

Aust J Crop Sci. 19(01):10-17 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.01.p105
Antifungal and antiocratoxigenic effects of the essential oils of Rosmarinus officinalis and Callistemon viminalis on fungi of the genus Aspergillus
Carolina Salles Freire1, Maria das Graças Cardoso*1, Eduardo Alves, Gabriela Aguiar Campolina3, Gislaine Cristina Peixoto de Carvalho1, Luís Roberto Batista3
1Chemistry Department, Federal University of Lavras (UFLA), Lavras, MG, Brazil
2Department of Phytopathology, Federal University of Lavras (UFLA), Lavras, MG, Brazil
3Food Sciences Department, Federal University of Lavras (UFLA), Lavras, MG, Brazil
*Corresponding author: Maria das Graças Cardoso 
ORCID: https://orcid.org/0000-0001-8075-1725
Abstract: The chemical constituents of essential oils exhibit significant biological activities and can be used to inhibit insects and microorganisms. The objectives of the study were to extract and characterize the essential oils of Rosmarinus officinalis and Callistemon viminalis and to evaluate their antifungal and anticratoxigenic potential against Aspergillus ochraceus, Aspergillus carbonarius and Aspergillus westerdijkiae. The essential oils were obtained by hydrodistillation and characterized by GC–MS and GC–FID. The antifungal and anticratoxigenic activities were determined by the fumigation method. Scanning electron microscopy was used to analyse the effects of essential oils on the fungal cell membrane. The major compounds found were 1,8-cineole and camphor (R. officinalis) and 1,8-cineole and α-pinene (C. viminalis). The mycelial growth of the studied fungi was significantly inhibited by the essential oils and 1,8-cineole at the concentrations tested. A reduction in the synthesis of ochratoxin A for the studied fungi was observed in the presence of the essential oils and 1,8-cineole. The electron micrographs showed deleterious effects on fungal morphology after the treatments. The results suggest that the essential oils of R. officinalis and C. viminalis have antifungal and anticratoxigenic potential against toxigenic fungi that contaminate food.
Keywords: Aspergillus carbonarius; Aspergillus ochraceus; Aspergillus westerdijkiae; mycotoxins; ochratoxin A.
Abbreviations: FID_flame ionization detector; GC_gas chromatography; MS_mass spectrometer; OTA_ochratoxin A; SEM_scanning electron microscopy.
Introduction
Food contamination is a major threat to food safety and a worldwide problem endangering human and animal health. According to the World Health Organization (WHO), approximately 600 million people fall ill each year due to the consumption of contaminated food (FAO and WHO, 2018). Fungal species are among the main causes of contamination in food, leading to its deterioration. These microorganisms are still capable of producing mycotoxins (Silva et al., 2020).
Mycotoxins are secondary metabolites produced by filamentous fungi, and the genus Aspergillus is one of the main producers. These compounds have high toxicity, causing acute and chronic problems for human and animal health, in addition to resulting in economic losses. In foods, mycotoxin production can occur in the soil during harvest, processing, storage, and transportation (Brandão et al., 2021). Among the mycotoxins produced by the genus Aspergillus, aflatoxins and ochratoxin A (OTA) stand out, being considered carcinogenic and possibly carcinogenic to humans, respectively, according to the International Agency for Research on Cancer (IARC) (International Agency for Research on Cancer – IARC, 2012).
Aspergillus ochraceus, Aspergillus carbonarius and Aspergillus westerdijkiae produce OTA in grapes, coffee, wine and cereals (Iamanaka et al., 2010). Several measures have been applied to prevent and control the growth of these fungi, as well as inhibit the production of mycotoxins. The use of synthetic chemicals is the most common way to reduce food contamination. However, the continuous and exacerbated use of these synthetic products can increase the risk of toxic residues in food and in the environment and may cause resistance of microorganisms (Hua et al., 2014; Moon et al., 2016).
The search for alternative methods to synthesize chemical additives highlights natural resources. Essential oils, formed by a complex mixture of organic compounds, stand out for having proven biological activities (Bakkali et al., 2008). The diverse chemical composition of essential oils guarantees fungicidal and bactericidal potential, hindering the selection of resistant pathogens (Brandão et al., 2020; Camargo et al., 2020). Furthermore, essential oils have lower toxicity than synthetic substances, with relative food safety, because their chemical compounds are, for the most part, classified as agents generally recognized as safe (GRAS) by the Food and Drug Administration (FDA).
Among the numerous plants that produce essential oils, Rosmarinus officinalis, belonging to the Lamiaceae family, and Callistemon viminalis, belonging to the Myrtaceae family, present as major constituents 1,8-cineole, camphor, α-pinene, borneol and limonene (Caetano et al., 2022; Lunguinho et al., 2021). Studies have reported the biological potential of these species, especially in fungal control, promoting great interest in the food, pharmaceutical and agricultural industries (Mekonnen et al., 2016; Sales et al., 2017).
The present study aimed to extract and chemically characterize the essential oils of R. officinalis and C. viminalis, to evaluate their antifungal and antimycotoxigenic activities on A. ochraceus, A. carbonarius and A. westerdijkiae and to analyse their effects on the morphology of both. the fungi.
Results and Discussion
Moisture content, yield and chemical characterization of essential oils
The moisture content and the yield value of the essential oils extracted from R. officinalis and C. viminalis are described in Table 1.
According to the data described in Table 1, the yields of essential oils in the moisture-free base of R. officinalis and C. viminalis were 0.85% and 1.02%, respectively.
Moussii et al. (2020) and Al Zuhairi et al. (2020) studied the essential oil of fresh leaves of R. officinalis and obtained 1.35% and 0.98% yields, respectively. These values were higher than those found in the present study. Sales et al. (2017) and Lunguinho et al. (2021), in turn, evaluated the yield of essential oil of C. viminalis and obtained values of 1.14% and 0.94%, respectively, which corroborate those obtained in the present study.
