

Aust J Crop Sci. 19(01):37-43 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.01.p156
Hydrogen peroxide and Ascophyllum nodosum promote vigor in Cordia alliodora seeds at different maturation stages
Aline das Graças Souza1, Oscar José Smiderle2*, Edna Ursulino Alves1
1Graduate Program in Agronomy, Center for Agricultural Sciences, Federal University of Paraíba, Campus II, Areia, Paraíba, Brazil
2Department of Seeds, Brazilian Agricultural Research Corporation, Boa Vista, RR, Brazil
*Corresponding author: Oscar José
Smiderle 
Abstract: Cordia alliodora is a species from the humid American tropics that produces valuable wood and is suitable for large-scale artificial regeneration. Its main areas of exploration and participation in production systems are in Central America and in the western and northwestern countries of the Brazilian Amazon. Cordia alliodora is a pioneer species that produces a large quantity of seeds annually. Seedling emergence begins between 17 and 20 days after sowing, and may continue for another 20 days. Using solutions of hydrogen peroxide and phytohormones has been shown to be an important tool in the pre-germination stage of seeds of forest species, as it results in seedlings with greater vigor when the seeds are subjected to this chemical treatment. The objective was to determine the best concentrations of hydrogen peroxide and Ascophyllum nodosum for Cordia alliodora seeds at different maturation stages aiming at maximum seedling vigor. Experiment I: The first experiment was conducted in a completely randomized design, in a 2x4 factorial scheme with two seed colors (green and brown) and four concentrations o Ascophyllum nodosum (0, 5.0, 10.0 and 15.0 ml L-1), with four replicates, each of which composed of 25 seeds. The variables evaluated in experiment I and II were obtained from the data collection performed by daily counts of emerged seedlings until the stabilization of emergence (28 DAS). The variables were: Emergence speed index (ESI), emergence percentage (E%) and mean time of emergence (MTE) Ascophyllum nodosum at concentration of 4.0 ml L-1 applied in green seeds of Cordia alliodora promotes 76.3% of seedling emergence. For brown seeds of Cordia alliodora, the use of hydrogen peroxide and Ascophyllum nodosum is not recommended. Experiment II: The second experiment was conducted in a completely randomized design, in a 2x4 factorial scheme with two seed colors (green and brown) and four concentrations of hydrogen peroxide (H2O2) (0, 20, 30 and 40 mM), with four replicates, each of which composed of 25 seeds. Treatments consisted of Cordia alliodora seeds (100 seeds per treatment) soaked in H2O2 solutions at concentrations of 0, 20, 30 and 40 mM for 30 minutes. The results obtained in this study show the existence of activation or inhibition of processes linked to root protrusion and seedling vigor, which is paramount to the success of the species under study. For green seeds of Cordia alliodora, the hydrogen peroxide concentration of 20 mM is indicated to obtain 90.8% of seedling emergence and increase uniformity in the stand of normal seedlings, promoting higher yield and vigor in Cordia alliodora seedlings. While the control showed 67,5% emergence of Cordia alliodora seedlings.
Keywords: Freijó; reactive oxygen species; acadian®; pre-germination treatment.
Introduction
Studies related to forest species native to the northern Amazon are important both economically and ecologically, as they make it possible to know the potential for supplying plant resources, since propagation by seeds is the main form of dissemination of species (Smiderle and Souza 2022a).
In this context, Cordia alliodora, a component of the local flora of the Roraima state, belonging to the Boraginaceae family and popularly known as Freijó, stands out among the native species of the northern region of Brazil (Souza et al., 2023a).
Cordia alliodora trees stand out for producing wood of excellent quality and comparable to that of species already established in the international market, such as mahogany (Swietenia macrophylla King.) and teak (Tectona grandis L.F.). Nonetheless, there is limited data in the literature regarding the seed technology sector for Cordia alliodora. In a study conducted in the Province of Limón, Costa Rica, Liegel and Stead (1990) worked with freshly harvested seeds (brown color) of Cordia alliodora and obtained 60% of emerged seedlings.
In this context, knowledge on the maturation of Cordia alliodora seeds is important to assist in decision making at the time of harvest and hence obtain better quality material aiming at maximum vigor. To do so, it is necessary to know the morphophysiological characteristics of the species and always consider the factors that modulate production, such as time and year of harvest, climatic conditions and plant characteristics (Smolikova et al., 2021; Cruz et al., 2021). All these factors should be considered in seed harvest planning, as this is a both economically and operationally costly activity in forestry (Bewley et al., 2013).
