

Aust J Crop Sci. 19(01):111-118 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.01.p249
Micro-morphological, phytochemical and molecular characteristics of aquatic pennywort (Hydrocotyle verticillata) accessions in Vietnam
Hai Thi Hong Truong1*, Nhi Thi Hoang Ho1, Han Ngoc Ho1, Thao Xuan Hoang2
1Institute of Biotechnology, Hue University, Hue city, Thua Thien Hue, Vietnam
2Faculty of Biology, Hue University of Education, Hue University, Hue city, Thua Thien Hue, Vietnam
*Corresponding author: Hai Thi Hong Truong 
ORCID 0000-0002-6591-5485
Abstract: Hydrocotyle verticillata, known as aquatic pennywort, is commonly found in wetlands. Recently, it is widely cultivated as an ornamental plant in Vietnam. In this work, we characterised a four-accession germplasm of H. verticillata, gathered across Vietnam with a maximal distance of 620 km. The accessions were identified based on ITS sequencing, using primers ITSu1 and ITS2R. The four accessions displayed variations in morphological and micro-morphological traits such as stolon diameter, thickness of runner cortex, runner stele diameter, number of runner cortex cell layer, number of runner vascular bundles, diameter of runner xylem vessel elements, diameter of young root, root cortex thickness, diameter of root stele, number of cortex cell layer of root, number of xylem bundles of root, diameter of xylem vessel elements of root, leaf thickness and palisade mesophyll thickness. In addition, phytochemical contents, including Vitamin C, phenolic, flavonoid and saponin, were found to be significantly different among H. verticillata accessions, while reducing sugar, carotenoid and tannin contents were comparable. Finally, genetic diversity was demonstrated within the germplasm using RAPD genotyping. While the number of accessions was small (four accessions), this report showcased the diversity of aquatic pennywort accessions in Vietnam, warranting further studies into this vegetatively propagated species. These findings formed the basis for further research on breeding, cultivation and extraction of bioactive compounds from H. verticillata.
Keywords: Aquatic pennywort, Hydrocotyle verticillata, morphological, micro-morphological, RAPD.
Introduction
Aquatic pennywort (Hydrocotyle verticillata), also known as whorled pennywort, is a member of the ginseng family (Araliaceae). It is a fast-growing aquatic herb that is also an invasive aquatic weed in water bodies across the world. It can outcompete native plants, affect water quality and alter aquatic populations. While it is strictly managed in some countries, it is sold as an aquarium plant in others. At the same time, aquatic pennywort is considered as an environmental remedy in polluted water bodies as it functions as a natural oxygenator and removes both divalent iron and total iron (Chu et al., 2020). H. verticillata is still under-investigated worldwide (Daminar and Bajo, 2013; Emeka et al., 2022; Downie et al., 2014; Lim et al., 2014; Umate and Deogade, 2020).
More recently, H. verticillate is considered as a source of medicinal compounds since its extract contained bioactive compounds that are useful in treating leukemia (Daminar and Bajo, 2013; Emeka et al., 2022) and other diseases (Umate and Deogade, 2020). Useful bioactive compounds in H. verticillata leaves include 3. beta.17. beta.-dihydroxyestr-4-ene (10.89%); hexadecanoic acid, ethyl ester (7.49%), and 1-Methylbicyclo [3.2.1] octane (7.28%) (Emeka et al., 2022). These compounds may underlie antibacterial activity against both gram-positive and gram-negative bacteria exhibited by leaf extracts (Dhivya et al., 2023). H. verticillata was introduced into Vietnam, it is now found near canals, water bodies and is commonly grown as an ornamental plant. Despite their potential to be used as a medicinal plant and as a remedy for environmental pollution, H. verticillata remained under-investigated. No effort to characterize H. verticillata accessions in Vietnam has been attempted. To understand variations among H. verticillata accessions in Vietnam, we collected four accessions with a maximal distance of 620 km (Table 1, Fig. 1). The germplasm was extensively characterized in terms of morphological, micro-morphological traits, phytochemical contents and genetics. While the number of H. verticillata accessions in this study was small, this pioneering work forms the basis for future analysis of aquatic pennywort in Vietnam, and their potential to be used in medicine and environmental remedies.
Results
ITS identification
The four accessions collected in this study had been identified as H. verticillata based on the ITS sequences (GenBank accessions: OM943954.1, OM943955.1, OM943956.1 and OM943957.1), using primers ITSu1 (Cheng et al., 2016) and ITS2R (Chen et al., 2010). The sizes of the sequences were 799-802 bp, with roughly 58% GC. There were two polymorphic sites and a low rate of variable sites, 0.25%.
Morphological characterization
Among aquatic pennywort accessions, there were significant differences in quantitative morphological features but no differences in qualitative morphological traits. With their spreading leaf arrangement, orbicular leaf shape, crenate leaf edge, dark green color, glabrous leaf surface, long, thin, and light green petioles at the base, as well as their white stolons, greenish white flowers, and compound inflorescence, all four accessions exhibited good regenerability. With the exception of dry matter, there were notable variations between aquatic pennywort accessions in all quantitative morphological characteristics (Table 2, Figure 2). HUIB_HV23, an aquatic pennywort accession, had the longest and widest leaves (5.63 cm × 4.15 cm), whereas HUIB_HV17 had the shortest leaves (2.74 cm × 2.55 cm). The leaf
Table 1. Aquatic pennywort accessions collected in Vietnam. These accessions were collected while we were studying the diversity of pennywort (C. asiatica) across Vietnam.
