

Aust J Crop Sci. 19(02):136-144 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.02.p143
Effects of applying Bacillus subtilis before harvest on tomato fruit physicochemical properties
Isabelly Cristina da Silva Marques1*, Dayane Mércia Ribeiro Silva2, Eduardo Santana Aires1, Fernanda Nery Vargens1, Francisco Gilvan Borges Ferreira Freitas Júnior1, Vinicius Alexandre Ávila dos Santos1, José Wilker Germano de Souza2, Wesley de Oliveira Galdino2, Francisco de Assis de Oliveira3, Elizabeth Orika Ono4, João Domingos Rodrigues4
1Department of Horticulture, School of Agronomy, São Paulo State University (Unesp), Botucatu 18618-000, Brazil
2Department of Agricultural Science, Federal University of Alagoas (Ufal), Arapiraca 57309-005, Brazil
3Department of Agricultural and Forestry Sciences, Federal Rural University of the Semi-Arid (Ufersa), Mossoró 59625-900, Brazil
4Department of Botany, Institute of Biosciences, São Paulo University (Unesp), Botucatu 18618-000, Brazil
*Corresponding author: Isabelly Cristina da Silva
Marques 
ORCID: https://orcid.org/0000-0002-2138-5849
Abstract: Plant growth-promoting bacteria (PGPB) naturally occur in the environment and offer various plant benefits. The Bacillus genus, part of the PGPB group, is effective in post-harvest disease control and may influence fruit quality mechanisms. The objective was to evaluate the pre-harvest application of different doses of a commercial B. subtilis formulation on the physicochemical and qualitative characteristics of tomato fruits. Conducted in a randomized block design with five treatments and four blocks, increasing doses of the biofungicide Serenade® (B. subtilis) were applied via foliar spray: 0.0 (Control), 2.0, 4.0, 6.0, and 8.0 L ha-1. The study measured soluble solids (SS), pH, titratable acidity (TA), ratio (SS/TA), ascorbic acid (AA), citric acid (CA), firmness, total phenolic compounds (TPC), antioxidant capacity (DPPH), lipid peroxidation (LP), hydrogen peroxide (H2O2), antioxidant enzyme activity (superoxide dismutase - SOD, catalase - CAT, and peroxidase - POD), total soluble sugars (TSS), reducing sugars (RS), starch, respiration, and ethylene content. Doses of 4 and 6 L ha-1 of B. subtilis increased SS (13%), TA (58%), AA (30%), CA (17%), TPC, DPPH (50%), H2O2 (30%), CAT activity (11%), POD, starch (46%), and respiration, while the ratio (SS/TA) was reduced by 27%, as well as LP (20%), SOD activity (6%), and RS content (28%) compared to the control. Pre-harvest application of the B. subtilis-based biofungicide at doses of 4 and 6 L ha-1 improved tomato fruits' physicochemical and qualitative characteristics, enhancing the antioxidant system and reducing membrane damage to maintain tissue integrity.
Keywords: Antioxidant enzymes; Biofungicide; Physicochemical analyses; Post-harvest analysis; Solanum lycopersicum.
Abbreviations: O.M._Organic matter; P(resin)_Phosphor; K+_Potassium; Ca2+_Calcium; Mg2+_Magnesium; CEC_Cation exchange capacity; V_Base saturation; SS_Solube solids; TA_Titrable acidity; AA_Ascorbic acid; CA_Citric acid; TPC_Total phenolic compounds; DPPH_Antioxidant capacity; LP_lipid peroxidation; H2O2_Hydrogen peroxide; SOD_superoxide dismutase; CAT_Catalase; POD_Peroxidase; RS_Reducing sugars; TSS_Total soluble sugars; RESP_Respiration; ET_Ethylene; FIRM_Firmness.
Introduction
One of the most significant vegetables in terms of area cultivated, production, yield, commercial use, and consumption, is the tomato plant (Solanum lycopersicum) (Sinha et al., 2019). The fruits of the tomato plant are widely consumed as a source of vitamin C and minerals, as they are rich in antioxidants and bioactive compounds, such as phenolic compounds, ascorbic acid, and lycopene (Collins et al., 2022).
However, tomatoes are highly perishable due to their climacteric fruit pattern, making them prone to rapid deterioration after harvest and more susceptible to microbial infection. This perishability is attributed to various physiological and physicochemical modifications, including respiration, mass loss, and pulp softening among others, during post-harvest handling (Meiramkulova et al., 2023).
Climacteric fruits, such as tomatoes, are commercially harvested after completing their growth, and their detachment from the plant is directly associated with ethylene production (Al-Dairi et al., 2021).
The quality of agricultural products is strongly influenced by various pre-harvest factors, considering that agronomic practices are recognized as a determining factor in the integrity of the tomato fruits, especially due to biotic factors (Chandrasekaran et al., 2019). However, producing fruits with reduced use of synthetic chemicals and limiting post-harvest losses poses a significant challenge, prompting research into the use of technologies based on natural molecules.
Because of this, the use of biological products has emerged as an effective alternative to address the increasing number of microorganisms resistant to chemical fungicides (Lahlali et al., 2022). These biocontrol agents, including bacteria from the PGPB group (Plant Growth-Promoting Bacteria) such as Bacillus, play a prominent role in plant establishment and growth. In tomato
Table 1. Load matrix, eigenvalues, and proportion of variation associated with PCA's two principal components (PC) of 19 fruit characteristics at 5 doses of Bacillus subtilis-based biofungicide in tomato cultivation.
| Variables | PC1 | PC2 |
|---|---|---|
| Soluble solids (SS) | 0.362 | 0.052 |
| pH (pH) | - 0.208 | - 0.097 |
| Titratable acidity (AT) | 0.322 | - 0.201 |
| Ratio (RATIO) | - 0.304 | 0.237 |
| Ascorbic acid (AA) | 0.304 | - 0.075 |
| Citric acid (AC) | 0.274 | - 0.253 |
| Firmness (FIRM) | 0.013 | - 0.394 |
| Total phenolic compounds (TPC) | 0.295 | 0.196 |
| Atioxidant capacity (DPPH) | 0.168 | 0.088 |
| Lipid peroxidation (LP) | - 0.221 | - 0.244 |
| Hydrogen peroxide (H2O2) | 0.237 | 0.259 |
| Superoxide dismutase (SOD) | - 0.039 | 0.424 |
| Catalse (CAT) | 0.048 | - 0.423 |
| Peroxidase (POD) | 0.113 | 0.198 |
| Total soluble sugars (TSS) | - 0.215 | - 0.034 |
| Reducing sugars (RS) | - 0.320 | 0.081 |
| Starch (STA) | 0.113 | - 0.103 |
| Respiration (RESP) | 0.219 | 0.094 |
| Ethylene (ET) | 0.122 | 0.259 |
| Eigenvalue | 7.359 | 5.456 |
| Percent (%) | 38.737 | 28.717 |
| Cum Percent (%) | 38.737 | 67.453 |
Fig 1. Tomato fruits treated pre-harvest with biofungicide based on Bacillus subtilis.
plants, they appear to be significantly involved in fruit quality, extending the post-harvest shelf life (Chandrasekaran et al., 2019). This suggests that these microorganisms may be linked to the physiological and biochemical metabolism of fruits (Lastochkina et al., 2019).
Several studies highlight the beneficial role of B. subtilis in the biological control of pathogens in tomato plants and fruits (Cawoy et al., 2011; Punja et al., 2016; Samaras et al., 2021). However, its impact on post-harvest quality is not well-documented. Given this gap, this study aimed to assess the pre-harvest application effect of different doses of a commercial formulation based on B. subtilis on the physicochemical and qualitative characteristics of tomato fruits. We hypothesized that the beneficial effects of this PGPR, in the appropriate quantity, control physiological processes that regulate fruit ripening.