According to Smiderle and Souza (2022a), the germination process of native forest seeds is associated with several metabolic events that are triggered from the beginning of seed imbibition to the protrusion of the radicle.
Currently, several studies reveal that forest seeds subjected to some pre-germination treatment can activate physiological mechanisms to the point of improving germination under adverse conditions (Smiderle and Souza, 2021; Smiderle and Souza 2022a; Souza et al., 2023a). Solutions based on hydrogen peroxide and Ascophyllum nodosum employed in the seed technology sector have acted as a stimulant in the germination process and vigor of seedlings of forest species (Souza et al., 2023b; Smiderle and Souza, 2022b; Fagundes et al., 2023). Su et al. (2016), in a study with Hedysarum scoparium seeds, obtained germination of 21% without H2O2 application and 84% when the seeds were inoculated with 50 mM of H2O2. In view of the above, the objective was to determine the best concentrations of hydrogen peroxide and Ascophyllum nodosum for Cordia alliodora seeds at different maturation stages aiming at maximum seedling vigor.
Results and discussion
Experiment I: Cordia alliodora seeds at different maturation stages subjected to Ascophyllum nodosum (Acadian®) concentrations
Analysis of variance
According to the analysis of variance, there was a significant interaction between seed color and the algae-based extract Acadian® for all seedling vigor variables evaluated (Table 1)
As described in Table 2, Cordia alliodora seeds were characterized at two maturation stages, with green and brown seed coat, and biometric analysis was also performed for length and width, expressed in mm. The fresh mass of green and brown seeds of Cordia alliodora was 0.29 g and 0.17 g, respectively.
Relations between Ascophyllum nodosum doses and maturation stage seeds of Cordia alliodora
The water content in the initial maturation stage (green seeds) was high (48.3%) and, as maturation progressed, it drastically decreased to 11.8%, observed in brown seeds (Table 1). Concomitant with this effect, fresh mass accumulation increased up to the maximum point of 0.29 g for green seeds (Table 1), being a characteristic dependent on each species. According to Cruz et al. (2021), at this stage of fresh mass accumulation, seeds would theoretically have maximum potential for seedling emergence. This result was observed in the present study, as Cordia alliodora seeds of green color led to higher percentages of seedling emergence when compared to brown seeds, regardless of Ascophyllum nodosum concentrations (Figure 1A). Fagundes et al. (2023), establishing the following research problem: can Ascophyllum nodosum applied to African mahogany seeds at different doses be effective in promoting emergence and vigor of seedlings, revealed the percentage of emergence of African mahogany seedlings as a function of the doses established for the bioregulator Acadian®, and the dose of maximum technical efficiency (DMTE) found was 0.2 ml L-1, which led to a 31.2% increase in seedling emergence when compared to the control.
Figure 1A presents the percentage of seedling emergence from green seeds, showing that the Ascophyllum nodosum concentration of 0.6 ml L-1 promoted a 11.14% increase in seedling emergence compared to the zero dose, that is, control of green seeds (Figure 1A). A study conducted by Souza and Smiderle (2022b) revealed that the Ascophyllum nodosum concentration of 8.5 ml L-1 inoculated in Hymenaea courbaril seeds promoted an increase in seedling emergence.
Nonetheless, for all concentrations of Ascophyllum nodosum applied in brown seeds of Cordia alliodora, emergence percentages were lower than 38.5%, while in the control the emergence percentage was 46.5% in brown seeds, contributing decisively to the inhibitory or reductional effect on this variable.
When comparing the speed of emergence of Cordia alliodora seedlings from green seeds among the Ascophyllum nodosum concentrations tested, higher ESI values were observed at all tested concentrations compared to the control, as shown in Figure 1B.
In turn, the maximum value for the emergence speed index of Cordia alliodora seedlings from green seeds (Figure 1A) was obtained with the Ascophyllum nodosum concentration of 6.0 ml L-1. It is also worth pointing out the higher speed of emergence with green seeds, regardless of the Ascophyllum nodosum concentrations used, when compared to brown seeds (Figure 2B).