| No. | Accession code | Location of collection | Coordinate |
| 1 | HUIB_HV17 | Ngo May, Kon Tum | 14°21'25.4"N 107°59'59.5"E |
| 2 | HUIB_HV22 | Hung Nguyen, Nghe An | 18°40'14.9"N 105°36'42.8"E |
| 3 | HUIB_HV23 | Quynh Luu, Nghe An | 19°10'51.2"N 105°39'39.9"E |
| 4 | HUIB_HV24 | Tinh Gia, Thanh Hoa | 19°32'20.3"N 105°47'40.0"E |
Table 2. Quantitative morphological traits observed in four aquatic pennywort accessions.
| Accession code | Leaf length (cm) | Leaf width (cm) | Leaf petiole length (cm) | Number of primary lateral veins | Runner length (cm) | Fresh yield at the first harvest (g) | Plant weight (g) | Dry matter (%) |
| HUIB_HV17 | 2.74 ± 0.27c | 2.56 ± 0.24b | 13.79 ± 1.09b | 13.63± 0.93b | 8.65 ± 0.82a | 542.41 ± 88.02a | 1.24 ± 0.04b | 16.800a ± 0.400 |
| HUIB_HV22 | 3.81 ± 0.36b | 2.80 ± 0.29b | 16.95 ± 1.64a | 13.17 ± 1.12b | 5.75 ± 0.77c | 549.82 ± 38.86a | 1.26 ± 0.04b | 16.267a ± 0.231 |
| HUIB_HV23 | 5.63 ± 0.62a | 4.15 ± 0.70a | 13.21 ± 1.54b | 16.03 ± 1.47a | 6.65 ± 0.71b | 474.24 ± 13.31a | 0.69 ± 0.01c | 16.467a ± 0.306 |
| HUIB_HV24 | 5.50 ± 0.60a | 4.25 ± 0.67a | 10.97 ± 1.01c | 16.37 ± 1.50a | 6.29 ± 0.64b | 295.58 ± 11.29b | 2.02 ± 0.02a | 16.400a ± 0.400 |
The same upper-case letters within columns indicate the lack of significant difference (p ≥ 0.05). Errors represent standard deviation.
petioles of HUIB_HV22 and HUIB_HV24 were the longest and shortest, respectively (16.95 cm vs. 10.97 cm).
There were 13.17 to 16.37 main lateral veins in total. With 16.37 primary lateral veins, HUIB_HV24 had the greatest, while HUIB_HV22 had the fewest (13.17). At the first harvest, there were significant differences in both plant weight and fresh yield, which ranged from 0.69 g to 2.02 g and 295.58 g to 549.82 g, respectively. At the first harvest, HUIB_HV22 (549.82 g) had the highest fresh yield while HUIB_HV24 (295.58 g) had the lowest. HUIB_HV23 had the lowest plant weight (2.02 g and 0.69 g, respectively), while HUIB_HV24 had the highest plant weight. across 16.27% and 16.80%, there was no discernible variation in dry matter across the four aquatic pennywort accessions.
Micro-morphological identification
The anatomical features of leaves, runners and roots of four aquatic pennywort accessions were showed in Figure 3. The epidermis (1) consisted of a layer of rectangular cells with uneven thickness. Inside the epidermis lied the cortex of the runner and root (2). The runner cortex comprised approximately 9-13 layers of cells, with an outer layer consisting of 1-2 collenchyma cells having thick walls, followed by parenchyma cells (8-11 layers) that were either round or polygonal, with slightly curved edges and varying sizes. The stem cortex contained large air spaces. The root cortex consisted solely of parenchyma cells, typically with 3-5 cell layers, with small air spaces. The innermost layer of the root cortex was the endodermis (6). Inside the cortex was the cylindrical region of the runner and root. The xylem (3) and phloem (4) were arranged in vascular bundles in the runner and alternate in the root. Toward the innermost part of the runner lied the pith (5), composed of parenchyma cells with thin walls, exhibiting diverse shapes and sizes. The runner typically contained 10-11 vascular bundles, while the root had 2-3 xylem branches alternating with phloem. In leaves, both the upper and lower epidermal layers consisted of flattened or oval-shaped cells with uneven dimensions. Beneath the upper epidermal cell layer was the palisade mesophyll (9), which comprised two layers of elongated, rectangular cells arranged vertically. The spongy mesophyll (10), characterized by large intercellular spaces and a variety of cell shapes and sizes, lied immediately below the palisade mesophyll.
Micro-morphological traits varied among the four aquatic pennywort accessions except for NRCOCL, ISCRu, ISCR and NPMCL (Table 3). SD varied from 1.26 mm (HUIB_HV23) to 1.72 mm (HUIB_HV17). TRC ranged from 305.00 µm (HUIB_HV22) to 502.25 µm (HUIB_HV17). RSD was the largest (802.50 µm) in HUIB_HV24 and smallest (578.50 µm) in HUIB_HV23. All aquatic pennywort accessions had 1.5 runner collenchyma cell layer (NRCOCL). NRCCL ranged from 9.50 (HUIB_HV24) to 13.00 (HUIB_HV22). The ISCRu was present in all four aquatic
pennywort accessions. HUIB_HV24 had 11 NRVB and the rest had 10 NRVB. HUIB_HV17 had the thinnest DXVE and DYR (31.55 μm and 0.17 mm, respectively) while the thickest DXVE and DYR were observed in HUIB_HV24 (48.48 μm and 0.26 mm, respectively). RCT ranged from 0.06 μm (HUIB_HV22) to 0.08 μm (HUIB_HV17 and HUIB_HV23). DRS ranged from 0.05 μm (HUIB_HV17) to 0.08 μm (HUIB_HV24). HUIB_HV17 and HUIB_HV24 had the highest NCCLR (4.5) and HUIB_HV22 had the lowest one (3.5). HUIB_HV17 having two xylem bundles of root (NXBR) while the rest had three NXBR. All the aquatic pennywort accessions had DXVER of 0.01 μm. The presence of ISCR was observed in most accessions except HUIB_HV22. LT ranged from 0.13 mm (HUIB_HV23) to 0.18 mm (HUIB_HV24). All aquatic pennywort accessions had two palisade mesophyll cell layers (NPMCL) and 0.07 μm of PMT, except HUIB_HV23 (0.06 μm of PMT).