Results
According to the analysis of variance, there was a significant effect of the doses of biofungicide based on Bacillus subtilis for the following variables: soluble solids (SS), titratable acidity (TA), ratio, ascorbic acid (AA), citric acid (CA), total phenolic compounds (TPC), antioxidant capacity (DPPH), lipid peroxidation (LP), hydrogen peroxide (H2O2), superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), reducing sugars (RS), starch and respiration, while pH, firmness, total soluble sugars (TSS) and ethylene showed no significant response to the treatments.
Physicochemical analyzes
A quadratic effect was observed for the TA, SS, and Ratio variables (Fig 2.) as a function of the doses of B. subtilis-based biofungicide applied to the tomato fruit. TA and SS increased up to the dose of 4.60 and 5.40 L ha-1 of product, with an increase of 58 and 13% compared to the control, respectively (Fig 2a. and 2b.). On the other hand, there was a reduction in the Ratio (Fig 2c.) due to the increase in product doses. This reduction was observed up to the 4.49 L ha-1 dose, 27% lower than the control.
Organic acids
The AA and CA values were adjusted to the quadratic model and increased up to the doses of 5.82 and 4.44 L ha-1 of B. subtilis-based product, with maximums of 11.66 mg g-1 of the sample and 0.41 g 100-1 of the sample, with gains of 30 and 17% compared to the control treatment, respectively (Fig 3a. and 3b.).
Total phenolic content and antioxidant capacity
The TPC content of the tomato fruit showed a positive linear response due to the increase in the doses applied, while DPPH increased up to the dose of 5 L ha-1 of the product, resulting in a higher percentage of antioxidant capacity (50%) compared to the control (Fig 4a. and 4b.).
Lipid peroxidation and hydrogen peroxide
LP decreased in the presence of B. subtilis, showing a decreasing linear response due to the doses of the biofungicide (Fig 5a.), with an average reduction of 20% compared to the control. However, the H2O2 content increased as a function of the doses of B.
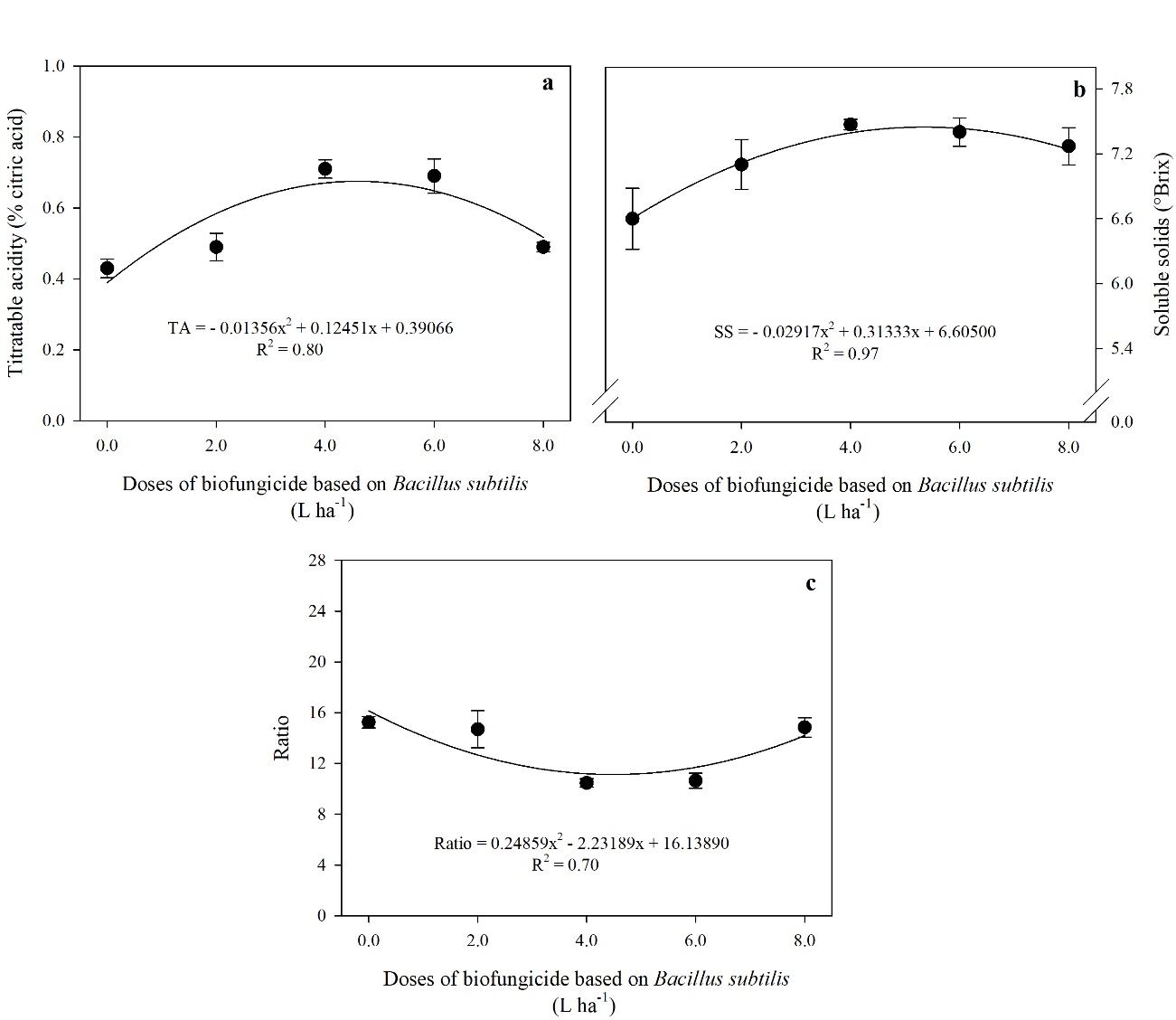
Fig 2. Titratable acidity levels-TA (a), soluble solids-SS (b), and Ratio (c) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
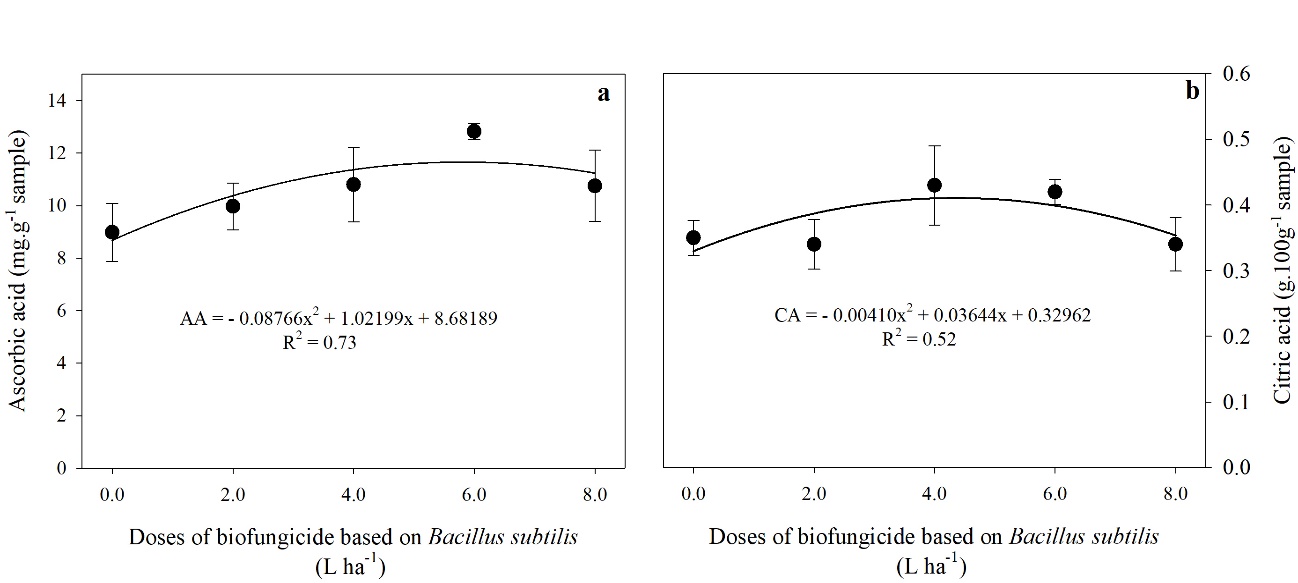
Fig 3. Ascorbic acid levels-AA (a) e citric acid-CA (b) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
subtilis applied, with a more significant increase at the highest dose (8 L ha-1) of the product, 30% higher than the control (Fig 5b.).