According to Marcos Filho (2015), seeds in early stages of maturation can germinate because they already have their structures formed, that is, the embryo is already morphologically
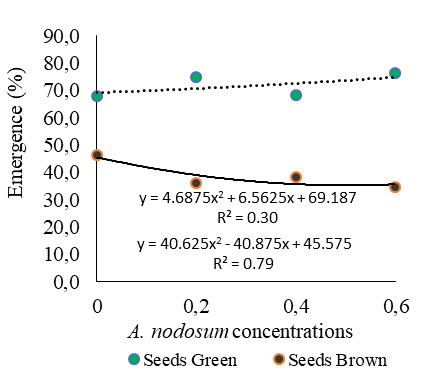
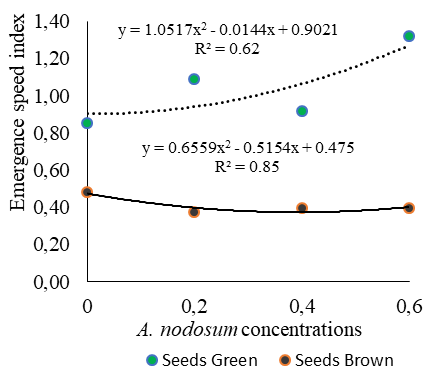
Fig 1. Mean values of seedling emergence (A) and emergence speed index (B) as a function of green and brown seeds of Cordia alliodora under different concentrations of Ascophyllum nodosum (0, 0.2, 0.4 and 0.6 ml L-1) applied to the seeds.
formed. However, physiological maturity will only be confirmed for a given species when the seeds have high vigor. This was observed for Cordia alliodora with the use of green seeds, as they led to higher vigor of seedlings when compared to brown seeds, regardless of the Ascophyllum nodosum concentrations.
It is also worth mentioning that three ranges of emergence speed index for green seeds were found in the present study, as shown in Figure 1B, which revealed the following order for ESI: 1.32 (6.0 ml L-1 Ascophyllum nodosum) > 1.09 (2.0 ml L-1 Ascophyllum nodosum) > 0.91 (4.0 ml L-1 Ascophyllum nodosum), demonstrating that the different concentrations of Ascophyllum nodosum (Figure 1B) promote the capacity and speed of emergence of Cordia alliodora seedlings.
According to Smiderle and Souza (2022b), seeds with higher ESI are subject to adverse conditions found in the soil, such as temperature variation, water stress, and attack of pests and pathogens for a shorter time. In this case, the use of green seeds and extract based on algae, such as Ascophyllum nodosum, was favorable to promote the speed of emergence of Cordia alliodora seedlings, being indicated under the conditions of the present study.
On the other hand, brown seeds exhibited emergence speed indices below 0.48, regardless of Ascophyllum nodosum concentrations (Figure 1B).
Table 1. Experiment I. Summary of the analysis of variance for emergence percentage (E%), emergence speed index (ESI), mean time of emergence (MTE) and percentage of normal seedlings (%NS) of Cordia alliodora as a function of seed color (C) and Acadian® (Ascophyllum nodosum) (A)
| Sources of Variation | DF | E% | ESI | MTE (days) | %NS |
|---|---|---|---|---|---|
| Color (C) | 1 | 10766.60** | 4.0068** | 455.625** | 2847.656** |
| Acadian® (A) | 3 | 23.789* | 0.0866** | 57.552** | 405.937** |
| Interaction (C x A) | 3 | 211.497** | 0.1456** | 34.479** | 556.510** |
| CV (%) | 4.71 | 8.94 | 6.91 | 6.92 |
**,*, ns Significant at 1 and 5 % probability levels and not significant by the F test.
Table 2. Mean values of length (mm), width (mm), fresh mass (g) and water content (%) of Cordia alliodora seeds as a function of seed coat color
| Seed Color | Length | Width | Fresh Mass | Water Content |
|---|---|---|---|---|
| Green | 7.1 | 4.1 | 0.29 | 48.3 |
| Brown | 7.0 | 3.2 | 0.17 | 11.8 |
Fig 2. Mean values of mean time of emergence (A) and percentage of normal seedlings (B) as a function of green and brown seeds of Cordia alliodora under different concentrations of Ascophyllum nodosum (0, 0.2, 0.4 and 0.6 ml L-1) applied to the seeds.
In addition, the highest percentage of normal seedlings (45%) from green seeds was observed at the Ascophyllum nodosum concentration of 0.6 ml L-1 (Figure 2A), with a mean time of 10.0 days (Figure 2B), resulting in uniformity in the stand of Cordia alliodora seedlings. It is known that, in parallel to the emergence test, seedling vigor is attested by the capacity for emergence and production of normal seedlings (Smiderle et al., 2024), pointing to differences in the quality of seeds in the lot according to the emergence percentage, thus making it possible to classify them as of high and low vigor (Leal et al., 2020; Smiderle and Souza 2022b; Smiderle et al., 2024).
On the other hand, green seeds without addition of Ascophyllum nodosum, i.e. control (Figure 2B), led to the lowest percentage of normal seedlings (13.8%) as a function of the Ascophyllum nodosum concentrations described in Figure 2B.