Phytochemical analysis
We found significant differences in the contents of Vitamin C, phenolic, flavonoid and saponin but not in reducing sugar, carotenoid and tannin contents among four aquatic pennywort accessions (Tables 4 and 5). HUIB_HV23 had the highest Vitamin C content (0.401% of dry weight), while HUIB_HV22 had the lowest (0.337% of dry weight). Reducing sugar ranged from 7.639% of dry weight (HUIB_HV22) to 8.534% of dry weight (HUIB_HV24). Carotenoid ranged from 0.65% of dry weight (HUIB_HV24) to 0.71% of dry weight (HUIB_HV17). Tannin content varied from 3.37% of dry weight (HUIB_HV17) to 3.81% of dry weight (HUIB_HV22). The phenolic content varied from 0.31% (HUIB_HV23) to 0.48% (HUIB_HV24) of dry weight. HUIB_HV24 had the highest flavonoid content (6.590% of dry weight), while HUIB_HV17 had the lowest flavonoid content (4.914% of dry weight). The saponin content varied from 1.541 mg GYE/g of dry weight (HUIB_HV22) to 2.339 mg GYE/g of dry weight (HUIB_ HV17).
Genetic diversity analysis
A total of 100 UBC RAPD primers were used to screen four aquatic pennywort accessions. Out of eighteen polymorphic RAPD primers, only four primers (Table 6) yielding clear DNA bands were selected to genotype the germplasm (Fig. 4).
A total of 160 DNA bands were recorded. The highest number of bands were observed in HUIB_HV22, with 49 DNA bands (30.6%). On the other hand, the lowest number of bands were observed in HUIB_HV17 with 33 DNA bands (20.6%). The total number of amplified DNA bands with each primer ranged from 28 (UBC#384) to 45 (UBC#376) DNA bands, averaging from 7 to 11 DNA bands per primer. A total of 20 polymorphic DNA bands were obtained using four polymorphic RAPD primers from the
Table 3. Micro-morphological features observed in four aquatic pennywort accessions collected in Vietnam.
| SD | TRC | RSD | NRCOCL | NRCCL | ISCRu | NRVB | DXVE | DYR | |
| HUIB_HV17 | 1.72 ± 0.061 | 502.25±0.816 | 773.25±2.573 | 1.5±0 | 12.50±0.424 | P | 10±0 | 31.55±0.374 | 0.17±0.007 |
| HUIB_HV22 | 1.43±0.007 | 305.00±2.449 | 783.00±1.633 | 1.5±0 | 13.00±0.00 | P | 10±0 | 43.58±0.383 | 0.19±0.002 |
| HUIB_HV23 | 1.26±0.009 | 337.75±1.225 | 578.50±1.633 | 1.5±0 | 11.50±0.00 | P | 10±0 | 37.80±0.408 | 0.23±0.002 |
| HUIB_HV24 | 1.60±0.002 | 435.75±0.816 | 802.50±0.816 | 1.5±0 | 9.50±0.408 | P | 11±0 | 48.48±2.419 | 0.26±0.016 |
| p (Kruskal-Wallis) | 0.015 | 0.016 | 0.016 | 1 | 0.021 | 1 | 0.012 | 0.016 | 0.015 |
| RCT | DRS | NCCLR | NXBR | DXVER | ISCR | LT | PMT | NPMCL | |
| HUIB_HV17 | 0.08±0.003 | 0.05±0.002 | 4.5±0 | 2±0 | 0.01±0.001 | P | 0.17±0.005 | 0.07±0.004 | 2±0 |
| HUIB_HV22 | 0.06±0.002 | 0.06±0.001 | 4.0±0 | 3±0 | 0.01±0.000 | A | 0.17±0.002 | 0.07±0.00 | 2±0 |
| HUIB_HV23 | 0.08±0.003 | 0.06±0.002 | 3.5±0 | 3±0 | 0.01±0.000 | P | 0.13±0.001 | 0.06±0.001 | 2±0 |
| HUIB_HV24 | 0.07±0.008 | 0.08± 0.008 | 4.5±0 | 3±0 | 0.01±0.001 | P | 0.18±0.008 | 0.07±0.002 | 2±0 |
| p (Kruskal-Wallis) | 0.03 | 0.022 | 0.012 | 0.012 | 1 | 0.012 | 0.027 | 0.432 | 1 |
P: Present, A: Absent; Stolon diameter (mm): SD; Thickness of runner cortex (μm): TRC; Runner stele diameter (μm): RSD Number of runner collenchyma cell layer: NRCOCL Number of runner cortex cell layer: NRCCL Intercellular spaces in cortex of runner: ISCRu Number of runner vascular bundles: NRVB Diameter of runner xylem vessel elements (μm): DXVE Diameter of young root (mm): DYR Root cortex thickness(μm): RCT Diameter of root stele (μm): DRS Number of cortex cell layer of root: NCCLR Number of xylem bundles of root: NXBR Diameter of xylem vessel elements of root (μm): DXVER Intercellular spaces in cortex of root: ISCR Leaf thickness (mm): LT Palisade mesophyll thickness (μm): PMT Number of palisade mesophyll cell layer: NPMCL
Table 4. Vitamin C content, reducing sugar and carotenoid contents in four aquatic pennywort accessions.
