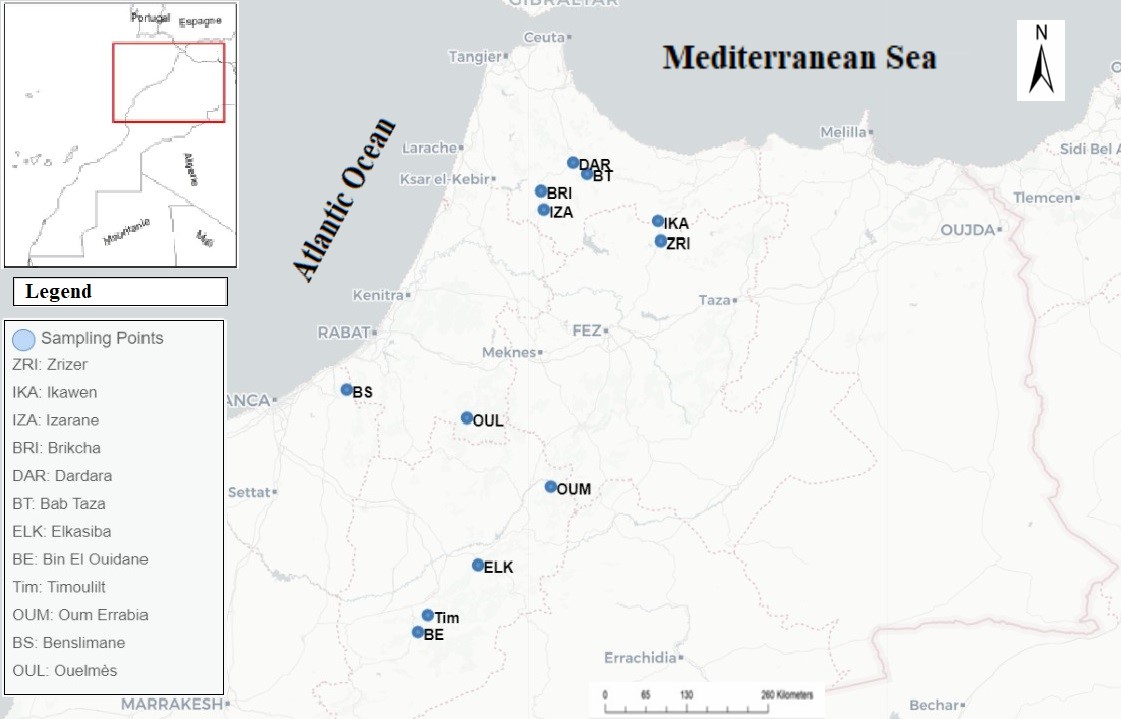
Map 1. Distribution of natural populations of Arbutus Unedo L. in Morocco collected for the study.
inhibition zones were measured in millimeters. The sensitivity to the different extracts was classified according to the diameter of the inhibition zones as follows: not sensitive (-) for diameters below 8 mm; sensitive (+) for diameters from 9 to 14 mm; very sensitive (++) for diameters from 15 to 19 mm; and extremely sensitive (+++) for diameters above 20 mm (Ponce et al., 2003).
Determination of Minimum Inhibitory Concentration (MIC)
The determination of MIC is carried out by microdilution method in medium, using a sterile 96-well microplate (8 × 12 wells) (Balouiri et al 2016). The extract was prepared at a concentration of 100 mg/mL in dimethyl sulfoxide (DMSO), followed by a series of one-half dilutions in the Muller-Hinton broth (MHB), by adding 100 μl of the extract into 100 μl of Muller-Hinton broth. The final concentration of extracts ranged from 50 mg/ml to 0.4 mg/ml. The inoculum is prepared in the same manner as described previously for the disk diffusion test in solid medium and used to inoculate the microplates with a volume of 100 μl for each well, resulting in a final volume of 200 μl for each well. In parallel three negative controls are prepared in the first three lines, the first line contains 200 μl of sterile BMH is served as sterility control (Ts), the second line contains 100 μl of BMH and 100 μl of bacterial suspension is served as growth control (Tc) and the third line contains DMSO diluted in BMH and 100 μl of bacterial suspension to confirm that DMSO has no antibacterial effect. The microplates were incubated for 24 hours at 37°C. After this incubation time, observation was made with the naked eye, and the lowest concentration for which no bacterial growth was observed corresponded to the Minimum Inhibitory Concentration (MIC).