In addition, Figure 2B shows that brown seeds inoculated with Ascophyllum nodosum at a concentration of 4.0 ml L-1 resulted in a higher percentage of normal seedlings compared to the other concentrations.
According to Souza et al. (2023a), higher percentage of normal seedlings with adequate commercial characteristics is one of the primary factors for the success of the species, resulting in the economic return of the capital invested by producers and nurseries.
Experiment II: Cordia alliodora seeds with green and brown color under different concentrations of hydrogen peroxide (H2O2)
Analysis of variance
According to the analysis of variance, there was a significant interaction between seed maturation stage (green seeds and
brown seeds) and the different concentrations of hydrogen peroxide (H2O2) evaluated for the variables studied (Table 3).
Relations between H2O2 concentrations and maturation stage (green seeds and brown seeds) of Cordia alliodora
Figure 3A shows the emergence percentage of Cordia alliodora seedlings from green seeds as a function of H2O2 concentrations, and it is possible to observe that the H2O2 concentration of 20 mM resulted in a 25.6% increase in seedling emergence compared to the control (Figure 1A). These results showed that the different H2O2 concentrations applied to Cordia alliodora seeds may be related to cell signaling. That is, reactive oxygen species (ROS) interact with other molecules especially in the regulation of phytohormones (Stein et al., 2021), which are involved in the activation or inhibition of the growth and development processes that occur early in embryogenesis (Souza et al., 2023b), as well as in the mechanisms linked to root protrusion and seedling emergence (Hernández Cortés, 2022).
A study conducted by Souza et al. (2023b) revealed that 40 mM of H2O2 applied to Khaya ivorensis seeds caused reduction in seedling emergence, as also found by Miranda et al. (2014) in Prosopis juliflora seeds.
In turn, in the present study, 40 mM of H2O2 applied to green seeds of Cordia alliodora caused an emergence percentage of approximately 81%, resulting in a 16.6% increase in seedling emergence when compared to the control (Figure 3A).
On the other hand, for brown seeds all H2O2 concentrations led to emergence percentages below 38%, while the control showed 50% emergence of Cordia alliodora seedlings.
The results obtained in this study reinforce those obtained with brown seeds of Cordia alliodora by Liegel and Stead (1990), who found seedling emergence percentage of approximately 60%. Smiderle and Souza (2022a), in a study conducted in Boa Vista,
Table 3. Experiment II. Summary of the analysis of variance for emergence percentage (E%), emergence speed index (ESI), mean time of emergence (MTE) and percentage of normal seedlings (%NS) of Cordia alliodora as a function of seed color (C) and hydrogen peroxide (P).
| Sources of Variation | DF | E% | ESI | MTE (days) | %NS |
|---|---|---|---|---|---|
| Seed color (C) | 1 | 19305.039** | 5.0126** | 1279.726** | 14774.414** |
| Hydrogen peroxide (P) | 3 | 180.039** | 0.0506** | 97.018** | 1563.476** |
| Interaction (C x P) | 3 | 779.205** | 0.3571** | 153.789** | 2111.289** |
| CV (%) | 3.78 | 8.66 | 6.34 | 7.54 |
**, ns Significant at 1% probability level and not significant by the F test.
Roraima, working with freshly harvested seeds (brown color) of Cordia alliodora, obtained 60% of emerged seedlings in substrate consisting of medium sand.
However, in the present study, the test of emergence of Cordia alliodora in medium sand substrate proved to be a good indicator to differentiate seed vigor for different maturation stages (green seeds and brown seeds), because brown seeds of Cordia alliodora subjected to treatments with different H2O2 concentrations led to low seedling emergence (E%: 27%), compared to green seeds (E%: 90.8%) (Figure 3A). The use of a hydrogen peroxide-based solution in the seed technology, (Anand et al., 2019), sector has been used as a stimulant in the germination process and seedling vigor (Souza et al., 2023b; Limão et al., 2024). Research carried out by Wojtyla et al. (2016) with Jatropha curcas seeds, without application of H2O2, they obtained 31% germination and, when the seeds were inoculated with 30 mM H2O2, they exhibited 88% germination
This result was also obtained for the emergence speed index of Cordia alliodora seedlings (Figure 3B) with brown seeds under the different concentrations of H2O2 (Figure 3B), which led to ESI lower than 0.38, while in the control the ESI was 0.64 for brown seeds.