| No. | Accession | Vitamin C content (% dry weight) |
Reducing sugar (% dry weight) |
Carotenoid (mg/100 g dry weight) |
|---|---|---|---|---|
| 1 | HUIB_HV17 | 0.380b ± 0.009 | 7.883a ± 1.338 | 0.707a ± 0.074 |
| 2 | HUIB_HV22 | 0.337a ± 0.012 | 7.639a ± 0.899 | 0.666a ± 0.073 |
| 3 | HUIB_HV23 | 0.401b ± 0.013 | 8.331a ± 0.694 | 0.685a ± 0.051 |
| 4 | HUIB_HV24 | 0.346a ± 0.020 | 8.534a ± 1.084 | 0.650a ± 0.005 |
The same lower-case letters within columns indicate the lack of significant difference (p ≥ 0.05). Errors represent standard deviation.
Table 5. Tannin, phenolic, flavonoid and saponin contents in four aquatic pennywort accessions.
| No | Accession | Tannin content (% dry weight) | Phenolic content (mg GAE/g of dry weight) | Flavonoid content (mg CE/g of dry weight) | Saponin content (mg GYE/g of dry weight) |
|---|---|---|---|---|---|
| 1 | HUIB_HV17 | 3.370a ± 0.254 | 17.379a ± 0.495 | 4.914a ± 0.644 | 2.339c ± 0.046 |
| 2 | HUIB_ HV22 | 3.813a ± 0.096 | 18.110ab ± 0.792 | 4.990a ± 0.555 | 1.541a ± 0.033 |
| 3 | HUIB_HV23 | 3.592a ± 0.440 | 17.332a ± 0.235 | 5.314a ± 0.649 | 1.593a ± 0.062 |
| 4 | HUIB_HV24 | 3.658a ± 0.240 | 18.951b ± 0.548 | 6.590b ± 0.144 | 1.939b ± 0.038 |
The same lower-case letters within columns indicate that the lack of significant difference (p ≥ 0.05). GAE: gallic acid equivalents, CE: catechin equivalents, GYE: gypenoside XVII equivalents. Error bars represent standard deviation.
four different aquatic pennywort accessions (the average number of polymorphic DNA bands per primer was 11.75). The band size ranged from 200 to 1600 bp.
Theses polymorphic DNA bands were employed in the development of lineage trees and the investigation of genetic diversity. According to the PIC values (Table 7) indicated that primers UBC#354, UBC#362 and UBC#376 were able to detect polymorphisms at medium levels in four aquatic pennywort accessions, however primer UBC#384 showed low level polymorphism detection. The highest values were obtained with primer UBC#354, according to the results of the MI index (the capacity to identify polymorphic loci between genotypes in each primer) and the Rp index (which is based on the distribution of alleles in the sampled genotypes; primers with the larger polymorphic bands, the higher the Rp value). As a result, this primer was the best option for identifying polymorphism in the population. Examination of each person's genetic diversity coefficient within the population. The examined samples had a high degree of diversity, as evidenced by the analysis of the genetic diversity coefficient of individuals in the population (h), which ranged from 0.049 (UBC#384) to 0.238 (UBC#354), with an average of 0.135. Between UBC#384 to UBC#354, the Shannon genetic diversity index (I) varied from 0.136 to 0.347. The four RAPD primers have a mean of 1.426 and a range of observed alleles (na) of 1.250 (UBC#384) to 1.600 (UBC#354). Furthermore, there was variation in the number of effective alleles (ne) between primers, with an average value of 1.220 and a range of 1.130 (UBC#362) to 1.431 (UBC#354). To summarize, out of the four aquatic pennywort accessions that were gathered
throughout Vietnam, primer UBC#354 displayed the highest level of genetic diversity.
Individuals of aquatic pennywort accessions had genetic similarity coefficients ranging from 0.67 to 1.00 (Fig. 5). The phylogenetic tree separated the four aquatic pennywort accessions into two major groups according to the genetic similarity coefficient. The remaining aquatic pennywort accessions were in group II, while the single aquatic pennywort accession in group I was HUIB_HV22. The aquatic pennywort accessions in this group were split into two subgroups with a genetic separation of 0.896. HUIB_HV17 was found in the first grouping, and the genetic distances of the other two subgroups (HUIB_HV23 and HUIB_HV24) were identical (Fig. 5).
Discussion
This study is the first characterization of micro-morphology, phytochemicals and molecular diversity of aquatic pennywort (H. verticillata) accessions collected across Vietnam. The phytochemical characterization of the four H. verticillata accessions collected in this study showed that Vitamin C, reducing sugar, carotenoid, saponins, tannins, flavonoids and phenolic contents were comparable with the previous study (Emeka et al., 2022) and similar to those in C. asiatica (Truong et al., 2024), an important medicinal plant that was used to treat a range of ailments (Brinkhaus et al., 2000).