Antioxidant enzymes assay
The activity of the SOD enzyme showed a quadratic adjustment as a function of the doses applied, showing a reduction in this activity up to the dose of 3.15 L ha-1 of the commercial product, with a subsequent increase. When 3.15 L ha-1 of the biofungicide was applied, there was a 6% reduction in SOD activity compared to the control (Fig 6a.). On the other hand, the activity of the CAT enzyme increased with increasing doses up to 3.18 L ha-1, 11% more activity than the control (Fig 6b.).
In addition to CAT, the enzyme POD also plays an important role in eliminating H2O2 from the cellular medium, and in this study, the activity of POD in tomato fruit increased linearly due to the increase in doses of B. subtilis (Fig 6c.).
Reducing sugars, starch and respiration
The presence of B. subtilis in the biofungicide led to a reduction in RS content, with the lowest RS content observed at a dose of 5.14 L ha-1, with a 28% reduction compared to the fruit not treated with B. subtilis (Fig 7a.). On the other hand, the starch content increased as a function of the doses applied, with an increase up to the dose of 3.67 L ha-1 of the product, showing a 46% increase compared to the control (Fig 7b.). Additionally, the
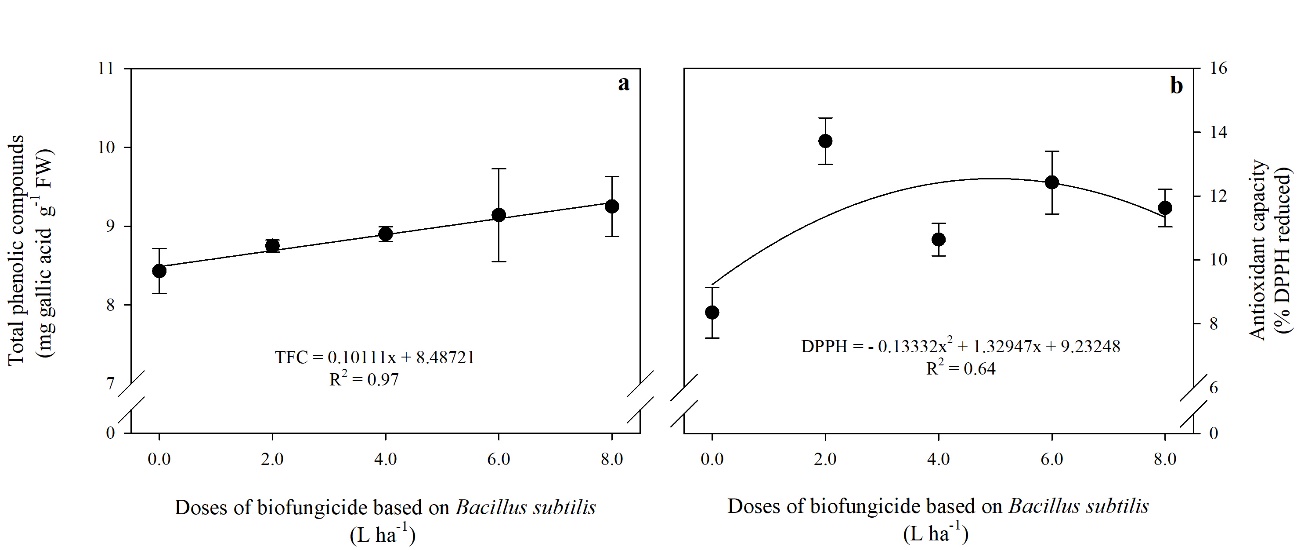
Fig 4. Levels of total phenolic compounds-TPC (a) and antioxidant capacity-DPPH (b) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
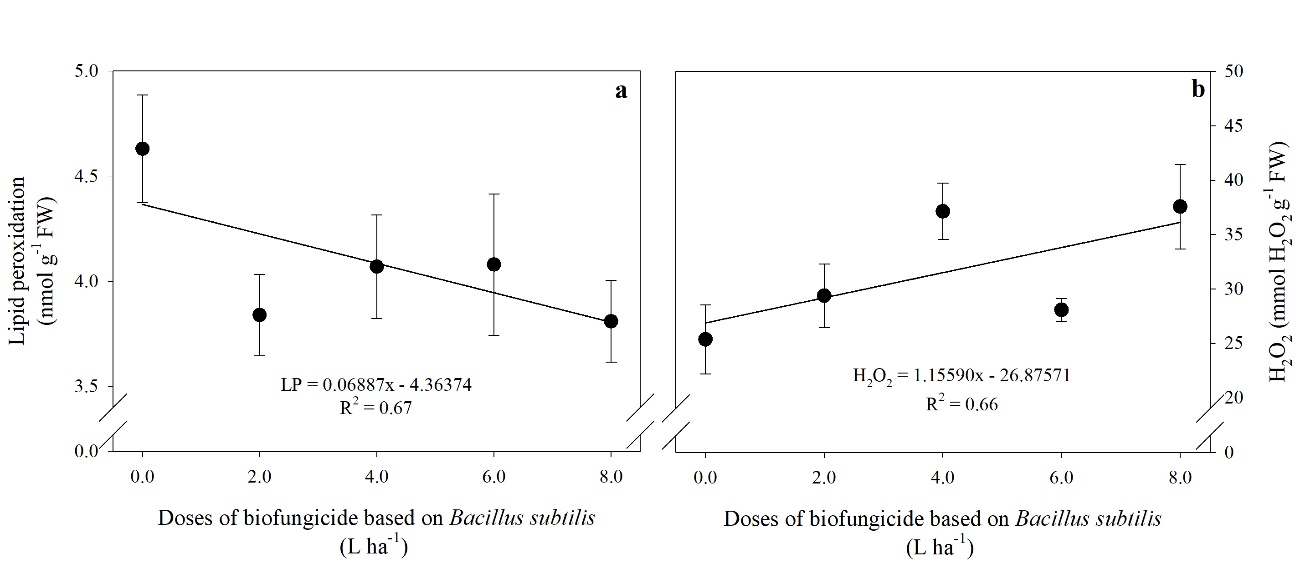
Fig 5. Lipid peroxidation-LP (a) and hydrogen peroxide content-H2O2 (b) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
fruit treated with the doses of biofungicide showed greater respiration compared to the control (Fig. 7c).
Principal component analysis
The Principal Component Analysis (PCA) of 19 tomato fruit characteristics under 5 doses of biofungicide based on B. subtilis allowed for the overall observation of data in reduced dimensions (Table 1).
The total variability was explained by four principal components (PC). However, two of them were considered the most important, as they exhibited eigenvalues > 4.0. Out of these four PCs, the first two (PC1 and PC2) represented 67.45% of the total variation. PC1 accounted for 38.7% of the total variation and effectively separated fruits treated with 4, 6, and 8 L ha-1 from other biofungicide doses (Fig 8a.). The analysis of PC1 loadings (Fig 8b.) suggests that this separation is attributed to parameters such as soluble solids (SS), ascorbic acid (AA), citric acid (CA), firmness (FIRM), total phenolic compounds (TPC), antioxidant capacity (DPPH), hydrogen peroxide (H2O2), catalase (CAT), peroxidase (POD), starch (STA), and respiration (RESP), as they exhibited positive loadings. PC1 scores and loadings revealed that doses of 4 and 6 L ha-1 led to higher CAT enzyme activity, FIRM, the concentration of AA, CA, and STA, as well as higher AT in tomato fruits due to strong positive correlations, while the 8 L ha-1 dose of biofungicide showed increased POD and DPPH activity, SS and TPC concentration. However, it resulted in higher ET content, RESP rate, as well as increased H2O2 levels.