The chemical composition of the essential oils of R. officinalis and C. viminalis is presented in Table 2. Sixteen chemical constituents were identified for R. officinalis, and 11 chemical constituents were identified for C. viminalis.
The major constituent of the essential oil of R. officinalis was 1,8-cineole (49.24%), followed by camphor (17.28%), α-pinene (8.24%) and borneol (5.33%). The main components of the essential oil of C. viminalis were 1,8-cineole (56.70%), α-pinene (15.32%), limonene (8.60%) and α-terpineol (6.50%). The essential oils of R. officinalis and C. viminalis presented similar constituents, differing in the percentages and in the compounds present in smaller amounts.
The data obtained for the essential oil of R. officinalis corroborate those found by Rezende et al. (2017), who studied the essential oil of R. officinalis and found the same main components, but at different concentrations, being 62.26% 1,8-cineole, 17.34% camphor and 9.07% α-pinene. Al Zuhairi et al. (2020) studied the essential oil of R. officinalis and identified 10 compounds, the majority being 1,8-cineole (17.16%), α-pinene (16.95%) and verbenone (15.78%), differing in percentage and in some compounds found in the present study.
Lunguinho et al. (2021) evaluated the main constituents of the essential oil of C. viminalis and obtained 1,8-cineole (78.10%), α-pinene (12.50%) and limonene (3.36%). These compounds corroborate those found in this study, with variation in percentages. Sales et al. (2017) also studied the essential oil of C. viminalis, obtaining 1,8-cineole (84.60%), α-pinene (10.28%) and α-terpineol (2.59%) as major constituents.
These differences observed in the yield values and chemical characterization of essential oils in the present study can be explained by several factors, such as place, time and time of collection of plant material. In addition, the soil type and nutrients present there interfere with the production of secondary metabolites of the species. The stage of plant development, site humidity, temperature and other factors may also influence the biosynthesis of secondary metabolites (Gobbo-Neto and Lopes, 2007).
Antifungal activity
The effect of different concentrations of the essential oils of R. officinalis and C. viminalis and of the monoterpene 1,8-cineole on the mycelial growth of the fungi A. ochraceus, A. carbonarius and A. westerdjikae is shown in Figure 1. The results indicated a fungistatic effect, in which the lowest concentrations showed significant inhibition of mycelial growth (p < 0.05) compared to the negative control effect.
For the fungus A. ochraceus, all treatments inhibited mycelial growth. At the highest concentration studied (3000 µL. L -1), the essential oil of C. viminalis showed the highest percentage of inhibition (68.89%), but there was no significant difference with the monoterpene 1,8-cineole (66.30%). The essential oil of R. officinalis differed statistically from that of C. viminalis, showing a percentage of mycelial inhibition of 65.00%.
The fungus A. carbonarius was more sensitive to treatment with the essential oil of R. officinalis, showing 55% inhibition of mycelial growth at a concentration of 3000 µL. L -1. This value differed statistically from the treatments with C. viminalis essential oil (47.50%). and with the monoterpene 1,8-cineole (39.17%). In the mycelial growth inhibition test for A. westerdjikae, it was observed that the monoterpene 1,8-cineole and the essential oil C. viminalis showed statistically the highest percentages of inhibition, 56.67% and 56.18%, respectively, for the highest concentration studied.
These results for the three Aspergillus species show that the concentrations studied by the fumigation method were not dose-dependent at each concentration increase, demonstrating that working at lower concentrations ensures significant percentages of mycelial growth inhibition.
The results obtained indicate that the antifungal activity of essential oils is linked to their chemical constituents but that those present in higher concentrations are not always responsible for the antifungal action, and it is possible that minor substances or even the ability to have a synergistic effect are present. and interfere with the final action (Baldim et al., 2018).
The mechanism that explains the antifungal action of the constituents of essential oils has not yet been fully elucidated. Studies have described that the lipophilic nature of essential oil compounds and their low molecular weight allow them to cross the lipid layer of the fungal cell membrane, causing damage to its integrity (Kisová et al., 2020; Lyu et al., 2019). In addition, the functional groups present in the structure of the essential oil molecules also interfere with their antimicrobial effect. Constituents with functional groups such as alcohols, phenols, aldehydes, ketones, ethers and esters can interact with the fungal cell membrane, inducing damage to the microorganism (Yang et al., 2023; Kalemba and Kunicka, 2003).
Thus, exposure of the fungus to essential oil compounds can cause changes in membrane permeability, resulting in extravasation of vital cellular constituents (Ca 2+, Mg 2+ and K+), reduced ergosterol biosynthesis, altered fluidity and morphology, exacerbated production of reactive oxygen species and DNA damage. All these factors, individually or in combination, can cause fungal cell death (Chaudhari et al., 2019; Brandão et al., 2020; Kumar et al., 2022; Tian et al., 2012).
However, the direct use of essential oils in food preservation has certain limitations because of their high volatilities, characteristic aromas and flavors, and instability in the presence of light and heat. Thus, the nanoencapsulation of volatile compounds present in essential oils emerges as an option to extend their efficacy and enhance their utilization. Qiu et al. (2017) observed that the antioxidant and antimicrobial activities of nanoparticles containing essential oil were prolonged. Brandão et al. (2023) evaluated the antifungal potential of grape packaging containing nanofibers encapsulated with essential oil from Ocimum gratissimum L. and Ocimum basilicum L. and observed that these nanofibers preserved the quality and shelf life of the fruits for a longer period. Hasheminejad et al. (2019) observed that the encapsulation of clove essential oil in chitosan nanoparticles provided a controlled release of the oil, as well as greater antifungal activity than free oil.