Interestingly, Dhivya and co-workers (2023) reported that H. verticillata extracts inhibited the growth of bacteria such as Staphylococcus aureus, Bacillus subtilis, Escherichia coli and
Table 6. The sequence of RAPD polymorphic primers.
| No. | Primer name | Sequence |
| 1 | UBC#354 | CTAGAGGCCG |
| 2 | UBC#362 | CCGCCTTACA |
| 3 | UBC#376 | CAGGACATCG |
| 4 | UBC#384 | TGCGCCGCTA |
Table 7. Genetic diversity of four aquatic pennywort accessions.
| Primer | na* | ne* | h* | I* | PIC index | Rp coefficient | MI coefficient |
| UBC#354 | 1.600 | 1.431 | 0.238 | 0.347 | 0.238 | 3.500 | 1.164 |
| UBC#362 | 1.427 | 1.130 | 0.100 | 0.169 | 0.161 | 3.000 | 0.413 |
| UBC#376 | 1.400 | 1.194 | 0.123 | 0.192 | 0.158 | 3.500 | 0.380 |
| UBC#384 | 1.250 | 1.163 | 0.049 | 0.136 | 0.090 | 1.000 | 0.047 |
| Average | 1.426 | 1.220 | 0.135 | 0.208 | - | - | - |
| SD | 0.500 | 0.327 | 0.177 | 0.260 | - | - | - |
Note: na, Number of observed alleles; ne, Number of effective alleles (Kimura and Crow, 1964); h, Nei's genetic diversity (1973); I, Shannon genetic diversity index (Lewontin, 1972).
Figure 1. Collection sites of four Hydrocotyle verticillata accessions: HUIB_HV17, HUIB_HV22, HUIB_HV23 and HUIB_HV24.
Klebsiella pneumoniae. Therefore, H. verticillata may contain valuable medicinal compounds that warrant further investigations. Furthermore, H. verticillata is highly tolerant to waterlogging and thrives in flooding areas (Kozeko et al., 2023). Genetic investigation into the mechanism of waterlogged resistance in H. verticillata will be useful for breeding programs aiming to generate waterlogged tolerant vegetables.
Genetic diversity was assessed among the four H. verticillata accessions. Among the 100 RAPD primers used, four primers revealed high polymorphism. Primer UBC#376 showed the highest number of amplified DNA bands (45 DNA bands) while UBC#384 primer yielded the least number of amplified DNA bands (28 DNA bands) (Table 7). All primers amplified six polymorphic bands, except for primer UBC#382 with two polymorphic bands. According to Nei (1978), the greater the number of amplified DNA bands, the greater the ability to distinguish samples on the pedigree tree and the more accurate the pedigree tree can be built. Genetic diversity was detected among the H. verticillata accessions. The four aquatic pennywort accessions were divided into two groups. Group I contained only one accession (HUIB_HV22) and group II contained three accessions (HUIB_HV17, HUIB_HV23 and HUIB_HV24; Fig. 5). While accessions HUIB_HV22 and HUIB_HV23 were collected in the same province in central Vietnam, they differed in terms of genetics, micro-morphological traits and phytochemical contents. On the other hand, HUIB_HV17 was genetically closer to HUIB_HV23 and HUIB_HV24, even though it was distributed roughly 600 km away from HUIB_HV23 and HUIB_HV24.
Overall, genetic diversity is consistent with the diversity in quantitative morphological and micro-morphological traits, and phytochemical contents.
Aquatic pennywort remains largely under-investigated worldwide. In the genus Hydrocotyle, H. sibthorpioides is widely used in traditional medicine to treat fever, edema, psoriasis and liver diseases (Huang et al., 2013a). It contained valuable compounds that displayed anti-tumour, liver-protective and neuro-protective effects (Yu et al., 2007; Huang et al., 2013b; Hazarika et al., 2022). While preliminary work by Emeka and co-workers (2022) demonstrated the abundance of tannins and alkaloids in H. verticillata leaf extracts, it remains to be explored whether H. verticillata contained other valuable compounds that can be used or serve as precursors for drug development programs. Towards this direction, this study contributes to a greater understanding of aquatic pennywort in Vietnam and more broadly in Asia.
Materials and methods
Plant collection, cultivation and identification using ITS sequencing
Four aquatic pennywort accessions were collected from different locations in Vietnam (Table 1). The soil that used for planting collected aquatic pennywort accessions was taken from Quang Tho, Thua Thien Hue (16°32'06.2"N, 107°31'39.7"E), a popular pennywort producing location in Vietnam (Truong et al., 2024). A mother plant was planted in a plastic tray (W40 cm x L65 cm

Figure 2. Variations in leaf morphological traits and runner length of four aquatic pennywort accessions. (A) HUIB_HV17, (B) HUIB_HV22, (C) HUIB_HV23 and (D) HUIB_HV24.