In the score plot, PC2 was crucial in separating treatments 2 and 8 L ha-1 from the others, as these treatments exhibited positive loadings (Fig 8a.). PC2 represented 28.7% of the total variation and was primarily associated with the activity of the SOD enzyme, RS content, and Ratio in fruits treated with 2 L ha-1 (Fig
8b.). The control treatment (A) caused higher membrane damage (LP), pH, and TSS concentration.
Discussion
As a climacteric fruit, tomato ripening during the post-harvest period can cause a series of physicochemical transformations that are characterized by physiological and biochemical changes in the fruit, such as changes in color, firmness, appearance, soluble solids content, pH, and titratable acidity, among others. Therefore, these parameters are great indicators when it comes to fruit quality.
The pH of the tomatoes, normally associated with loss of acidity during ripening, was not affected by the treatments, indicating that all the fruit were in the green-ripe stage. Titratable acidity (TA), a crucial factor in fruit flavor, was higher in fruit treated with 4 and 6 L ha-1 of the B. subtilis-based biofungicide. This suggests that the pre-harvest application of this agent contributes to an increase in organic acids in the fruit, positively influencing its sensory characteristics, such as flavor and acidity, as observed by Nascimento et al. (2013).
Similarly to TA, the soluble solids (SS) content, an important indicator of ripeness and sensory quality (Al-Dairi et al., 2021), increased with the doses of B. subtilis, reflecting the higher quality of the fruit. On the other hand, the SS/TA (Ratio) decreased with increasing doses of B. subtilis due to the high levels of SS and TA, indicating a possible influence on the respiratory activity of the fruit. In summary, the application of B. subtilis improved the sensory quality of the tomatoes, increasing the content of organic acids, SS, and influencing the SS/TA (Ratio). These results corroborate the research by Chitarra and Chitarra (2005), which highlights the influence of organic
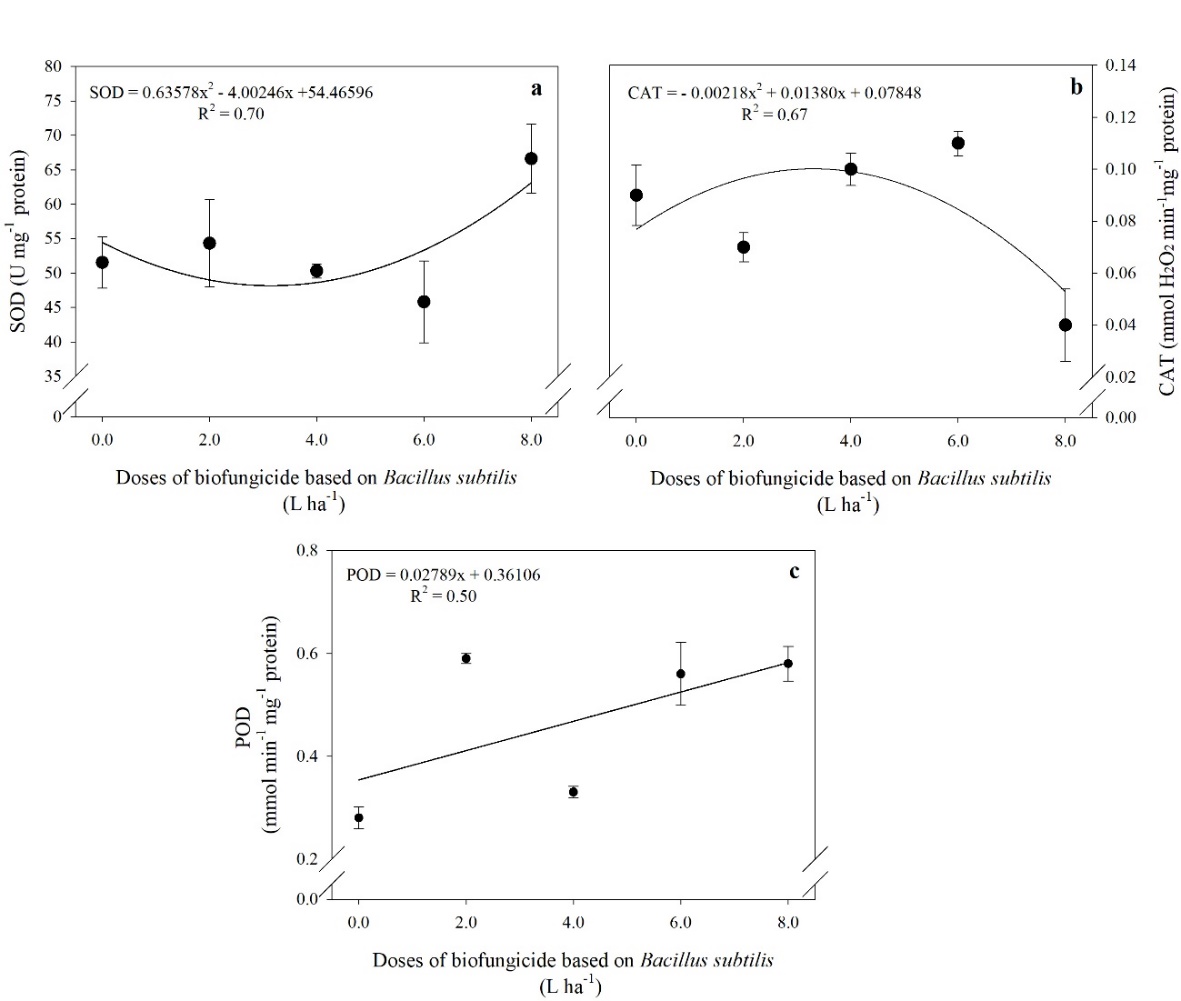
Fig 6. The activity of the enzymes superoxide dismutase-SOD (a), catalase-CAT (b), and peroxidase-POD (c) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
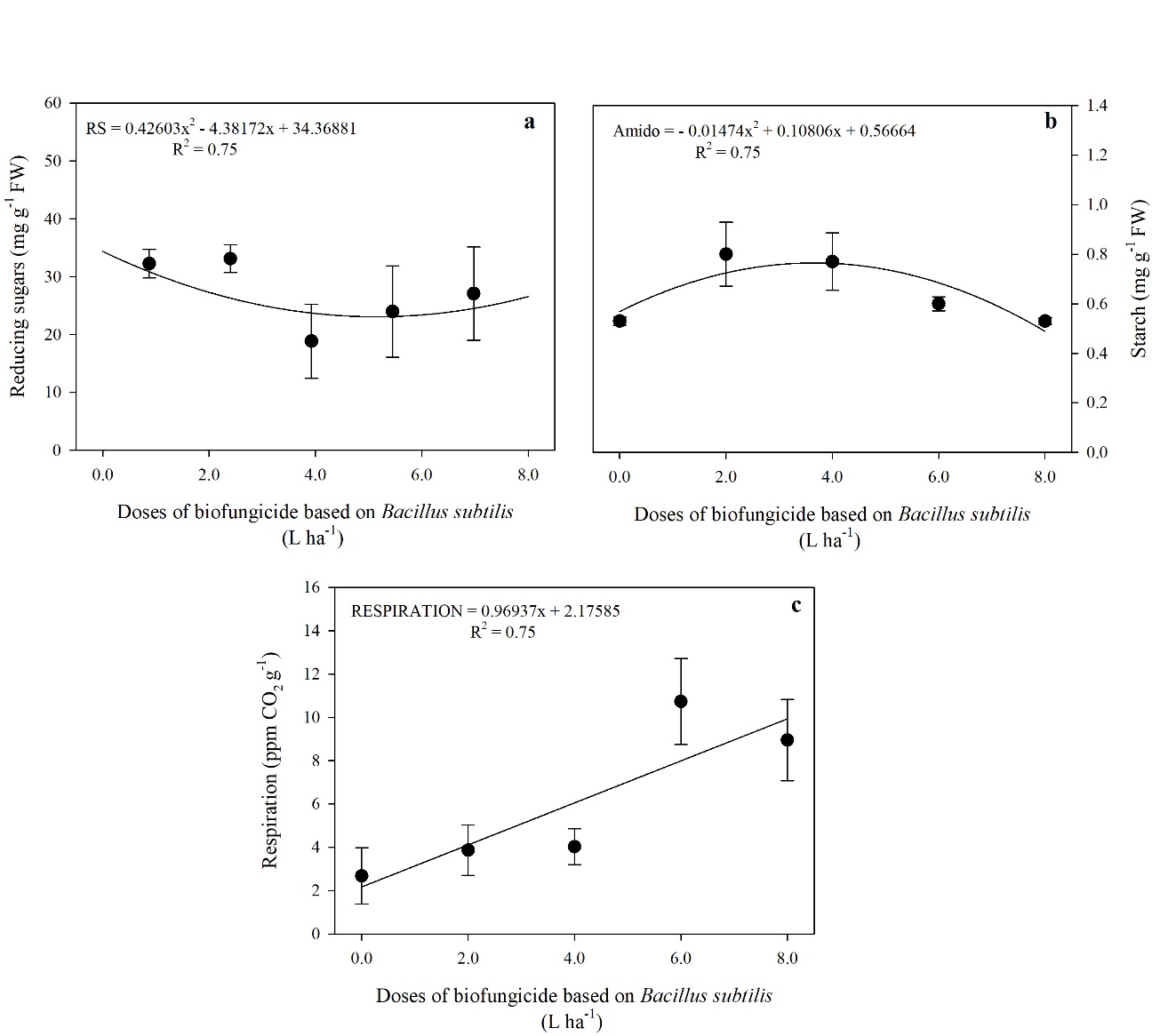
Fig 7. Contents of reducing sugars-RS (a), starch (b), and respiration (c) in tomato fruits treated with different doses of a biofungicide based on Bacillus subtilis. The bars show the standard deviation. n = 4 (number of replicates).
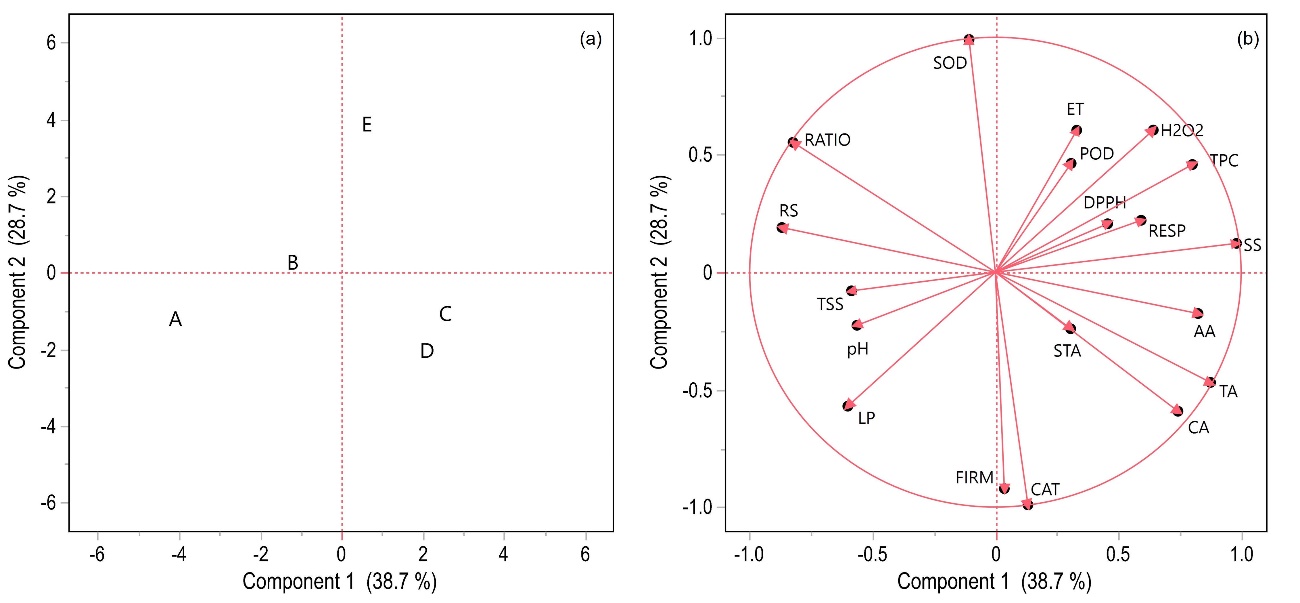