Antiocratoxigenic activity
The results for the inhibition of the synthesis of ochratoxin A of A. ochraceus, A. carbonarius and A. westerdjikae by the essential oils R. officinalis and C. viminalis and by the monoterpene 1,8-cineole are presented in Table 3. Significant (p < 0.05) inhibition of OTA synthesis in all treatments under study when compared to the fungal control (sample containing only the fungus in the culture medium).
Combining the values obtained with the fungal control, A. ochraceus inhibited OTA production ranging from 24.69% to 53.66% for the three treatments. With A. carbonarius, there was a variation of 32.51% to 91.00% in the reduction of OTA synthesis, while the amount of OTA inhibited for A. westerdijkiae had values that ranged from 41.99% to 64.09%. The percentages of inhibition of the three treatments differed statistically for all fungal species studied.
Table 1. Moisture content and yield of the essential oil of R. officinalis and C. viminalis.
| Plant | Moisture (%) |
Essential oil yield (% p/p) |
|
|---|---|---|---|
| R. officinalis | 7.35 | 0.85 | |
| C. viminalis | 39.16 | 1.02 | |
Table 2. Chemical composition of the essential oil of R. officinalis and C. viminalis.
| IRtab. | IRcalc. | Compound | Peak area (%) | |
|---|---|---|---|---|
| R. officinalis | C. viminalis | |||
| 924 | 923 | α-thujene | - | 0.08 |
| 932 | 929 | α-pinene | 8.24 | 15.32 |
| 946 | 950 | Camphene | 2.80 | - |
| 974 | 979 | β-pinene | 0.41 | 0.90 |
| 998 | 998 | Myrcene | 0.65 | 0.28 |
| 1002 | 1005 | α-phellandrene | - | 0.58 |
| 1007 | 1007 | Isoamyl butyrate | - | 2.25 |
| 1014 | 1016 | α-terpinene | 0.27 | - |
| 1020 | 1019 | p-cymene | 2.44 | - |
| 1024 | 1021 | Limonene | 2.18 | 8.60 |
| 1025 | 1023 | β-phellandrene | 0.11 | - |
| 1026 | 1027 | 1,8-cineole | 49.24 | 56.70 |
| 1095 | 1101 | Linalool | 0.73 | 0.37 |
| 1141 | 1140 | Camphor | 17.28 | - |
| 1165 | 1163 | Borneol | 5.33 | - |
| 1174 | 1171 | Terpinen-4-ol | - | 1.98 |
| 1186 | 1188 | α-terpineol | 3.44 | 6.50 |
| 1298 | 1296 | Carvacrol | 0.34 | - |
| 1408 | 1402 | β-caryophyllene | 0.44 | - |
| Composition | ||||
Monoterpene hydrocarbons Oxygenated monoterpenes Sesquiterpene hydrocarbons |
17.10 76.36 0.44 |
25.76 67.80 - |
||
| Identified total (%) | 93.90 | 93.56 | ||
Comparing the results of mycelial growth inhibition of the fungus with the inhibition of OTA production, it can be observed that for A. ochraceus, the essential oil of C. viminalis showed the highest percentage of inhibition in both cases at the concentration of 3000 µL L -1 when compared to the other treatments. The same did not happen for A. carbonarius, since the monoterpene 1,8-cineole resulted in the lowest antifungal potential for the concentration of 3000 µL L -1, while for the inhibition of OTA synthesis, it showed the highest percentage (91.00%), which differed statistically from the samples with essential oil.
In addition, for A. westerdijkiae, the monoterpene 1,8-cineole had the greatest effect of inhibiting mycelial growth, a value that did not differ statistically from the essential oil of C. viminalis at the concentration of 3000 µL L -1, but for the production of OTA, this one presented a lower anticratoxigenic activity (41.99%) in relation to the other two treatments. These results show that preventing only the mycelial growth of the fungus does not imply the inhibition of OTA production. In addition, although the essential oils under study presented the same major compound (1,8-cineole), they varied in the antiocratoxigenic potential for the same microorganism. This can be explained by the presence of other compounds that probably interfere with the final activity of the essential oil.
A study by El Khoury et al. (2016) helps to understand this difference observed between the inhibition of mycelial growth and OTA production. The authors studied the effect of ten essential oils on OTA production by A. carbonarius. They observed that six of the essential oils tested, including R. officinalis oil, significantly reduced OTA production but did not equally reduce fungal growth. These results suggested that the reduction in OTA was associated with the repression of biosynthesis-related genes, such as acpks, acOTApks, acOTAnrps, laeA and veA, involved in the synthesis of appropriate enzymes for OTA biosynthesis. Likewise, Murthy et al. (2009) also observed that the reduction in A. ochraceus growth and OTA production was not linear with increasing concentrations of the ethanolic extract of Trachyspermum ammi when they used concentrations of 50 and 150 µL g -1. These authors concluded that the inhibition of OTA production was greater than the inhibition of the mycelial growth of the fungus.
According to Passone et al. (2012), Rucky et al. (2015) and Santos et al. (2010), OTA production capacity depends on several physical, chemical and biological factors, such as incubation time, water activity, temperature, pH, incidence of light, fungal lineage, and substrate composition. Passamani et al. (2014) reported that the temperature ranges for OTA production are more limited than those for fungal growth. Thus, it can be inferred that the differences observed between the mycelial growth of the fungus and OTA production are related to all these factors mentioned.
Scanning electron microscopy - SEM
The structural morphologies of A. ochraceus, A. carbonarius and A. westerdijkiae treated and untreated with the essential oils of R. officinalis and C. viminalis and with the monoterpene 1,8-cineole are shown in Figure 2.
The fungal controls A. ochraceus (Fig. 2A), A. carbonarius (Fig. 2E) and A. westerdijkiae (Fig. 2I) exhibited regular morphology, with development of conidia and conidiophores. The hyphae exhibited uniform, smooth, robust, constant diameter, linear and regular characteristics.