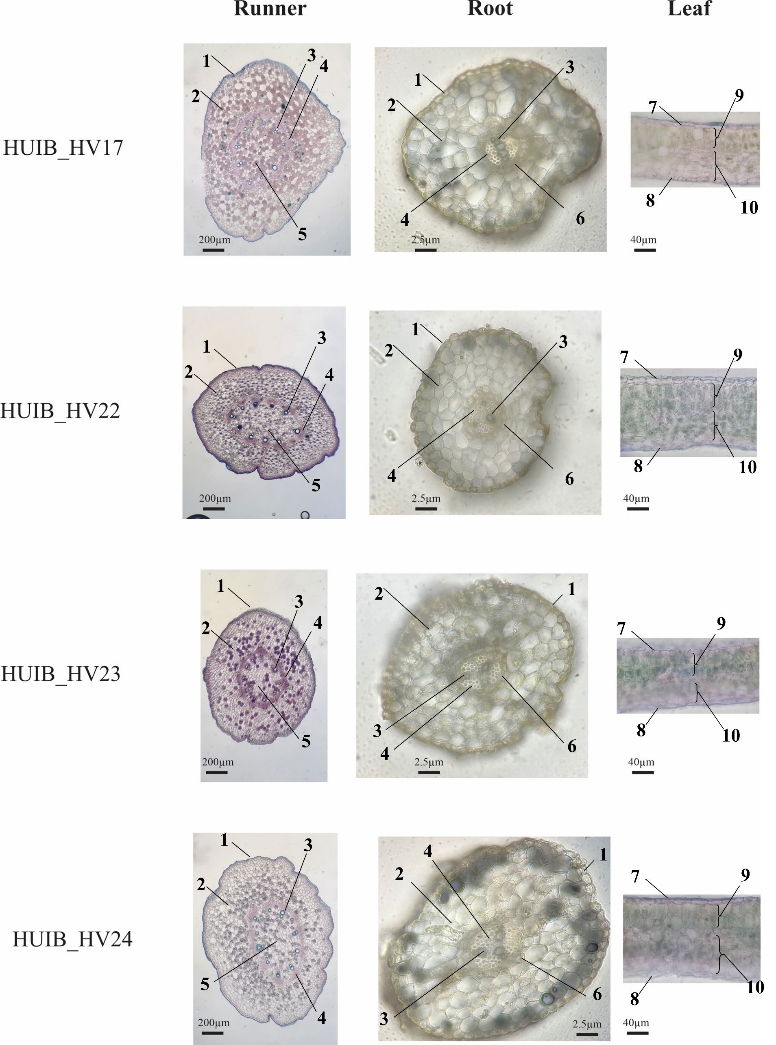
Figure 3. Anatomical features of leaves, runners and roots of the four aquatic pennywort accessions collected throughout Vietnam. (1) Epidermis, (2) Cortex, (3) Xylem, (4) Phloem, (5) Pith, (6) Endodermis, (7) Upper epidermis, (8) Lower epidermis, (9) Palisade mesophyll and (10) Spongy mesophyll.
x H18 cm) filled with 30 kg of soil and maintained water level daily up to marked point of 5 cm from tray surface. The trays were placed in full sun position (sunlight from roughly 6 AM to 6 PM) at the Institute of Biotechnology (16°29'35.7"N, 107°36'20.1"E) in Hue city, Vietnam with temperature ranging from 20oC to 35oC during the day. After five months, nine runner cuttings of each accession were planted to a new W40 cm x L65 cm x H18 cm tray with three replications for each accessions. The water level in the trays was maintained daily up to the marked point of 5 cm from the tray surface.
Genomic DNA was extracted from leaves using CTAB (cetyltrimethylammonium bromide) extraction protocol as described previously (Nguyen et al., 2023). DNA was further purified using silica columns (Biobasic, Canada). The quality of the DNA was examined on agarose gel. ITSu1-2R primer (ITSu1: GGAAGGACAAGTCGTAACAAGG (Cheng et al., 2016); ITS2R: GACGCTTCTCCAGACTACAAT (Chen et al., 2010)) were used for molecular identification of aquatic pennywort accessions. PCR were performed with ITS primers in a SimpliAmp™ Thermal Cycler (Thermo Fisher Scientific, USA). Each 15 µL reaction mixture included 7.5 µL of 2x MyFi Mix (Meridian bioscience, USA), 10 pmol of ITSu1-2R primers, 10-20 ng of template DNA. The thermocycling program involved a cycle of initial denaturation (94°C for 5 min), 30 cycles of amplification (94°C for 30 s, 60°C for 30 s and 72°C for 45 s) and a final extension (72°C for 5 min). PCR products were examined on 1% agarose gels and sequenced using Sanger sequencing method. Raw sequences for the ITSu1-4 gene region were edited in BioEdit v7.2.5 prior to depositing on GenBank (Table 2). Edited sequences were then aligned by
Figure 4. Electrophoresis of PCR products obtained from polymorphic UBC RAPD primers. M: DNA marker; white stars mark polymorphic bands. HV17 (HUIB_HV17), HV22 (HUIB_HV22), HV23 (HUIB_HV23), HV24 (HUIB_HV24).
Figure 5. Dendrogram of the genetic relationship between four aquatic pennywort accessions, based on RAPD analysis.
ClustalW in MEGA X and the non-overlapping sequence regions at the 5′- and 3′-ends were trimmed (Kumar et al., 2018).
Morphological identification
Quantitative morphological traits included leaf length, leaf width, leaf petiole length, number of primary lateral veins, runner length, fresh yield at the first harvest, plant weight and dry matter. Qualitative morphological traits included plant regenerability, leaf arrangement, leaf shape, leaf margin, leaf colour, leaf surface, petiole length, petiole thickness, petiole pigmentation at the base, stolon colour, flower colour, type of inflorescence. These traits were scored at full foliage stage (three plants per accession) at five months post transplanting. Results represent averages of three repeats, with ten randomly selected leaves each repeat.