Fig 8. Score Graph (a) and Load Graph (b) of the principal component analysis of 19 characteristics of tomato fruits under 5 doses of Bacillus subtilis-based biofungicide. Score Graph legend: 0 L ha-1 (control – A), 2 L ha-1 (B), 4 L ha-1 (C), 6 L ha-1 (D), and 8 L ha-1 (E). See Table 3 for load table characteristics legend.
acids, soluble solids, and the ratio between them on the sensory quality of the fruit.
The levels of AA and CA in tomato fruit increased progressively with the doses of the B. subtilis-based biofungicide. Ascorbic acid, the predominant form of vitamin C, is sensitive and used as a quality indicator (Babu et al., 2015). It also plays a role in cell expansion and as a cofactor for enzymes involved in ethylene synthesis (Foyer et al., 2020). Citric acid, dominant in tomatoes, decreases in the post-harvest period, but its presence contributes to reducing microbial contamination, preventing browning, and maintaining quality (Liu et al., 2016). The increase in AA and CA levels, in conjunction with the biological control potential of B. subtilis, suggests a possible extension of fruit shelf life, particularly at doses of 4 and 6 L ha-1 of the biofungicide, as evidenced by Marques et al. (2024).
According to Ruiz-Cisneros (2022), the presence of Bacillus or other non-pathogenic microorganisms can contribute significantly to the synthesis of TPC due to their influence on plant growth. As investigated in this study, Chandrasekaran et al. (2019) also found that tomato fruits treated with B. subtilis had a higher amount of TPC.
Antioxidant capacity, mainly made up of phenolic compounds, is essential for eliminating free radicals, providing better fruit quality (Vega-López et al., 2022). Therefore, an increase in DPPH indicates a higher rate of free radical elimination, an essential factor in preventing tissue damage and organ aging during the post-harvest period.
During the post-harvest period, fruits tend to increase the production of reactive oxygen species (ROS) as a result of ripening, which can result in a shorter shelf life (Meitha et al., 2020). Excess ROS damages cell tissues, especially to peroxidation of the plasma membrane, causing damage to the cell membrane system (Ma et al., 2022). The elimination of free radicals by bioactive compounds in fruit is inversely related to the levels of lipid peroxidation (LP) (Suzuki and Mittler, 2006). This research shows a reduction in malondialdehyde (MDA) levels in tomato fruit treated with the biofungicide tested, suggesting that B. subtilis can delay the deterioration of tomato fruit. Therefore, a reduction in MDA indicates the prevention of oxidative damage, protection of membrane components, and maintenance of their functionality (El-Gendi et al., 2022).