Several morphological changes were observed in the samples treated with the essential oils of R. officinalis and C. viminalis and the standard 1,8-cineole when compared to the fungal controls. It can be noted that there was loss of integrity of the conidial head, with ruptures (Fig. 2B, C, F, H, J and K) and nonformation (Figs. 2D, G and L). The hyphae also showed morphological deformation after the treatments, with smaller diameters than the controls and characteristic wrinkled, flattened, collapsed and empty hyphae (Fig. 2B, D, G, H and L).
Brandão et al. (2021) and Rezende et al. (2021) found results similar to the present study, in which treatments with essential oil caused morphological abnormalities in the fungi, with a
Table 3. Inhibition of ochratoxin A synthesis in the fungi A. ochraceus, A. carbonarius and A. westerdijkiae treated with essential oil of R. officinalis and C. viminalis and 1,8-cineole
| Percentage of inhibition of Ochratoxin A production | |||
|---|---|---|---|
| Treatment | A. ochraceus | A. carbonarius | A. westerdijkiae |
| R. officinalis | 51.76 ±0.14 b | 32.51 ±0.74 c | 52.83 ±1.52 b |
| C. viminalis | 53.66 ±0.10 a | 79.65 ±0.06 b | 64.09 ±0.18 a |
| 1,8-cineole | 24.69 ±0.01 c | 91.00 ±0.02 a | 41.99 ±1.27 c |
*Identical lowercase letters in the column do not differ statistically by Tukey's Test at the 5% level of probability.
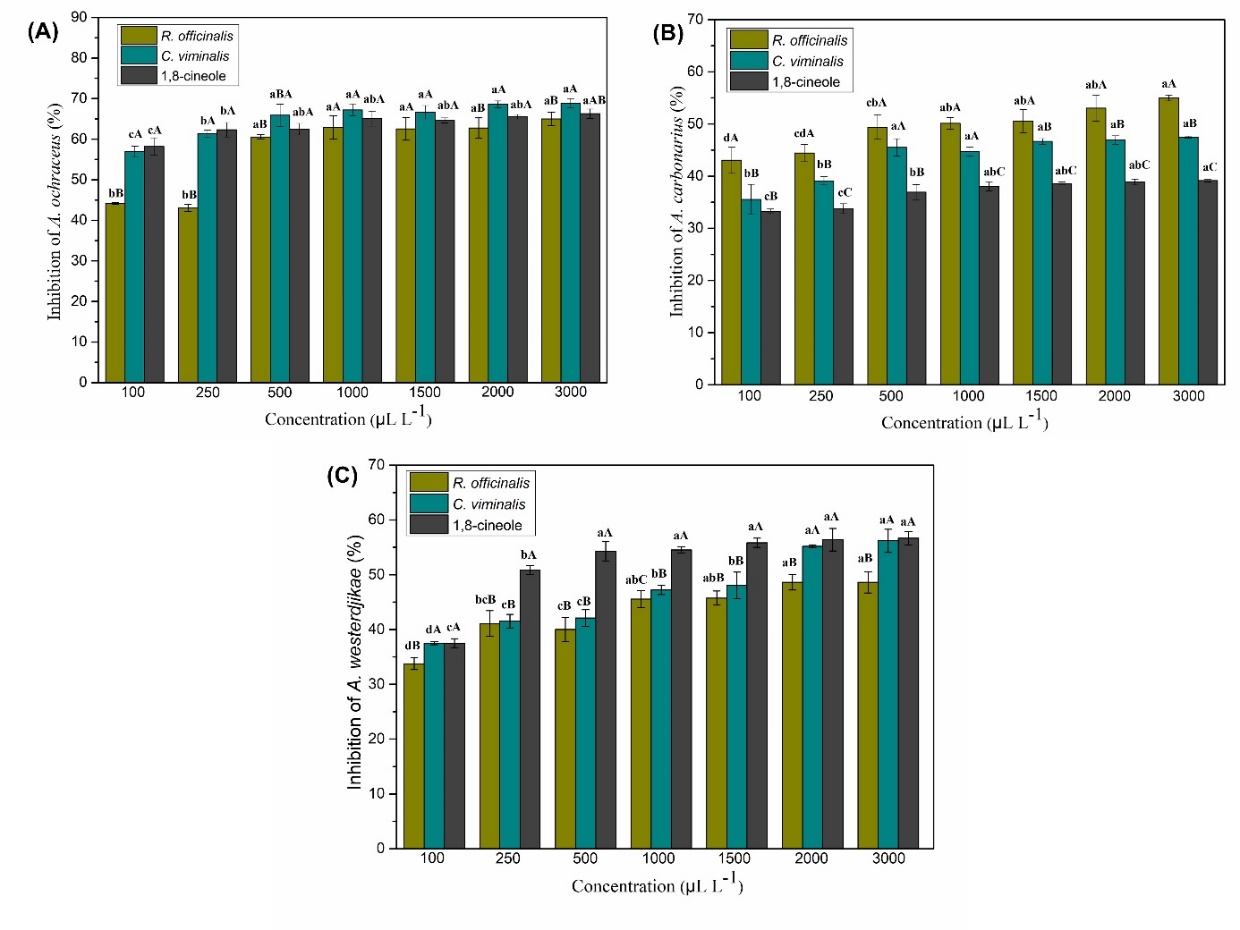
Figure 1. Percentage inhibition of mycelial growth by A. ochraceus, A. carbonasrius and A. westerdjikae on the effect of essential oils R. officinalis and C. viminalis and the monoterpene 1,8-cineole. (A) A. ochraceus,(B) A. carbonasrius and (C) A. westerdjikae (C) Lowercase letters indicate significant differences between the concentrations tested in each treatment and uppercase letters indicate significant differences between the three treatments, according to Tukey test at the 5% level of probability.
consequent decrease in spore production. These changes in the fungal structure may be related to the lipophilic characteristics of the essential oil constituents, which may interact with molecules present in the cell membrane. The proteins present in the fungal membrane play an important role in the vital functions of the microorganism (Li et al., 2021), and essential oils can interact with these proteins, as well as affect the synthesis of ergosterol, causing changes in the permeability of the membrane, hindering fungal growth, inhibiting metabolic processes and leakage of cellular materials (Lin et al., 2023; Nerilo et al., 2016; Brandão et al., 2021).