Determination of Minimum Bactericidal Concentration (MBC)
The Minimum Bactericidal Concentration (MBC) was determined after reading the MIC by inoculating the media of wells where no visible growth was observed with the naked eye on Mueller-Hinton agar plates and incubating them for 18 to 24 hours. The MBC was defined as the lowest concentration of samples tested giving negative subcultures on solid medium.
Determination of total polyphenol content
Total polyphenols were determined using the Folin-ciocalteau method (Singleton and Rossi 1965) following the protocol developed by Laouicha et al. (2020). A 0.3 ml of extract (1 mg/ml) was mixed with 1.5 ml of Folin-Ciocalteu reagent for 4 minutes. Subsequently, 1.2 ml of 7.5% sodium carbonate was added. After one hour of incubation at room temperature, the absorbance was measured at 750 nm using a spectrophotometer. UV-Visible spectrophotometer with automatic wavelength selection and the wavelength range is 190-1100 nm. All tests were performed in triplicate, and the concentration of total phenolic compounds was expressed as microgram gallic acid equivalent per milligram extract (μg GAE/mg extract).
Determination of antioxidant activity
The analysis of the anti-radical activity of the methanolic extract of the strawberry tree on DPPH was carried out following the protocol of Bouyahya et al. (2016). In tubes, 0.3 ml of different concentrations (200, 100, 50, 25, 12.5 μg/ml) of each extract were introduced and 2.7 ml of freshly prepared methanolic DPPH solution (0.1 mM DPPH) were added. Mixtures were vigorously vortexed, and placed in the dark at room temperature for 30 min. After incubation, the color change was measured by absorbance at 517 nm.
The anti-free radical activity of the extracts was expressed as IC50 (concentration enabling 50% inhibition of free radicals). The mean IC50 values were calculated by linear regressions of the three separate assays, where the abscissa is represented by the extract concentration tested and the ordinate by the percentage inhibition (PI) of the DPPH radical, which is calculated by the following formula:
PI % = [(Abs Control negative- Abs Sample)/Abs Control negative] X 100
With PI %: Percentage of free radical scavenging activity; Abs Sample: Absorbance of sample; Abs Negative Control: Absorbance of negative control (DPPH solution only).
Ascorbic acid was used as a positive control to evaluate the antioxidant activity of the extracts studied.
Statistical analysis
The data obtained were subjected to statistical analysis to define the variability of antibacterial activities under the effects of methanolic extracts of arbutus leaves collected from natural populations in Morocco, and the mechanisms of action of the extracts on this antibacterial activity. The descriptive statistics were used to calculate the levels of variation of the means by calculating the standard deviation (±). One-way analysis of variance (provenance effect) was used to compare means for inhibition diameters, MIC and MBC at different concentrations. All the traits studied were used for principal component analysis (PCA) and hierarchical classification on the matrix of their mean values. The statistical analyses were carried out using SPSS version 26 and XLSTAT soft.
Acknowledgements
We thank the dean of the Faculty of Science and Technology of Béni Mellal for the means of transport in order to carry out the collection of samples. We also strongly thank Professor WAHID N. for the personal funding for the travel and for the costs of collecting plant material and laboratory products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to
influence the work reported in this paper.
References
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 6(2): 71-79.
Bebek Markovinović A, Brčić Karačonji I, Jurica K, Lasić D, Skendrović Babojelić M, Duralija B, Šic Žlabur J, Putnik P, Bursać Kovačević D (2022) Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae 8(10): 881. https://doi.org/10.3390/horticulturae8100881.