On the other hand, the hydrogen peroxide (H2O2) content increased. H2O2 is a ROS commonly produced in cells under normal conditions or stress and can act, depending on its concentration, as an intracellular signaling molecule (Polychroniadou et al., 2022). In previous studies, the increase in H2O2 after harvest was associated with oxidative and membrane damage (Guo et al., 2020; Qin et al., 2009; Ren et al., 2016),
however, in this study, the increase in H2O2 content did not negatively affect the membrane integrity or antioxidant capacity of the fruit, suggesting that this increase may be related to ripening signaling.
In addition to bioactive compounds such as flavonoids, total phenolic compounds, and organic acids, the antioxidant system includes various antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) (Bautista-Rosales et al., 2022). SOD plays a primary role in the defense against oxidative damage, converting the superoxide radical into H2O2. The CAT and POD enzymes act synergistically to break down this H2O2 into water and O2, contributing to cellular detoxification (Tan et al., 2020).
In this study, the increase in H2O2 levels with increasing doses of B. subtilis was observed, despite the greater activity of the CAT and POD enzymes, whose function is to remove H2O2. However, despite the increase in H2O2 levels, lipid peroxidation was reduced with increasing doses of the biofungicide. As indicated by Padró et al. 2021, this can be attributed to the ability of B. subtilis microorganisms to modulate ROS formation. Therefore, the doses of B. subtilis at the beginning of the post-harvest period of the tomato fruit maintained the redox state of the cell tissue, the integrity of the plasma membrane, and the antioxidant capacity.
During this research, the firmness and total soluble sugar content of tomato fruit were not affected by the doses of B. subtilis, showing that it does not compromise the structural integrity of the fruit and does not negatively affect its soluble carbohydrate content. In post-harvest quality, sugars act as energy substances and signaling molecules, regulating the degree of sweetness of the fruit and various physiological processes, ranging from ripening to senescence (Li et al., 2021).
The reduction in reducing sugar (RS) content and increase in starch content in response to doses of B. subtilis suggest a positive impact of the biofungicide on the post-harvest quality of the fruit. Considering that the RS content tends to accumulate during ripening, and the starch content decreases throughout ripening, this variation points to a potential extension of the shelf life of tomato fruits and, consequently, a longer shelf life (Gao et al., 2019).
According to Chitarra (1998), an increase in the respiration rate is generally associated with a reduction in shelf life, and in this study, a higher respiration rate was observed in response to the doses of B. subtilis. On the other hand, the ethylene content was not affected by the treatments, making it difficult to identify the role of the biofungicide as an inhibitor of the synthesis of this hormone, considering that in the post-harvest period, climacteric fruits, such as tomatoes, show a peak in ethylene production, accompanied by an increase in the respiration rate (Gamrasni et al., 2020). In contrast, the results show that the presence of this microorganism positively influences the other physiological responses of the fruit in the synthesis of biochemical compounds and maintenance of antioxidant capacity, contradicting the traditional relationship between respiration rate and post-harvest quality.
Given the high perishability of tomato fruits and the need for technologies to enhance their post-harvest quality, ensuring a longer shelf life for these fruits, B. subtilis boosted the antioxidant system, reduced membrane damage while maintaining the integrity of the plant tissue, and contributed to maintaining the content of organic acids and sugars in tomato fruits.
Materials and Methods
Experimental area and cultivation conditions
The experiment was conducted in Mogi Guaçu-SP (22º22'15" S, 46º56'38" W, and 638 m above sea level), characterized by a Cwa climate as mesothermal, with a dry winter, and average temperatures ranging from 18ºC to 22ºC, and precipitation index between 1100 and 1700 mm, according to the Koppen classification (Sparovek et al., 2007).
The experiment selected the tomato variety STVE4466, a table tomato with indeterminate growth, developed by the Seminis® group. Seedlings were transplanted into beds with a planting spacing of 1.2 m in width and 4.5 m in length, containing 26 plants per plot, arranged in 2 rows with a spacing of 75 cm between rows and 35 cm between plants. The average temperature and relative humidity during the experiment were 26°C and 50%, respectively.
The soil of the experimental area was characterized as Sandy Red Latosol, presenting the following chemical properties: pH (CaCl2) = 5.1; O.M. = 23.0 g dm-3; P(resin) = 29.0 mg dm-3; H+Al = 28.0 mmolc dm-3; K+ = 2.5 mmolc dm-3; Ca2+ = 22.0 mmolc dm-3; Mg2+ = 10.0 mmolc dm-3; CEC = 62.7 mmolc dm-3, and V = 55%. Fertilization was carried out according to soil analysis and recommendations for tomato cultivation (Trani et al., 2015). The irrigation system was implemented using drip irrigation, with a control station and a flexible polyethylene tube system.
Experimental design and application of treatments
The research employed a randomized block design with 5 treatments and 4 blocks. The treatments consisted of increasing doses of a biofungicide based on Bacillus subtilis: T1 – (Control – no application), T2 – (2.0 L ha-1), T3 – (4.0 L ha-1), T4 – (6.0 L ha-1), and T5 – (8.0 L ha-1). The commercial product Serenade® (Bayer S.A) was used for the treatments, containing the biological agent Bacillus subtilis QST713 at a concentration of 13.68 g L-1 of the active ingredient.
The pre-harvest applications of Serenade® were applied by foliar spray and conducted weekly, starting from the fruit developmental phenological stage. Fruits, identified in the 4th cluster with the same age, underwent four product applications before harvest. All applications took place between 8:00 and 10:00 am, utilizing a pressurized sprayer with an operating pressure of 2.5 kgf cm-2, equipped with nozzle sizes 110.01, nozzle spacing of 40 cm, boom width of 1.2 m, and boom height of 40 cm.
Harvesting of fruit and analyzed variables
The harvest was conducted when the fruits reached the "turning" stage, with 30 to 50% red coloration. After the harvest, the fruits were washed with distilled water and dried with paper towels for the following analyses: soluble solids content, pH, titratable acidity, ratio, ascorbic acid and citric acid content, firmness, total phenolic compounds content, antioxidant capacity (DPPH), lipid peroxidation, hydrogen peroxide content, activity of antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), total soluble sugars and reducing sugars content, starch content, respiration rate, and ethylene content (Fig 1.).
The physicochemical characteristics of the tomato fruits were assessed by homogenizing two fruits per experimental unit. The soluble solids (SS) content was determined using an Atago digital refractometer, expressed in ºBrix. The pH was measured in an aqueous extract of the fruit pulp using a Micronal model B-221 potentiometer. Titratable acidity (TA) was calculated in grams of citric acid per 100 g of pulp by titration with a standardized 0.1 N sodium hydroxide solution (Brasil, 2005). The ratio (SS/TA) was determined as the relationship between soluble solids and titratable acidity. The ascorbic acid (AA) content was obtained by titration in 0.5% oxalic acid with 0.01 N 2,6-dichlorophenolindophenol (DCPI) (Brasil, 2000). The quantification of citric acid (CA) was carried out according to the methodology proposed by Choy et al. (1963). The results were expressed in different units for each parameter evaluated.
Fruit firmness was measured using a digital penetrometer model PTR-500 from Instrutherm®, at two central points of two whole fruits with skin, and the results were expressed in kgf cm-2.
For the biochemical analyses, including total phenolic compounds, antioxidant capacity (DPPH), lipid peroxidation, hydrogen peroxide, superoxide dismutase, catalase and peroxidase enzyme activity, total soluble sugars, reducing sugars and starch content, the fruits were collected and frozen instantly in liquid nitrogen. In the laboratory, the fruits were stored in an ultra-freezer at -80°C until analysis.
The concentration of total phenolic compounds (TPC) was determined by the Folin-Ciocalteu spectrophotometric method (Singleton et al., 1999), using a UV-visible spectrophotometer. The results were expressed in μg of gallic acid per g of fresh weight.
The total antioxidant capacity (DPPH) was determined following the methodology of Brand-Williams et al. (1995). The 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution was prepared in 80% methanol and the samples were extracted, centrifuged, homogenized, and stored before reading in a UV-visible spectrophotometer. The results were expressed as %DPPH reduced.