In turn, green seeds subjected to zero concentration of H2O2 showed ESI of 0.83. It is worth mentioning that green seeds at H2O2 concentration of 20 mM exhibited better ESI, 1.34, with gains of 38% compared to the control (Figure 3B), indicating that the absorption of the H2O2 solution at the aforementioned concentration was efficient to promote the regulation and balance of phytohormones, as well as greater vigor of Cordia alliodora seedlings.
In addition, the highest percentage of normal seedlings (70%) was observed at the H2O2 concentration of 20.0 mM (Figure 4B), with a mean time of 5.0 days (Figure 3A), resulting in greater uniformity in the stand of Cordia alliodora seedlings. It is known that, in parallel to the germination test, seed vigor is attested by the capacity for germination and production of normal seedlings, pointing to differences in the quality of seeds in the lot according to germination percentage, thus making it possible to classify them as of high and low vigor (Smiderle and Souza 2022a). In this case, it can be said that Cordia alliodora seeds at the maturation stage with green color had a fully formed embryo with high vigor.
On the other hand, for brown seeds of Cordia alliodora, as they were subjected to different concentrations of H2O2, the mean time of emergence increased up to 21.8 days, while the percentage of normal seedlings was drastically reduced to only 5.8%. These values are considered unsatisfactory according to Brasil (2009). Thus, it is suggested that seeds with brown color may decisively contribute to the low percentage of normal seedlings of Cordia alliodora.
In view of the above, for the species Cordia alliodora, aiming at the optimization of seedling emergence, green seeds should be used to enable the production of seedlings on a commercial scale.
In addition, using adequate H2O2 concentrations for green seeds represents an advantage to promote uniform emergence and better stand of normal plants in the nursery, resulting in efficient use of propagative material aiming at the quality of the seedlings produced.
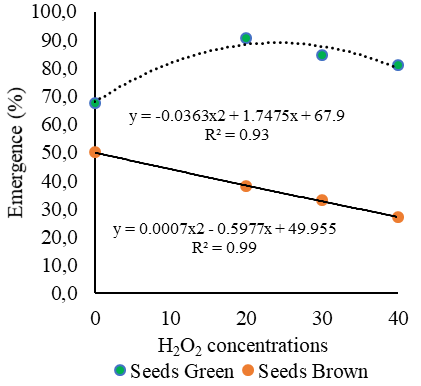
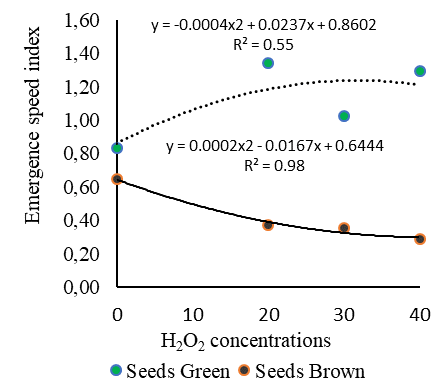
Fig 3. Mean values of seedling emergence percentage (A) and emergence speed index (B) as a function of green and brown seeds of Cordia alliodora under different concentrations of hydrogen peroxide (0, 20, 30 and 40 mM) applied to the seeds.
Materials and Methods
Plant material
The study was conducted in the laboratory and greenhouse of Embrapa Roraima, and the species used was Cordia alliodora. Fruits were collected manually from trees present at the headquarters of Embrapa Roraima, located in the municipality of Boa Vista – RR, Brazil. The local climate is Am (tropical monsoon climate). The warmest month has an average temperature of 27.2 °C and the coldest month has an average temperature of 23.3 °C, with average annual temperature of 25.4 °C. The average annual rainfall is up to 1808 mm, with the highest values in June (365 mm) and the lowest values in February (26 mm).
Cordia alliodora fruits were harvested from ten productive trees at Embrapa Roraima, when they were at the beginning of natural dehiscence. After harvesting, the fruits were taken to the seed laboratory, where they were processed manually with opening of the perianth. After processing, the seeds were separated into two
Fig 4. Mean values of mean time of emergence (A) and percentage of normal seedlings (B) as a function of green and brown seeds of Cordia alliodora under different concentrations of hydrogen peroxide (0, 20, 30 and 40 mM) applied to the seeds.
color classes (green seeds and brown seeds). Cordia alliodora seeds were characterized as ellipsoidal, with 7 mm length and 3 mm width for brown seeds and 7 mm length and 4 mm width for green seeds, measured in the middle portion using a digital caliper with 0.01 mm precision. In addition, the fresh mass (g) of green and brown seeds was determined on a precision scale (0.001 g). Seed water content was determined for each mass class, in an oven (105 ± 3 ºC) where the seeds were kept for 24 hours, according to the procedure described by Brasil (2009), with four replicates of 10 seeds.