Micro-morphological identification
Quantitative micro-morphological traits included stolon diameter (SD), thickness of runner cortex (TRC), runner stele diameter (RSD), number of runner collenchyma cell layer (NRCOCL), number of runner cortex cell layer (NRCCL), number of runner vascular bundles (NRVB), diameter of runner xylem vessel elements (DXVE), diameter of young root (DYR), root cortex thickness (RCT), diameter of root stele (DRS), number of cortex cell layer of root (NCCLR), number of xylem bundles of root (NXBR), diameter of xylem vessel elements of root (DXVER), leaf thickness (LT), palisade mesophyll thickness (PMT), number of palisade mesophyll cell layer (NPMCL). Qualitative micro-morphological traits included intercellular spaces in cortex of runner (ISCRu) and intercellular spaces in cortex of root (ISCR). Cross-sections of the runners, root and leaves were prepared and stained as described by Tran et al. (2022). Briefly, the cross-sections were immersed in 5% sodium hypochlorite for 20
minutes before being rinsed with distilled water. The cross-sections were then soaked in 1% acetic acid for about 2 minutes and rinsed with distilled water several times. The cross-sections were stained with 1% methylene blue solution for about 15-30 seconds and rinsed with distilled water several times. The sections were further stained with 10% carmine red for about 30 minutes and rinsed with distilled water several times. The stained samples were observed, photographed, and the microscopic morphological features were described using a light microscope. Microscopic dimensions were measured using a microscope eyepiece measurement. For roots with a small diameter, cross-sections were directly observed and described under a microscope. Image analysis was performed in CorelDRAW.
Phytochemical analysis
Intact healthy leaves were washed with tap water, air-dried before being dried in an oven (50oC) until the moisture content was less than 10%. The dried samples were then ground in a mortar and filtered through a sieve (pore size ≤ 1 mm). The fine powder was subject to phytochemical analysis. The total phenolic content of aquatic pennywort leaves was determined using the Folin–Ciocalteu assay as previously described (Singleton & Rossi, 1965); the total flavonoid content was measured by the aluminium chloride colorimetric assay as previously described (Zhishen et al., 1999); the total saponins content of pennywort leaves was determined using the vanillin-sulphuric acid assay as previously described (Le et al., 2018); the Vitamin C content was performed as previously described (Satpathy et al., 2021); the reducing sugar content (RSC) was determined using the 3,5-dinitrosalicylic acid (DNSA) assay as previously described (Krivorotova and Sereikaite, 2014); the total carotenoid content in aquatic pennywort leaves was determined using a colorimetric assay as previously described (Biswas et al., 2011); the total tannin content in aquatic pennywort leaves was measured as previously described (Atanassova & Christova-Bagdassarian, 2009). All the methods mentioned above were used with modifications as described in detail by Truong and co-workers (2023).
RAPD analysis
An initial screen was carried out using 100 RAPD UBC (University of British Columbia) primers. Preliminary screen showed that four RAPD primers yielded high polymorphism among aquatic pennywort accessions. PCR mixture (15 μL) included 5x buffer (3 µL), 25 mM MgCl2, 10 mM dNTP, 10 pmol of RAPD primer, 0.2 µL of Taq polymerase and 50 ng DNA template. The thermocycling program involved an initial denaturation (94 oC for 3 min), 40 cycles of amplification (94 oC for 1 min, 37 oC for 1 min and 72 oC for 2 min) and a final extension at 72oC for 7 min. PCR products were examined by gel electrophoresis (2% agarose gel in 0.5X TBE). The RAPD-PCR products electrophoresis spectrum of samples with primers were analyse according to the principle of the presence (“1”) or absence (“0”) of bands. The genetic diversity coefficient was calculated as described by Verma and co-workers (2007). Coefficient of PIC (Polymorphism Information Content), Rp (Resolving power) and MI (Marker Index) of each RAPD primer were calculated as described by Serrote and co-workers (2020). The level of genetic diversity was analyse in PopGen 3.2. The pedigree chart was built in NTSYS 2.1.
Data analysis
ANOVA analysis of variance was performed, and Duncan's test was employed to compare the mean of morphological traits. Statistical significance (p < 0.05) of micro-morphological traits was evaluated with Kruskal-Wallis test. All statistical analyses were performed in SPSS (Version 20).
Conclusion
In this work, a four-accession H. verticillata germplasm collected throughout Vietnam was characterized. The germplasm displayed variations in morphological, micro-morphological traits, phytochemical contents and genetics. This study forms the basis for further understanding H. verticillata botany, phenotypes and genotypes in Asia, where the species remains largely under-investigated.
Acknowledgement
This study was funded by the Ministry of Science and Technology of Thua Thien Hue Province (Grant No. TTH.2020-KC.09) using Thua Thien Hue province state budget. The authors also acknowledge partial support from the Core Research Program, Hue University (Grant No. NCTB.DHH.2024.03). We thank Dr. Dang Thanh Long for help with the phytochemical and genetic diversity analysis; Sonexay Rasphone, Nguyen Van Hoan for plant care.
Conflict of interest
The authors declare no conflicts of interest.
References
Atanassova M, Christova-Bagdassarian V (2009) Determination of tannins content by titrimetric method for comparison of different plant species. J. Univ. Chem. Technol. Metallurgy 44, 413-415.
Biswas A, Sahoo J, Chatli MK (2011) A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT-FOOD SCI TECHNOL 44, 1809-1813.
Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, harmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7, 427-448.
Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K, Li Y, Li X, Jia X, Lin Y, Leon C (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One 5(1), e8613.
Cheng T, Xu C, Lei L, Li C, Zhang Y, Zhou S (2016) Barcoding the kingdom Plantae: New PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Resour. 16, 138-149.