Materials and Methods
Plant material and analytical standard
The dried leaves of Rosmarinus officinalis were obtained from the local market in Lavras - MG/Brazil, and the fresh leaves of Callistemon viminalis were collected from the Campus of the Federal University of Lavras (UFLA), located in the city of Lavras - MG/Brazil. The analytical standard 1,8-cineole was obtained commercially from Sigma‒Aldrich with a purity of 99%.
Essential oil extraction
The extraction of essential oils was performed using the hydrodistillation method with a modified Clevenger apparatus (Anvisa, 2019). The leaves of R. officinalis and C. viminalis (250 g) were placed in a round-bottomed flask containing distilled water
and coupled to the Clevenger system. The hydrodistillation process was performed for a period of two hours. The hydrolysate was centrifuged for 15 minutes in a bench top centrifuge with a horizontal crosshead (Fanem Baby I Model 206-R) at 965.36 x g. Next, the essential oil of each plant was collected with the aid of a glass Pasteur pipette, placed in a glass flask and stored protected from light and heat.
Determination of moisture and yield of essential oils
The moisture content of the plant material was determined according to the method described by Pimentel et al. (2006). Five grams of the plant material was added to 80 mL of cyclohexane in a 250-mL round-bottom flask, and the flask was then attached to the Dean-Stark apparatus. The system was subjected to heating for two hours, and the volume of water present in the plant material was measured at the end. The moisture content was measured in triplicate. Yield was calculated as percentage weight/weight (%w/w) and moisture free basis (BLU).
Identification and quantification of essential oil constituents
The qualitative and quantitative chemical characterization of the essential oil constituents was performed at the Chromatography Laboratory of the Institute of Chemistry of the State University of Campinas – Unicamp. The identification of the essential oil compounds was performed using a gas chromatograph coupled to a Thermo Scientific TRACE 1300 Series mass spectrometer (GC–MS).
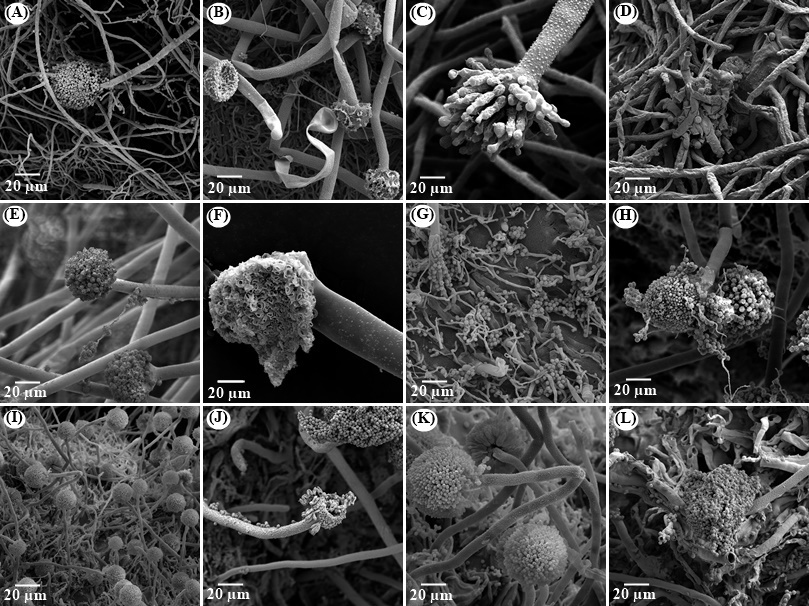
Figure 2. Scanning electron micrographs of A. ochraceus, A. carbonarius and A. westerdijkiae treated with essential oil of R. officinalis and C. viminalis and the monoterpene 1,8-cineole. (A), (E) and (I) are control from A. ochraceus, A. carbonarius and A. westerdijkiae, respectively; (B), (C) and (D) A. ochraceus treated with R. officinalis, C. viminalis and 1,8-cineole, respectively; (F), (G) and (H) A. carbonarius treated with R. officinalis, C. viminalis and 1,8-cineole, respectively; (J), (K) and (L) A. westerdijkiae treated with R. officinalis, C. viminalis and 1,8-cineole, respectively.
The experimental conditions were as follows: a fused silica capillary column (30 mx 0.25 mm) with DB5 bound phase (5% phenyl; 95% dimethylpolysiloxane) (0.25 µm film thickness); helium (He) was used as carrier gas with a flow rate of 1.0 mL min -1; the temperature was programmed, starting at 60 °C, followed by an increase of 3 °C min -1 until reaching 250 °C; the injector temperature was 250 °C and the detector temperature was 240 °C; the volume of injected sample was 0.5 µL, diluted in hexane; partition rate of the injected volume of 1:20 and column pressure of 71.0 kPa. The mass spectrometer conditions were as follows: detector scan 1,000; scanning interval of 0.50 fragments and fragments detected in the range of 45 to 500 Da.
The retention index was calculated using the equation of Van den Dool and Kratz (1963) with respect to the homologous series of n-alkanes (nC8-nC18). To identify the constituents, a comparison of the retention rates in the literature was performed with those obtained in the chromatogram (Adams, 2007). Two libraries of the NIST107 and NIST21 equipment were also used, which allowed the comparison of the spectral data with those existing in the libraries.
Quantitative analysis was performed by gas chromatography coupled to a Thermo Scientific TRACE 1300 Series flame ionization detector (FID). The experimental conditions were the same as those used for GC–MS, and the quantification of each constituent was obtained by means of the normalization of areas (%).