It is worth mentioning that two experiments were carried out, both to determine vigor in Cordia alliodora seeds at different maturation stages. For the studies of pre-germination treatments, Cordia alliodora seeds were subjected to the concentrations of hydrogen peroxide (H2O2) and Ascophyllum nodosum (Acadian®).
Experiment I- Cordia alliodora seeds at different maturation stages subjected to Ascophyllum nodosum (Acadian®) concentrations
Experimental design and conduction of study
The first experiment was conducted in a completely randomized design, in a 2x4 factorial scheme with two seed colors (green and brown) and four concentrations of Ascophyllum nodosum (0, 5.0, 10.0 and 15.0 ml L-1), with four replicates, each of which composed of 25 seeds. Seed treatment was performed according to the methodology recommended by Nunes (2005), in which the different concentrations of the bioregulator were applied directly to the bottom of a transparent plastic bag for each treatment, then the seeds were placed inside the plastic bag and shaken manually for 30 minutes so that the solution of Ascophyllum nodosum covered all the seeds.
In order to complement and elucidate the results of the present study, the seeds were sown in sand of medium particle size at 1.0 cm depth in plastic trays of 30 cm x 40 cm x 10 cm in a greenhouse with average temperature in the experimental period of 28 ± 5 °C and relative air humidity from 60% to 70%. The variables evaluated in experiment I and II were obtained from the data collection performed by daily counts of emerged seedlings until the stabilization of emergence (28 DAS). During the initial vigor stages of the seedlings, substrate moisture was kept by manual irrigation, with a total of four daily irrigation events. Emergence speed index (ESI), emergence percentage (E%) and mean time of emergence (MTE) were calculated. MTE was obtained according to Labouriau (1983) and ESI was calculated according to Maguire (1962).
The beginning of seedling emergence occurred at nine days after sowing (DAS). For seedling emergence percentage (E%), emerged
seedlings were considered to be the ones that had cotyledon leaves longer than five centimeters, after breaking the substrate surface. Vigor results were expressed as a percentage of normal seedlings, counted during the evaluations. Normal seedlings were considered to be those that, after breaking the substrate surface, showed well-differentiated and developed cotyledons and hypocotyls. Vigor results were expressed as a percentage of normal seedlings (%NS), obtained during the evaluations.
Experiment II: Cordia alliodora seeds with green and brown color under different concentrations of hydrogen peroxide (H2O2)
Experimental design and conduction of study
The second experiment was conducted in a completely randomized design, in a 2x4 factorial scheme with two seed colors (green and brown) and four concentrations of hydrogen peroxide (H2O2) (0, 20, 30 and 40 mM), with four replicates, each of which composed of 25 seeds. Treatments consisted of Cordia alliodora seeds (100 seeds per treatment) soaked in H2O2 solutions at concentrations of 0, 20, 30 and 40 mM for 30 minutes. Seed treatment was performed according to the methodology recommended by Souza et al. (2023b).
In order to complement and elucidate the results of the present study, Cordia alliodora seeds were sown according to the methodology of experiment I and under the same environmental conditions. The variables were obtained according to experiment I.
To check the assumptions of the analysis of variance (ANOVA), the data from experiments I and II were first assessed for: a) normality with the Shapiro-Wilk test (p > 0.05), and b) homoscedasticity by the Bartlett test (p > 0.05). When there was normality of the residuals and homogeneity of the variances, the data were subjected to analysis of variance (ANOVA). Quantitative variables were subjected to regression analysis in order to evaluate the response of seed vigor as a function of Ascophyllum nodosum and hydrogen peroxide concentrations. Data analysis was performed using the statistical program Sisvar (Ferreira, 2014).
Conclusions
The results obtained in this study show the existence of activation or inhibition of processes linked to root protrusion and seedling vigor, which is paramount to the success of the species under study. For green seeds of Cordia alliodora, the hydrogen peroxide concentration of 20 mM is indicated to obtain 90.8% of seedling emergence and increase uniformity in the stand of normal seedlings, promoting higher yield and vigor in Cordia alliodora seedlings. Ascophyllum nodosum at concentration of 4.0 ml L-1 applied in green seeds of Cordia alliodora promotes 76.3% of seedling emergence. For brown seeds of Cordia alliodora, the use of hydrogen peroxide and Ascophyllum nodosum is not recommended.
Acknowledgments
The authors would like to thank the funding for the realization of this study provided by the Brazilian agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico -Brasil), Senior Postdoctoral for the first research under process 101867/2024-7 second and third author research productivity grant.