Chu SY, Jing CX, Zhang XY, Huang ZD, Xiao JB (2020) Remediation performance and mechanism of aquatic plants for iron polluted water. Chin. J. Appl. Ecol. 31(2): 608-614.
Daminar N, Bajo L (2013) Isolation and partial characterization of the most bioactive metabolite from the Hexane extract of the aerial part of Hydrocotyle verticillata (WHORLED MARSHPENNYWORTH). GJSFR 13, 1-8.
Devkota A, Jha P (2011) Influence of water stress on growth and yield of Centella asiatica. International Agrophysics 25, 211-214.
Dhivya SM, Vijayashalini P, Sasmitha V, Dhivyasree M (2023) Antimicrobial Analysis of Centella asiatica L. and Hydrocotyle verticillata Thunb. Biological Forum – An International Journal 15(5a), 553-557.
Doyle JJ, Doyle JL (1986) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19, 11-15.
Downie SR, Peery RM, Jansen RK (2014) Another first for the Apiaceae: evidence for mitochondrial DNA transfer into the plastid genome. Istanbul Üniv. Eczac. Fak. Mecm. 44(2), 131-144.
Emeka CO, Ogunka-Nnoka CAU (2022) mRNA Expression of S1PR-1 in DMBA induced Leukemia in Wistar rats treated with ethanol extract of Hydrocotyle verticillata and Laportea aestuans leaves. Asian J. Oncol. 5, 24-33.
Hazarika I, Mukundan GK, Sundari PS (2022) Neuroprotective effect of Hydrocotyle sibthorpioides against monosodium glutamate-induced excitotoxicity. Nat Prod Res 36, 6156-6159.
Huang Q, Zhang S, Huang R, Wei L, Chen Y, Liang C, Tan S, Liang S, Zhuo L, Lin X (2013a) Isolation and identification of an anti-hepatitis B virus compound from Hydrocotyle sibthorpioides Lam. J. Ethnopharmacol. 150, 568-575.
Huang Q, Huang R, Zhang S, Lin J, Wei L, He M, Zhuo L, Lin X (2013b) Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol. Lett. 217, 102-110.
Khatun B, Rahman DMO, Syeda A, Sultana S (2010) Hydrocotyle verticillata Thunb. (Apiaceae) - A new angiospermic record for Bangladesh. Bangladesh J. Plant Taxon. 17, 105-108.
Kimura M, Crow JF (1964) The number of alleles that can be maintained in a finite population. Genetics 49, 725-38.
Kozeko L, Ovcharenko Y, Jurkoniene S, Kordyum E (2023) Understanding unique tolerance limits in Hydrocotyle verticillata: From submergence to water deficiency. Aquat. Bot. 190, 103725.
Krivorotova T, Sereikaite J (2014) Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 17, 329-333.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6): 1547-1549.
Le AV, Parks SE, Nguyen MH, Roach PD (2018) Improving the Vanillin-sulphuric acid method for quantifying total saponins. Technologies 6, 84.
Lewontin RC (1972) The Apportionment of Human Diversity. In: Dobzhansky T., Hecht
M.K., and Steere W.C., eds., Evolutionary Biology, vol. 6. New York: Springer, pp. 381–398.
Lim RCJ, Yee ATK, Xin NY, Tan HTW (2014) Whorled pennywort, Hydrocotyle verticillata Thunb. (araliaceae), a new record of a casual aquatic macrophyte in Singapore. Nature in Singapore, 7, 79-91.
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583-590.
Satpathy L, Pradhan N, Dash D, Baral PP, Parida SP (2021) Quantitative determination of vitamin C concentration of common edible food sources by redox titration using iodine solution. Lett. Appl. NanoBioScience 10, 2361-2369.
Serrote CML, Reiniger LRS, Silva KB, Rabaiolli SMDS, Stefanel CM (2020) Determining the polymorphism information content of a molecular marker. Gene, 726, 144175.
Singleton YI, Rossi JA (1965) Colorimetry of total phenolics with Phosphomolybdic- Phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144-158.
Singh S, Pinokiyo A (2023) Hydrocotyle verticillata (Araliaceae): A new record for the flora of North East India. Indian J. For. 46, 58-60.
Tran LTT, Nguyen TK, Nguyen HT, Nguyen PP, Dang TYN, Tran MH et al. (2022) Morpho-anatomical study and botanical identification of Pogostemon auricularius (L.) Hassk. (Lamiaceae). Sci. Prog. 105, 00368504221094156
Truong HTH, Ho NTH, Ho HN, Nguyen BLQ, Le MHD, Duong TT (2024) Morphological, phytochemical and genetic characterization of Centella asiatica accessions collected throughout Vietnam and Laos. Saudi J. Biol. Sci. 31, 103895.
Umate P, Deogade M (2020) Pharmacognostic study of Hydrocotyl verticillata Thunb. Journal of Indian System of Medicine 8, 122-129.
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valéro JR (2007) Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 37(1), 1-20.
Verloove F, Heyneman G (2021) A note on some alien species of Hydrocotyle (Araliaceae) in Belgium. Dumortiera 117, 26-29.
Weising K, Nybom H, Pfenninger M, Wolff K, Kahl G (2005) DNA Fingerprinting in Plants: Principles, Methods, and Applications, Second Edition (2nd ed.). CRC Press.
Yu F, Yu F, McGuire PM, Li R, Wang R (2007) Effects of Hydrocotyle sibthorpioides extract on transplanted tumors and immune function in mice. Phytomedicine 14, 166-171.
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 64, 555-559.