Lipid peroxidation (LP) was determined using the technique suggested by Heath and Packer (1968), using 300 mg of frozen plant material in 5 mL of a solution composed of 0.25% thiobarbituric acid (TBA) and 10% trichloroacetic acid (TCA). The samples were incubated in a water bath at 90ºC for 1 hour, followed by cooling in an ice bath and centrifugation at 10,000 rpm for 15 minutes at room temperature. The supernatant was collected for reading in a spectrophotometer at two wavelengths (560 and 600 nm), using the molar extinction coefficient of malondialdehyde (155 mmol L-1 cm-1).
Hydrogen peroxide (H2O2) levels were assessed as per Alexiava et al. (2001). Samples (100 mg) were centrifuged at 12,000 rpm for 15 minutes at 4°C. The reaction mix included extract, phosphate buffer, and potassium iodide. After incubation and spectrophotometric analysis, H2O2 concentration was determined using a standard curve.
To obtain the activity of the antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), enzyme extraction was carried out according to the methodology proposed by Kar and Mishra (1976), where 300 mg of the frozen plant material was extracted by centrifugation in 5 mL of chilled potassium phosphate buffer (0.1 M - pH 6.7) and 1% (w/v) polyvinylpolypyrrolidone at 10,000 rpm for 10 minutes at 4°C. The extract obtained was separated into 1.5 mL eppendorf tubes and stored at -80°C for later determination. From this extract, the total soluble protein content was determined using the methodology of Bradford (1976), SOD activity was determined using the method of Giannopolitis and Reis (1977), CAT followed the procedure suggested by Peixoto et al. (1999) and POD was quantified according to the methodology proposed by Teisseire and Guy (2000).
For the determination of sugar content, 100 mg of frozen and macerated fruit samples were weighed in 2 mL Eppendorf tubes. The total soluble sugar (TSS) and starch content were quantified according to the methodology described by (Yemm and Willis, 1954), and the results were calculated based on the glucose standard curve, expressed in mg g-1 fresh weight. The reducing sugars (RS) were quantified according to the methodology of (Miller, 1959), and the results were calculated based on the glucose standard curve, expressed in mg g-1 fresh weight.
To assess respiration rate and ethylene content, two tomatoes from each replication were stored for 12 hours in sealed jars at room temperature. Ethylene was determined by removing the air from the jars with a gas-tight syringe and injecting 2.0 mL of the air into a gas chromatograph (GC-FID Varian, model CP-3800) under specific conditions. The retention time for ethylene was 7.40 minutes, using an ethylene gas cylinder for calibration. For the analysis of released CO2, the air was removed with a gas-tight syringe, and manual injections of 2.0 mL were made into the chromatograph, with the CO2 retained for 4.5 minutes. A cylinder of CO2 gas (White Martins, 99.5% purity) was used for the calibration curve.
Statistical analysis
For the statistical analysis, the data were previously subjected to the Anderson-Darling homogeneity test using the Minitab program. Once the normality of the data had been verified an analysis of variance (F test) and polynomial regression were carried out using the Agroestat® program (Barbosa and Maldonado, 2015). Graphs were constructed using the SigmaPlot® software. Principal component analysis was performed using JMP 10 statistical software (SAS Institute Inc., USA).
Conclusions
This research highlighted the importance of using biofungicide based on B. subtilis before harvest, particularly in doses of 4 and 6 L ha-1, demonstrating improvements in the physicochemical and qualitative characteristics of the post-harvest quality of tomato fruits, through the regulation of the physiological metabolism of the fruit.
Acknowledgments
The authors would like to express their gratitude to the Faculty of Agricultural Sciences at São Paulo State University "Júlio de Mesquita Filho" (UNESP), Botucatu Campus, and to all its servers, who contributed to the development of this study. Special thanks are extended to Bayer-Brazil for their technical support throughout the experimental trial. Additionally, we would like to acknowledge the support provided by the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) Financing Code 001, for this research.
References
Al-Dairi M, Pathare PB, Al-Yahyai R (2021) Effect of Postharvest Transport and Storage on Color and Firmness Quality of Tomato. Horticulturae. 7(7):1-15. https://doi.org/10.3390/horticulturae7070163.
Alexiava V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24(12):1337–1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x.
Babu I, Ali MA, Shamim F, Yasmin Z, Asghar M, Khan AR (2015) Effect of calcium chloride treatments on quality characteristics of loquat fruit during storage. Int. J. Adv. Res. 3:602–610.
Barbosa JC, Maldonado JW (2015) AgroEstat—Sistema para Análises Estatísticas de Ensaios Agronômicos; FCAV/UNESP: Jabo-ticabal, Brazil.
Bautista-Rosales PU, Ochoa-Jiménez VA, Casas-Junco PP, Balous-Morales R, Rubio-Melgarejo A, Díaz-Jassi AE, Berumen-Varela G (2022) Bacillus mojavensis enhances the antioxidant defense mechanism of soursop (Annona muricata L.) fruits during postharvest storage. Arch. Microbiol. 204(9):1-10. https://doi.org/10.1007/s00203-022-03199-9.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7(72):248-254. https://doi.org/10.1016/0003-2697(76)90527-3.
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT. 28(1):25-30. https://doi.org/10.1016/S0023-6438(95)80008-5.
Brasil (2000) Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa nº1, de 7 de janeiro de 2000. Complementa padrões de identidade e qualidade para polpas de fruta. Brasília, 18p.
Brasil (2005) Ministério da Saúde. Agência Nacional de vigilância Sanitária. Métodos físico-químicos para análise de alimentos. Brasília, 1018p.
Cawoy H, Bettiol W, Fickers P, Ongena, M (2011) Bacillus-based biological control of plant diseases, in Pesticides in the Modern World - Pesticides Use and Management, ed Stoytcheva (InTech Academic Press), 273–302. https://doi.org/10.5772/17184.
Chandrasekaran M, Chun SC, Oh JW, Paramasivan M, Saini RK, Sahayarayan JJ (2019) Bacillus subtilis CBR05 for Tomato (Solanum lycopersicum) Fruits in South Korea as a Novel Plant Probiotic Bacterium (PPB): Implications from Total Phenolics, Flavonoids, and Carotenoids Content for Fruit Quality. Agronomy. 9(12):1-12. https://doi.org/10.3390/agronomy9120838.
Chitarra MIF (1998) Fisiologia e qualidade de produtos vegetais. In: BOREN, F.M. (Ed.). Armazenamento e processamento de produtos agrícolas Lavras: Ufla/SBEA, pp.1-57.
Chitarra MIF, Chitarra AB (2005) Pós-colheita de frutas e hortaliças: fisiologia e manuseio. 2ª. ed. Lavras: UFLA, 783p.
Choy TK, Quattrone JR, Elefant M (1963) Non-aqueous spectrophotometric determination of citric acid. Anal. Chim. Acta. 29:114-119. https://doi.org/10.1016/S0003-2670(00)88590-6.
Collins EJ, Bowyer C, Tsouza A, Chopra M (2022) Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology. 1(2):11-44. https://doi.org/10.3390/biology11020239.
El-Gendi H, Al-Askar AA, Király L, Samy MA, Moawad H, Abdelkhalek A (2022) Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae. 8(4):1-21. https://doi.org/10.3390/horticulturae8040301.
Foyer CH, Kyndt T, Hancock RD (2020) Vitamin C in plants: novel concepts, new perspectives and outstanding issues. Antioxid. Redox Signal. 32(7):1-70. https://doi.org/10.1089/ars.2019.7819.
Gamrasni D, Feldmesser E, Ben-Arie R, Raz A, Asiag AT, Glikman M, Aharoni A, Goldway M (2020) Gene Expression in 1- ethylcyclopropene (1-MCP) Treated Tomatoes during Pre-Climacteric Ripening Suggests Shared Regulation of Methionine Biosynthesis, Ethylene Production and Respiration. Agronomy. 10(11):1-15. https://doi.org/10.3390/agronomy10111669.