Bouyahya A, Moussaoui N, Abrini J, Bakri Y, Dakka N (2016) Determination of Phenolic Contents, Antioxidant and Antibacterial Activities of Strawberry Tree (Arbutus unedo L.) Leaf Extracts. British Biotechnology Journal. 14(3): 1–10. https://doi.org/10.9734/bbj/2016/26488.
Cornaglia G (2009) Fighting infections due to multidrug-resistant Gram-positive pathogens. Clinical Microbiology and Infection. 15(3): 209-211.
Cushnie TT, Hamilton VE, Lamb AJ (2003) Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiological research. 158(4): 281-289.
Cushnie TT, Lamb AJ (2005) Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 26(5): 343-356.
De Simón BF, Sanz M, Cervera MT, Pinto E, Aranda I, Cadahía E (2017) Leaf metabolic response to water deficit in Pinus pinaster Ait. relies upon ontogeny and genotype. Environmental and Experimental Botany. 140: 41-55.
Dib MA, Paolini J, Bendahou M, Varesi L, Allali H, Desjobert JM, Costa J (2010) Chemical composition of fatty acid and unsaponifiable fractions of leaves, stems and roots of Arbutus unedo and in vitro antimicrobial activity of unsaponifiable extracts. Natural Product Communications. 5(7): 1934578X1000500721.
Di Ferdinando M, Brunetti C, Agati G, Tattini M (2014) Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environmental and Experimental Botany. 103: 107-116.
Doudach L, Mrabti HN, Al-Mijalli SH, Kachmar MR, Benrahou K, Assaggaf H, Faouzi MEA (2023) Phytochemical, antidiabetic, antioxidant, antibacterial, acute and sub-chronic toxicity of moroccan Arbutus unedo leaves. Journal of Pharmacopuncture. 26(1): 27.
El Hartiti H, El Mostaphi A, Barrahi M, Ben Ali A, Chahboun N, Amiyare R, Ouhssine M (2020) Chemical composition and antibacterial activity of the essential oil of Myrtus communis leaves. Karbala International Journal of Modern Science. 6(3): 3.
Faida R, Aabdousse J, Boulli A, Bouda S, Wahid N (2019) Ethnobotanical uses and distribution status of Arbutus (Arbutus unedo L.) in Morocco. Ethnobotany Research and Applications. 18: 1-12. https://doi.org/10.32859/era.18.30.1-10.
Ferreira S, Santos J, Duarte A, Duarte AP, Queiroz JA, Domingues FC (2012) Screening of antimicrobial activity of Cistus ladanifer and Arbutus unedo extracts. Natural Product Research. 26(16): 1558-1560.
Guaouguaou FE, Taghzouti K, Oukabli M, Masrar A, Chabraoui L, Bouabdellah M, Es-Safi NE (2019) Acute and subchronic oral and dermal toxicological studies of salvia verbenaca extracts in mice and rats. Journal of Herbs, Spices & Medicinal Plants. 25(1): 33-42.
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant, Cell & Environment. 21(6): 535-553.
Hernández NE, Tereschuk ML, Abdala LR (2000) Antimicrobial activity of flavonoids in medicinal plants from Tafi del Valle (Tucumán, Argentina). Journal of Ethnopharmacology. 73: 317–322.
Huang WY, Cai YZ, Zhang Y (2010) Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutrition and Cancer. 62: 1–20. doi:10.1080/01635580903191585
Jurica K, Gobin I, Kremer D, Čepo D V, Grubešić R J, Karačonji I B, Kosalec I (2017) Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. Journal of Herbal Medicine. 8: 17-23.
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Frontiers in Plant Science. 6: 135066.
Laouicha S, Senator A, Kherbache A, Bouriche H (2020) Total Phenolic Contents and Antioxidant Properties of Algerian Arbutus unedo L. Extracts. Journal of Drug Delivery and Therapeutics. 10(3-s): 159–168. https://doi.org/10.22270/jddt.v10i3-s.4182.
Lobiuc A, Pavăl NE, Mangalagiu I I, Gheorghiță R, Teliban GC, Amăriucăi-Mantu D, Stoleru V (2023) Future antimicrobials: Natural and functionalized phenolics. Molecules. 28(3): 1114.