References
Anand A, Kumari A, Thakur M, Koul A (2019) Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep 9:8814.
Bewley JD, Bradford K, Hihorst H, Nonogaki H (2013) Development and maturation. In Seeds: Physiology of development, germination and dormancy. New York: Springer. 3rd ed., 27– 83p.
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p.
Cruz MFV, Malavasi MM, Ristau ACP, Malavasi UC, Dranski JÁ (2021) Maturidade de sementes de Anadenanthera colubrina (Vell.) Brenan. Cienc. Florestal 31:515-532.
Hernández JÁ, Martínez-Andújar C, Díaz-Vivancos P (2022) Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305. Seeds, 1:28–35.
Fagundes PRO, Smiderle OJ, Souza, AG. (2023) Bioregulators promote emergence of african mahogany seedlings? C. L. C. S, 16:5227-5236.
Ferreira DF (2014) Sisvar: a Guide for its Bootstrap procedures in multiple comparisons. Cienc. Agrotec. 38:109-112.
Labouriau LG (1983) A germinação de sementes. Washington DC.: Organização dos Estados Americanos, Programa Regional de Desenvolvimento Científico e Tecnológico.174 p
Limão MAR, Barbosa JL, Aldair de S. Medeiros ASM, Maia Júnior S , Magalhães ID , Pimenta TA, Gonzaga GBM , Sousa VFO , Siqueira GM , Marques JI , Sousa WS, Leite PG. (2024) Priming seeds with hydrogen peroxide attenuates damage caused by salt stress in sorghum. Rev. Bras. Eng. Agríc. Ambiental, 28: e279087, 2024.
Liegel LH, Stead JW (1990) Cordia alliodora (Ruiz & Pav.) Okén. Laurel, capá prieto. En: Burns RM, Honkala BH. eds. Silvics of North America: 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service. 270-277 p.
Leal YH, Sousa VFO, Dias TJ, Silva TI, Leal MPS, Souza AG, Lucena MFR, Rodrigues LS, Smiderle OJ (2020) Edaphic respiration in bell pepper cultivation under biological fertilizers, doses and application times. E.J.F.A 32:434-442.
Miranda RQ, Correia RM, Almeida-Cortez JS, Pompeli MF (2014) Germination of Prosopis juliflora (Sw.) D.C. seeds at different osmotic potentials and temperatures. Plant. Species. Biol, 29:9-20.
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci 2:176- 177.
Marcos-Filho J (2015) Fisiologia de sementes de plantas cultivadas. 2 ed. Londrina: Abrates, 660p.
Smiderle OJ, Souza AG (2022a). Cartilha de sementes de espécies florestais em Roraima. Embrapa Roraima, 60p.
Smiderle OJ, Souza AG. (2021) Do scarification and seed soaking periods promote maximum vigor in seedlings of Hymenaea courbaril? J. Seed Sci 43: e202143030.
Nunes JC (2005) Tratamento de semente- qualidade e fatores que podem afetar a sua performance em laboratório. Syngenta Proteção de Cultivos Ltda. 16p.
Smiderle OJ, Souza AG (2022b) Scarification and doses of Acadian®, Stimulate® and Trichoderma spp. promote dormancy overcoming in Hymenaea courbaril L. seeds? J. Seed Sci 44:e202244009.
Smiderle OJ, Souza AG, Lima-Primo HE, Fagundes PRO (2024) Efficiency of organomineral fertilizer and doses of Azospirillum brasilense on the morphophysiological quality of Mezilaurus itauba seedlings. Braz. J. Biol 84: 279851
Smolikova G, Leonova T, Vashurina N, Frolov A, Medvedev S (2021) Desiccation tolerance as the basis of long-term seed viability. Inter. J. Molec. Scien 22:101.
Souza AG, Smiderle OJ, Maia SS (2023a) Do Stimulate® and Ascophyllum nodosum seaweed promote the morphophysiological characteristics of Cordia alliodora seedlings? Aust. J. Crop. Sci 17:447-452.
Souza AG, Matera TC, Ecker AE, Smiderle OJ (2023b) Hydrogen peroxide influence on african mahogany seedling vigor. C. L. C. S, 16: 8090-8102.
Stein M, Serban C, Mccord P (2021) Exogenous ethylene precursors and hydrogen peroxide aid in early seed dormancy release in sweet cherry. J.A.S.H.S 146:50–55.
Su L, Lan Q, Pritchard HW, Xue H, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Biochem 109: 406-415.
Wojtyla L, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci 7:66-74.
Anand A, Kumari A, Thakur M, Koul A (2019) Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep 9:8814.