Gao YM, Tian P, Li J, Cao YN, Xu WR, Li JS (2019) Transcriptional changes during tomato ripening and influence of brackish water irrigation on fruit transcriptome and sugar content. Plant Physiol. Biochem. 145:21–33. https://doi.org/10.1016/j.plaphy.2019.10.025.
Giannopolitis CN, Reis SK (1977) Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 59(2):309-314. https://doi.org/10.1104/pp.59.2.309.
Guo DL, Wang ZG, Pei MS, Guo LL, Yu YH (2020) Transcriptome analysis reveals mechanism of early ripening in Kyoho grape with hydrogen peroxide treatment. BMC Genom. 21(784):1-18. https://doi.org/10.1186/s12864-020-07180-y.
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125(1):189-198. https://doi.org/10.1016/0003-9861(68)90654-1.
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57(2):315-319. https://doi.org/10.1104/pp.57.2.315.
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA (2022) Biological Control of Plant Pathogens: A Global Perspective. Microorganisms. 10(3):1-33. https://doi.org/10.3390/microorganisms10030596.
Lastochkina O, Seifikalhor M, Aliniaeifard S, Baymiev A, Pusenkova L, Garipova S, Kulabuhova D, Maksimov I (2019) Bacillus Spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants. 8(4):1-24. https://doi.org/10.3390/plants8040097.
Li J, Min D, Li Z, Fu X, Zhao X, Wang J, Zhang X, Li F, Guou Y (2022) Regulation of Sugar Metabolism by Methyl Jasmonate to Improve the Postharvest Quality of Tomato Fruit. J. Plant Growth Regul. 41:1615-1626. https://doi.org/10.1007/s00344-021-10415-1.
Liu KD, Liu JX, Li HL, Yuan CC, Zhong JD, Chen Y (2016) Influence of postharvest citric acid and chitosan coating treatment onripening attributes and expression of cell wall related genes incherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 198:1-11. https://doi.org/10.1016/j.scienta.2015.11.008.
Ma Q, Xu YQ, Li D, Wu XW, Zhang XC, Chen YP, Li L, Luo ZS (2022) Potential epigenetic regulation of RNA 5’-terminal NAD decapping associated with cellular energy status of postharvest Fragaria × ananassa in response to Botrytis cinerea invasion. Postharvest Biol. Technol. 186:1-11. https://doi.org/10.1016/j.postharvbio.2022.111840.
Marques ICS, Silva DMR, Aires ES, Freitas Júnior FGBF, Vargens FN, Santos VAA, Oliveira FA, Ono EO, Rodrigues JD (2024) Effect of pre-harvest application of Bacillus subtilis on the shelf life of tomato fruits. Sci. Hortic. 337:1-14. https://doi.org/10.1016/j.scienta.2024.113516.
Meiramkulova K, Devrishov D, Adylbek Z, Kydyrbekova A, Zhangazin S, Ualiyeva R, Temirbekova A, Adilbektegi G, Mkilima T (2023) The Impact of Various LED Light Spectra on Tomato Preservation. Sustainability. 15(2):1-22. https://doi.org/10.3390/su15021111.
Meitha K, Pramesti Y, Suhandono S (2020) Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020:1-11. https://doi.org/10.1155/2020/8817778.
Miller GL (1959) Modified DNS method for reducing sugars. Anal. Chem. 31:426-428.
Nascimento AR, Soares Júnior MS, Caliari M, Fernandes PM, Rodrigues JPM, Carvalho WT (2013) Qualidade de tomates de mesa cultivados em sistema orgânico e convencional no estado de Goiás. Hortic. Bras. 31(4):628-635. https://doi.org/10.1590/S0102-05362013000400020.
Padró MDA, Caboni E, Morin KAS, Mercado MAM, Olalde-Portugal V (2021) Effect of Bacillus subtilis on antioxidant enzyme activities in tomato grafting. PeerJ. 9:1-28. https://doi.org/10.7717/peerj.10984.
Peixoto PHP, Cambraia J, Sant’Anna R, Mosquim PR, Moreira MA (1999) Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 11(3):137-143.
Polychroniadou C, Michailidis M, Adamakis IDS, Karagiannis E, Ganopoulos I, Tanou I, Bazakos C, Molassiotis A (2022) Mechanical stress elicits kiwifruit ripening changes in gene expression and metabolic status. Postharvest Biol. Technol. 194:1-18. https://doi.org/10.1016/j.postharvbio.2022.112102.
Punja ZK, Rodriguez G, Tirajoh A (2016) Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 84:98-104. https://doi.org/10.1016/j.cropro.2016.02.011.
Qin G, Meng X, Wang Q, Tian S (2009) Oxidative damage of mitochondrial proteins contributes to fruit senescence: a redox proteomics analysis. J. Proteome Res. 8(5):2449–2462. https://doi.org/10.1021/pr801046m.
Ren Y, He J, Liu H, Liu G, Ren X (2016) Nitric oxide alleviates deterioration and preserves antioxidant properties in ‘tainong’ mango fruit during ripening. Hortic. Environ. Biotechnol. 58:27–37. https://doi.org/10.1007/s13580-017-0001-z.
Ruiz-Cisneros MF, Ornelas-Paz JJ, Olivas-Orozco GI, Acosta-Muñiz CH, Salas-Marina MA, Molina-Corral FJ, Berlanga-Reyes DI, Fernández-Pavía SP, Cambero-Campos OJ, Rios-Velasco C (2022). Effect of rhizosphere inoculation with Bacillus strains and phytopathogens on the contents of volatiles and human health-related compounds in tomato fruits. Food Sci. Technol. 42:1-12. https://doi.org/10.1590/fst.51120.
Samaras A, Roumeliotis E, Ntasiou P, Karaoglanidis G (2021) Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants. 10(6):1-17. https://doi.org/10.3390/plants10061113.
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 299:152-178. https://doi.org/10.1016/S0076-6879(99)99017-1.
Sinha SR, Singha A, Faruquee M, Jiku MAS, Rahaman MA, Alam MA, Kader MA (2019) Post-harvest assessment of fruit quality and shelf life of two elite tomato varieties cultivated in Bangladesh. Bull. Natl. Res. Cent. 43(185):1-12. https://doi.org/10.1186/s42269-019-0232-5.
Sparovek G, Van Lier QDJ, Dourado Neto D (2007) Short Communication Computer assisted Koeppen climate classification: a case study for Brazil. Int. J. Climatol. 27:257–266. https://doi.org/10.1002/joc.1384.
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 126:45–51. https://doi.org/10.1111/j.0031-9317.2005.00582.x.
Tan XL, Zhao YT, Shan W, Kuang JF, Lu WJ, Su XG, Tao NG, Lakshmanan P, Chen JY (2020) Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 138:1-11. https://doi.org/10.1016/j.foodres.2020.109790.
Teisseire H, Guy V (2000) Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci., 153(1):65–72. https://doi.org/10.1016/S0168-9452(99)00257-5.
Trani PE, Kariya EA, Hanai SM, Anbo RH, Bassetto Júnior OB, Purquerio LFV, Trani AL (2015) Calagem e adubação do tomate de mesa. Campinas: Instituto Agronômico. (Boletim Técnico IAC, 215).
Vega-López B, Carvajal-Miranda Y, Brenes-Peralta L, Gamboa-Murillo M, Venegas-Padilla J, Rodríguez G, Jiménez-Bonilla P, Álvarez-Valverde V (2022) Phytonutraceutical evaluation of five varieties of tomato (Solanum lycopersicum) during ripening and processing. LWT. 164:1-8. https://doi.org/10.1016/j.lwt.2022.113592.
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plants extracts by anthrone. Biochem. J. 57(3):508-514. https://doi.org/10.1042/bj0570508