Microorganisms and spore solution preparation
The species of filamentous fungi Aspergillus ochraceus (CCDC 10490), Aspergillus carbonarius (CCCDA 10447) and Aspergillus westerdjikiae (CCDCA 11469) were studied. These samples were obtained from the Microorganism Culture Collection of the Laboratory of Mycotoxins and Food Mycology, Department of Food Science, UFLA. The fungal species were cultivated in Petri dishes (9 cm in diameter) containing malt extract agar (MEA) at 25 °C for seven days in a BOD incubator. After this period, the spore suspension of the fungi was prepared in distilled water at 1% Tween 80, and the final number of spores was counted in a Neubauer chamber (10 6 spores. mL -1).
Effect of essential oil on the mycelial growth of fungi
The percentage of mycelial inhibition of the fungi was evaluated by the in vitro fumigation method described by Guimarães et al. (2011). Plating was performed using 10 µL of the spore suspension in the centre of the plate containing 20 mL of yeast extract sucrose agar (YES) for A. ochraceus and Czapeck yeast agar (CYA) for A. carbonarius and A. westerdijkiae. Sterile 4-cm diameter filter paper disks were attached to the plate lid, and 250 µL of the essential oils and 1,8-cineole monoterpene 1,8-cineole diluted in dimethyl sulfoxide (DMSO) were added with the aid of an automatic pipette. 100, 250, 500, 1000, 1500, 2000 and 3000 µL L -1. As a comparison standard, 10 µL of the spore suspension was added to the plate containing only the culture medium (fungal control).
The plates were sealed and incubated in BOD at 25 °C for 10 days. Subsequently, measurements of the mycelial growth diameter of the colonies were performed. These analyses were performed in triplicate, and the percent inhibition of mycelial growth of the fungus was calculated according to the equation described by Brandão et al. (2020).
Effect of essential oil on OTA synthesis
The ochratoxigenic activity of the essential oils and the 1,8-cineole standard was assessed using the method described by Wang et al. (2012), evaluating the inhibition of OTA production by the fungi A. ochraceus, A. carbonarius and A. westerdijkiae in culture medium.
OTA extraction was performed on the 10th day of fungal spore incubation following the methodology described by Passamani et al. (2014). Initially, three colony plugs were removed from the centre, middle and edge of the plates. The plugs were weighed on an analytical balance and placed in a test tube wrapped in aluminium foil. Then, 1 mL of HPLC-grade methanol was added to each test tube, shaken vigorously for five seconds and kept at 25 °C for 60 minutes. The extracts were filtered through polytetrafluoroethylene (PTFE) membranes (0.22 µm; Millipore).
The quantification of OTA was determined using the method described by Passamani et al. (2014) using a high-performance liquid chromatograph (Shimadzu, model SPD-M20A, Kyoto, Japan) equipped with two high-pressure pumps, a degasser (DGU 20A3), interface (CBM-20A), automatic injector (SIL-10AF) and fluorescence detector (RF-10 AXL).
The column used was an Agilent-Zorbax Eclipse XDB-C18 (4.6 mm x 250 mm, 5 µm) connected to an Agilent-Zorbax Eclipse XDB-C18 precolumn (4.6 mm x 12.5 mm, 5 µm). The wavelengths used were excitation at 332 nm and emission at 476 nm. The flow rate was 0.8 mL min-1, and the injected volume of the samples was 20 µL. Elution was performed in an isocratic system of 35:35:29:1 methanol/acetonitrile/water/acetic acid. The quantification of OTA was performed by means of external standardization, with the construction of an analytical curve obtained by linear regression correlating the peak area of the chromatogram and the concentration of the standard. The coefficient of determination (R2) was 0.9999, and the limit of detection (LOD) and limit of quantification (LOQ) were 0.0004 and 0.0016 µg g-1, respectively. To calculate the percentage of inhibition of OTA production by the samples, the following equation was used:
\[I = \frac{O\text{c} - O\text{t}}{O\text{t}}x\ 100\]
where Oc corresponds to ochratoxin produced by the control and Ot to ochratoxin produced after treatment with essential oil (Brandão et al., 2020).
Morphological evaluation by scanning electron microscopy - SEM
The analysis of morphological changes of the fungi by the influence of the essential oils of R. officinalis, C. viminalis and the standard 1,8-cineole was evaluated by means of scanning electron microscopy (SEM) using a Leo EVO 040 electron microscope to obtain electron micrographs.
The samples were prepared according to the fumigation test described above to evaluate mycelial growth on the tenth day of incubation. The concentration used was the last one that had mycelium growth, that is, the concentration of 3000 µL L -1 for all treatments. Three plugs were removed from the colony and transferred to Eppendorf-type centrifuge microtubes containing the modified Karnovsky fixative solution (2.5% glutaraldehyde, 2% formaldehyde, 0.05 M coccodylate buffer, pH 7.2 and 0.001 M CaCl buffer). for a minimum period of 24 hours. The samples were washed three times for 10 minutes with 0.05 M sodium coccodalite buffer, pH 7.2, and then dehydrated using a series of increasing concentrations of acetone (25, 50, 75, 90 and 100%), with a concentration of 100% used twice for 10 minutes and dried in a critical point device (Bal-tec CPD 030). The dried samples were mounted on stubs with double-sided tape and pulverized with gold (Bal-tec SCD 050) (De Oliveira et al., 2017).
Statistical analysis
The results of essential oils and the standard on the percentage of inhibition of mycelial growth and on ochratoxin A synthesis were subjected to analysis of variance, and the means were compared using the Tukey’s test at 5% probability for each treatment with 3 replicates. A completely randomized design (DIC) was used, and the statistical program was RStudio, version 7872775e.