Bewley JD, Bradford K, Hihorst H, Nonogaki H (2013) Development and maturation. In Seeds: Physiology of development, germination and dormancy. New York: Springer. 3rd ed., 27– 83p.
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 399p.
Cruz MFV, Malavasi MM, Ristau ACP, Malavasi UC, Dranski JÁ (2021) Maturidade de sementes de Anadenanthera colubrina (Vell.) Brenan. Cienc. Florestal 31:515-532.
Fagundes PRO, Smiderle OJ, Souza, AG. (2023) Bioregulators promote emergence of african mahogany seedlings? C. L. C. S, 16:5227-5236.
Ferreira DF (2014) Sisvar: a Guide for its Bootstrap procedures in multiple comparisons. Cienc. Agrotec. 38:109-112.
Hernández JÁ, Martínez-Andújar C, Díaz-Vivancos P (2022) Hydrogen Peroxide Imbibition Following Cold Stratification Promotes Seed Germination Rate and Uniformity in Peach cv. GF305. Seeds, 1:28–35.
Labouriau LG (1983) A germinação de sementes. Washington DC.: Organização dos Estados Americanos, Programa Regional de Desenvolvimento Científico e Tecnológico.174 p
Leal YH, Sousa VFO, Dias TJ, Silva TI, Leal MPS, Souza AG, Lucena MFR, Rodrigues LS, Smiderle OJ (2020) Edaphic respiration in bell pepper cultivation under biological fertilizers, doses and application times. E.J.F.A 32:434-442.
Liegel LH, Stead JW (1990) Cordia alliodora (Ruiz & Pav.) Okén. Laurel, capá prieto. En: Burns RM, Honkala BH. eds. Silvics of North America: 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service. 270-277 p.
Limão MAR, Barbosa JL, Aldair de S. Medeiros ASM, Maia Júnior S , Magalhães ID , Pimenta TA, Gonzaga GBM , Sousa VFO , Siqueira GM , Marques JI , Sousa WS, Leite PG. (2024) Priming seeds with hydrogen peroxide attenuates damage caused by salt stress in sorghum. Rev. Bras. Eng. Agríc. Ambiental, 28: e279087, 2024.
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci 2:176- 177.
Marcos-Filho J (2015) Fisiologia de sementes de plantas cultivadas. 2 ed. Londrina: Abrates, 660p.
Miranda RQ, Correia RM, Almeida-Cortez JS, Pompeli MF (2014) Germination of Prosopis juliflora (Sw.) D.C. seeds at different osmotic potentials and temperatures. Plant. Species. Biol, 29:9-20.
Nunes JC (2005) Tratamento de semente- qualidade e fatores que podem afetar a sua performance em laboratório. Syngenta Proteção de Cultivos Ltda. 16p.
Smiderle OJ, Souza AG (2022a). Cartilha de sementes de espécies florestais em Roraima. Embrapa Roraima, 60p.
Smiderle OJ, Souza AG (2022b) Scarification and doses of Acadian®, Stimulate® and Trichoderma spp. promote dormancy overcoming in Hymenaea courbaril L. seeds? J. Seed Sci 44:e202244009.
Smiderle OJ, Souza AG, Lima-Primo HE, Fagundes PRO (2024) Efficiency of organomineral fertilizer and doses of Azospirillum brasilense on the morphophysiological quality of Mezilaurus itauba seedlings. Braz. J. Biol 84: 279851
Smiderle OJ, Souza AG. (2021) Do scarification and seed soaking periods promote maximum vigor in seedlings of Hymenaea courbaril? J. Seed Sci 43: e202143030.
Smolikova G, Leonova T, Vashurina N, Frolov A, Medvedev S (2021) Desiccation tolerance as the basis of long-term seed viability. Inter. J. Molec. Scien 22:101.
Souza AG, Matera TC, Ecker AE, Smiderle OJ (2023b) Hydrogen peroxide influence on african mahogany seedling vigor. C. L. C. S, 16: 8090-8102.
Souza AG, Smiderle OJ, Maia SS (2023a) Do Stimulate® and Ascophyllum nodosum seaweed promote the morphophysiological characteristics of Cordia alliodora seedlings? Aust. J. Crop. Sci 17:447-452.
Stein M, Serban C, Mccord P (2021) Exogenous ethylene precursors and hydrogen peroxide aid in early seed dormancy release in sweet cherry. J.A.S.H.S 146:50–55.
Su L, Lan Q, Pritchard HW, Xue H, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Biochem 109: 406-415.
Wojtyla L, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci 7:66-74.