Makarewicz M, Drożdż I, Tarko T, Duda-Chodak A (2021) The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants. 10(2): 188.
Malheiro R, Sá O, Pereira E, Aguiar C, Baptista P, Pereira JA (2012) Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Industrial Crops and Products. 37(1), 473-478.
Martins JF, Correia SI, Canhoto JM (2016) Somatic embryogenesis induction and plant regeneration in strawberry tree (Arbutus unedo L.). In Vitro Embryogenesis in Higher Plants. 329-339.
Martins J, Pétriacq P, Flandin A, Gomez-Cadenas A, Monteiro P, Pinto G, Canhoto J (2022) Genotype determines Arbutus unedo L. physiological and metabolomic responses to drought and recovery. Frontiers in Plant Science. 13: 1011542. https://doi.org/10.3389/fpls.2022.1011542.
Morgado S, Morgado M, Plácido A I, Roque F, Duarte AP (2018) Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. In Journal of Ethnopharmacology. 225: 90-102. https://doi.org/10.1016/j.jep.2018.07.004.
Nathan C (2004) Antibiotics at the crossroads. Nature. 431(7011): 899-902.
Nenadis N, Llorens L, Koufogianni A, Diaz L, Font J, Gonzalez J A, Verdaguer D (2015) Interactive effects of UV radiation and reduced precipitation on the seasonal leaf phenolic content/composition and the antioxidant activity of naturally growing Arbutus unedo plants. Journal of Photochemistry and Photobiology. 153: 435-444.
Oliveira I, Sousa AS, Ferreira ICFR , Bento A, Estevinho L, Pereira JA (2008) Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) greens husks. Food Chemistry and Toxicology. 46: 2326–2331.
Orak HH, Yagar H, Isbilir SS, Demirci AŞ, Gümüş T, Ekinci N (2011) Evaluation of antioxidant and antimicrobial potential of strawberry tree (Arbutus unedo L.) leaf. Food Science and Biotechnology. 20: 1249-1256.
Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade P B, Ferreira, ICFR, Ferreres F, Bento A, Seabra R, Estevinho L (2007) Walnut (Juglans regia L.) leaves: phenolic compounds, antimicrobial activity and antioxidant potential of different cultivars. Food Chemistry and Toxicology. 45: 2287–2295.
Pereira JA, Pereira APG, Ferreira ICFR, Valentão P, Andrade PB, Seabra R, Estevinho L, Bento A (2006) Table olives from Portugal: phenolic compounds, antioxidant potential and antimicrobial activity. Journal of Agriculture, Food and Chemistry. 54: 8425–8431.
Ponce AG, Fritz R, Del Valle C, Roura SI (2003) Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT-Food Science and Technology. 36(7): 679-684.
Rhattas M, Douira A, Zidane L (2016) Étude ethnobotanique des plantes médicinales dans le Parc National de Talassemtane (Rif occidental du Maroc). Journal of Applied Biosciences. 97(0): 9187. https://doi.org/10.4314/jab.v97i1.5
Salim N, Oussif I, Hamamouch N, Wahid N (2024) Comparative Study of Antioxidants in Methanolic Extracts Fruit of Natural Populations of Myrtus communis L. in Morocco in Relation to Fruit Size and Environmental Effects. Ecological Engineering & Environmental Technology. 25(5): 1–13.
Silici S, Ünlü M, Vardar-Ünlü G (2007) Antibacterial activity and phytochemical evidence for the plant origin of Turkish propolis from different regions. World Journal Microbiolgy and Biotechnology. 23: 1797–1803. doi:10.1007/s11274-007-9430-7
Süntar I (2020) Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochemistry Reviews. 19(5): 1199-1209.
Žlabur JŠ, Bogdanović S, Voća S, Babojelić MS (2020) Biological Potential of Fruit and Leaves of Strawberry Tree (Arbutus unedo L.) from Croatia. Molecules. 25(21): 5102. https://doi.org/10.3390/molecules25215102