Conclusion
The essential oils used in the present study were considered as the main constituents of 1,8-cineole. The antifungal activity of essential oils from R. officinalis and C. viminalis and 1,8-cineol against A. ochraceus, A. carbonarius and A. westerdijkiae was significant. Essential oils inhibited ochratoxin A biosynthesis and caused damage to fungal cell membrane integrity. These results suggest that trained essential oils can be used as a natural and promising alternative in food preservation.
Conflict of interest
The authors declare that no conflict of interests exists.
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG — Project CAG/APQ 02390/2018), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq— Project CNPQ 311183/2022-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance code 001) for the scholarships. The authors are thankful for the scholarships and financial support and thank the Chromatography Laboratory at the Chemistry Institute of the State University of Campinas and the Laboratory of Electron Microscopy and Analysis Ultrastructural of the Federal University of Lavras for supplying the equipment and technical support for experiments.
Author contributions
CSF was responsible for the conceptualisation, formal analysis, methodology, data curation and writing (original draft); MGC was responsible for the resources, project administration, writing (review and editing), funding acquisition, supervision; EA was responsible for the conceptualisation, methodology and investigation; GAC was responsible for the conceptualisation, investigation and data curation; GCPC was responsible for the conceptualization and investigation. LRB was responsible for the conceptualization, validation, investigation and data curation.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Pub Corp, Carol Stream, IL.
Agência Nacional de Vigilância Sanitária (ANVISA) (2019) Formulário nacional da Farmacopeia brasileira, 6th ed. Fiocruz, Brasília, Brasil.
Al Zuhairi JJMJ, Kashi FJ, Rahimi-Moghaddam A, Yazdani M (2020) Antioxidant, cytotoxic and antibacterial activity of Rosmarinus officinalis L. essential oil against bacteria isolated from urinary tract infection. Eur J Integr Med. 38:101192.
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils. Food Chem Toxicol. 46(2):446–475.
Baldim JL, Silveira JGF, Almeida AP, Carvalho PLN, Rosa W, Schripsema J, Chagas-Paula DA, Soaresa MG, Luis JHH (2018) The synergistic effects of volatile constituents of Ocimum basilicum against foodborne pathogens. Ind Crops Prod. 112:821-829.
Brandão RM, Batista LR, De Oliveira JE, Barbosa RB, Nelson DL, Cardoso MG (2023) In vitro and in vivo efficacy of poly(lactic acid) nanofiber packaging containing essential oils from Ocimum basilicum L. and Ocimum gratissimum L. against Aspergillus carbonarius and Aspergillus niger in table grapes. Food Chem. 400:134087.
Brandão RM, Ferreira VRF, Batista LB, Alves E, Lira NA, Bellete BS, Scolforo JRS, Cardoso MG (2020) Antifungal and antimycotoxigenic effect of the essential oil of Eremanthus erythropappus on three different Aspergillus species. Flavour Fragr J. 35(5):524-533.
Brandão RM, Ferreira VRF, Batista LB, Alves E, Santiago WD, Barbosa RB, Caetano ARS, Nelson DL, Cardoso MG (2021) Antifungal activity and the effect of the essential oil of Lippia origanoides Kunth on Aspergillus mycotoxins production. Aust J Crop Sci. 15(7):1005–1012.
Caetano ARS, Cardoso MG, Haddi K, Campolina GA, Souza BM, Lunguinho AS, Souza L, Nelson DL, Oliveira JE (2022) Rosmarinus officinalis essential oil incorporated into nanoparticles as an efficient insecticide against Drosophila suzukii (Diptera: Drosophilidae). Austral Entomology, 61(8):265-272.
Camargo KC, Batista LR, Alves E, Rezende DACS, Teixeira ML, Brandão RM, Ferreira VRF, Nelson DL, Cardoso MG (2020) Antibacterial action of the essential oil from Cantinoa carpinifolia benth. Against Escherichia coli and Staphylococcus aureus strains. Flavour Fragr J. 35(1):99-106.
Chaudhari AK, Dwivedy AK, Singh VK, Das S, Akanksha S, Dubey NK (2019) Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci Pollut Res. 26(25):25414-25431.
De Oliveira MMM, Brugnera DF, Alves E, Cardoso MG, Piccoli RH (2017) Morphological alterations in sessile cells of Listeria monocytogenes after treatment with Cymbopogon sp. essential oils. Magistra. 26:385-392.
El Khoury R, Atoui A, Verheecke C, Maroun R, El Khoury A, Mathieu F (2016) Essential oils modulate gene expression and ochratoxin A production in Aspergillus carbonarius. Toxins. 8:242.
FAO; WHO (2018) Codex Alimentarius: Understanding Codex. 5th ed. Rome.
Gobbo-Neto L, Lopes NP (2007) Plantas medicinais: fatores de
influência no conteúdo
de metabólitos secundários. Química Nova. 30:374-381.
Guimarães LGDL, Cardoso MG, De Sousa PE, De Andrade J, Vieira SS (2011) Atividades antioxidante e fungitoxica do óleo essencial de capim limão e do citral. Ver Ciên Agron. 42(2):464–472.
Hasheminejad N, Khodaiyan F, Safari M (2019) Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 275:113-122.
Hua H, Xing F, Selvarai JN, Wang Y, Zhao Y, Zhou L, Liu X, Liu Y (2014) Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. Plos One. 9(9):e108285.
Iamanaka BT, Oliveira IS; Taniwaki MH (2010) Micotoxinas em alimentos. Anais da Academia Pernambucana de Ciência Agronômica, 7:138-161.
International Agency for Research on Cancer (IARC) (2012). Chemical agents and related occupations. IARC Monographs on the evaluation carcinogenic risks to humans 100F:225-244.
Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem. 10:813-829.
Kisová Z, Soltýsová A, Buckoyá M, Beke G, Puskárová A, Pangallo D (2020) Studying the gene expression of Penicillium rubens under the effect of eight essential oils. Antibiotics. 9(6):343.
Kumar OS, Nattudurai G, Islam VIH, Ignacimuthu S (2022) The effects of some essential oils on Alternaria alternata, a post-harvest phyto-pathogenic fungus in wheat by disrupting ergosterol biosynthesis. Phytoparasitica. 50:513–525.
Li F, Egea PF, Vecchio AJ, Asial I, Gupta M, Paulino J, Bajaj R, Dickinson MS, Ferguson-Miller S, Monk BC, Stroud RM (2021) Highlighting membrane protein structure and function: A celebration of the Protein Data Bank. J. Biol. Chem. 296:100557.
Lin L, Chen S, Xia W, Li T, Dong L, Zhang Y, Zhang Y, Li H, Zhao Y, Fu X (2023) A new strategy: Inhibitory effect and mechanism of Cymbopogon martinii essential oil on Aspergillus flavus. Food Biosci. 51:102315.
Lunguinho AS, Cardoso MG, Ferreira VRF, Konig IFM, Gonçalves RRP, Brandão RM, Caetano ARS, Nelson DL, Remedio RN (2021) Acaricidal and repellent activity of the essential oils of Backhousia citriodora, Callistemon viminalis and Cinnamodendron dinisii against Rhipicephalus spp. Vet Parasitol. 300:109594.
Lyu X, Lee J, Chen WN (2019) Potential natural food preservatives and their sustainable production in yeast: terpenoids and polyphenols. J. Agric. Food Chem. 67:4397-4417.
Mekonnen, A, Yitayew B, Tesema A, Taddese S (2016) In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int J Microbiol, 2016:9545693.
Moon YS, Choi W, Park E, Bae IK, Choi SD, Paek O, Kim SH, Chun HS, Lee SE (2016) Antifungal and antiaflatoxigenic methylenedioxy-containing compounds and piperine-like synthetic compounds. Toxins. 8(8):2-10.
Moussii IM, Nayme K, Timinouni M, Jamaleddine J, Filali H, Hakkou F (2020) Synergistic antibacterial effects of Moroccan Artemisia herba alba, Lavandula angustifólia and Rosmarinus officinalis essential oils. Synergy. 10:100057.
Murthy PS, Borse BB, Khanum H, Srinivas P (2009) Inhibitory effects of ajowan (Trachyspermum ammi) ethanolic extract on A. ochraceus growth and ochratoxin production. Turk J Biol. 33:211-217.
Nerilo SB, Rocha GHO, Tomoike C, Mossini SAG, Grespan R, Mikcha JMG, Junior MM (2016) Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int J Food Sci Technol. 51(2):286-292.
Passamani FRF, Hernandes T, Lopes NA, Bastos SC, Santiago WD, Cardoso MG, Batista LR (2014) Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J Food Prot. 77:1947-1952.
Passone MA, Girardi NS, Etcheverry M (2012) Evaluation of the control ability of five essential oils against Aspergillus section Nigri growth and ochratoxin A accumulation in peanut meal extract agar conditioned at different water activities levels. Int J Food Microbiol. 159:198–206.
Pimentel FA, Cardoso MG, Salgado APSP, Aguiar PM, Silva VF, De Morais AR, Nelson DL (2006) A convenient method for the determination of moisture in aromatic plants. Quím Nova 29(2):373–375.
Qiu C, Chang R, Yang J, Ge S, Xiong L, Zhao M, Li M, Sun Q (2017) Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 221:1426-1433.
Rezende DACS, Cardoso MG, Alves E, Brandão RM, Ferreira VRF, Caetano ARS, Lunguinho AS, Campolina GA, Nelson DL, Batista LR (2021) Effect of the essential oils of Satureja montana L., Myristica fragrans H. and Cymbopogon flexuosus S. on mycotoxin producing Aspergillus flavus and Aspergillus ochraceus antifungal properties of essential oils. FEMS Microbiol Lett. 368(19):fnab137.
Rezende DACS, Souza RV, Magalhães ML, Caetano ARS, Carvalho MSS, De Souza EC, Guimarães GL, Nelson DL, Batista LR, Cardoso MG (2017) Characterization of the biological potential of the essential oils from five species of medicinal plants. Am J Plant Sci. 8:154-170.
Rucky KD, De Boevre M, Huybrechts I, De Saeger S (2015) Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat Res. 766:32-41.
Sales TA, Cardoso MG, Guimarães LGL, Camargo KC, Rezende DACS, Brandão RM, Souza RV, Ferreira VRF, Marques AE, Magalhães ML, Nelson DV (2017) Essential oils from the leaves and flowers of Callistemon viminalis: chemical characterization and evaluation of the insecticide and antifungal activities. Am J Plant Sci. 8(10):2516-2529.
Santos L, Kasper R, Gil-Serna J, Marín S, Sanchis V, Ramos AJ (2010) Effect of Capsicum carotenoids on growth and ochratoxin A production by chilli and paprika Aspergillus spp. Isolates. Int. J Food Microbiol. 142(3):354-359.
Silva AS, Pereira RGFA, Lira NA, Da Glória EM, Chalfoun SM, Batista LR (2020) Fungi associated to beans infested with coffee berry borer and the risk of ochratoxin A. Food Control. 113:107204.
Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y (2012) The mechanism of antifungal action of essential oil from Dill (Anethum graveolens L.) on Aspergillus flavus. Plos One. 7(1):e30147.
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas‐liquid parti‐tion chromatography. J Chromatogr A. 11:463‐471.
Wang H, Liu Y, Wei S, Yan Z (2012) Comparative seasonal variation and chemical composition of essential oils from the leaves and stems of Schefflera heptaphylla using microwave-assisted and conventional hydrodistillation. Ind Crops Prod. 36(1):229-237.
Yang S, He M, Li D, Shi J, Peng L, Jinjing L (2023) Antifungal activity of 40 plant essential oil components against Diaporthe fusicola from postharvest kiwifruits and their possible action mode. Ind Crops Prod. 194:116102.