

Aust J Crop Sci. 19(02):161-167 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.02.p241
Impact of accelerated ageing on seed germination capacity and antioxidant in Moroccan durum wheat
Malika Ouzouline*, Kawtar Jdaini, Halima Bouchentouf, Hana Serghini Caid*
Laboratory for Agricultural Productions Improvement, Biotechnology and Environment (LAPABE), Faculty Sciences, University Mohammed First, BP-717, 60000 Oujda, Morocco
*Corresponding authors: Malika Ouzouline 
ORCID: https://orcid.org/0009-0009-1509-3354
Abstract: The aim of this study was to investigate the effects of accelerated ageing on seed viability and vitality as well as the changes in certain chemical parameters such as electrical conductivity and the enzymatic and non-enzymatic antioxidant system of two durum wheat varieties (Isly and Marzaq). The wheat grains were placed under a temperature of 40°C and 100% humidity for a period of 2 to 8 days. The various measurements are repeated three times for each lot being studied. After a treatment exposure of 8 days, there was a significant decrease in germination capacity of 24.45% for IS and 41.67% for MZ as well as a decrease in total phenol content of 12.32% for IS and 21.07% for MZ and a decrease in carotenoid content -which is extracted in acetone- of 35.44% for IS and 7.44% for MZ. The enzymatic system was influenced by accelerated ageing, marked by a significant decrease in catalase activity of 45.59% for IS and 43.43% for MZ and a significant decrease in peroxidase activity of 62.81% for IS and 50.42% for MZ. After 24 hours of incubation in distilled water, both varieties and all lots saw an increase in electrical conductivity. Despite 8 days of treatment, there was no significant difference in the lipid composition analysis. The use of the accelerated ageing test enabled us to identify the MZ variety as the better performing variety, and the study of the antioxidant system is a good indicator of which variety is better able to withstand the effects of the accelerated ageing test.
Keywords: Accelerated ageing, germinative capacity, Saturated fatty acids, unsaturated fatty acids, carotenoids, phenols, catalase, peroxydase.
Abbreviations: IS_Isly; MZ_Marzaq; AA_Accelerated ageing.
Introduction
Given that the renewed war between Russia and Ukraine and given that 50 countries in Asia and Africa depend on Russia and Ukraine to meet 30% of their food needs for wheat and 26 countries meet 50% of their wheat needs without Russia and Ukraine (FAO, 2022), this could be a major factor in hunger and food insecurity; especially as the global economy has begun to recover from the turbulence caused by COVID-19.
Wheat must be stored in sufficient quantities under suitable, healthy conditions while ensuring the food needs of the world's growing population are met by carefully controlling temperature and relative humidity. The accelerated ageing test is considered a seed quality test and measures the deterioration of a seed lot's germination capacity caused by temporarily storing the seed under high temperature and humidity conditions. Artificial ageing protocols have been developed mainly for cereals, vegetables, forage crops and forestry crops Marcos (2015) such as rice, wheat, soybeans and tomatoes. The test is considered a method that simulates stress conditions, and these conditions lead to high respiration and consumption of reserves, resulting in degenerative changes in the metabolism of the seeds (Moraes et al., 2016), leading to their deterioration. Seed leakage also increased with storage under unfavorable conditions (Rao et al., 2006). Previous studies have shown that lipid peroxidation is the first event in the ageing process (Ebone, 2019), and there are also reports that lipid peroxidation and the destruction of cell membranes by free radicals are the main damages in the ageing process of seeds Fu (2015).
Phenolic compounds in food have recently attracted considerable attention from nutritionists, technologists and consumers alike Tsao (2010). Phenolics are among the most important antioxidant compounds in wheat. These components, which contain hydroxyl groups attached to aromatic rings, can efficiently react with and stabilize free radicals Di Silvestro (2017). Phenols are easily degraded during post-harvest storage. Their biological activity can be modified as a result De Oliveira (2017).
Carotenoids give the grains of durum wheat (Triticum turgidum) a yellowish colour. The yellow colour of pasta is an important quality feature Ficco (2014). In addition, different carotenoids are associated with different health benefits: epidemiological studies have linked the intake of carotenoids with protection against some chronic diseases such as cardiovascular disease and cancer (Cooper, 2004; Britton, 2009). Carotenoids exert a protective effect against free radical attack, and the decrease in carotenoid content in wheat seeds could make these seeds susceptible to more rapid tissue deterioration and depletion of stored reserves (Galleschi et al., 2002; Calucci et al., 2004).
The enzymatic activity of superoxide dismutase, catalase, peroxidase and glutathione reductase decreases, which reduces the respiratory capacity in older seeds and thus reduces the synthesis of ATP and assimilates of the germinating seed (Ghasemi et al., 2014).
This work aims to evaluate the effects of the accelerated ageing test on the germination capacity of two durum wheat cultivars "IS and MZ", leakage solute, lipid composition and on the enzymatic antioxidant system by measuring catalase and peroxidase and on the non-enzymatic system by monitoring phenol and carotenoid. This study aims to estimate the spoilage rate of the two durum wheat varieties to determine which variety is more suitable for storage and then develop safe storage guidelines by controlling the quality factors of moisture and temperature.
Results and discussion:
Germination capacity
Accelerated ageing is widely used in seed deterioration tests by exposing the seed to relatively high humidity and temperature conditions. Artificial ageing and natural ageing have been reported to produce similar results (Machado Neto, 2001; Kapoor, 2011; Basra, 2003). Vigour tests can be used to detect differences in the physiological quality of seed lots, especially those with similar germination times, and to estimate their ability to store and emerge seedlings in the field.
The germination capacity of stored grains is significantly impacted by accelerated ageing. It decreases significantly in the two durum wheat varieties (P < 0.05) (Fig. 1, A). It increased from 100% in the control lot to the following values in the AA8J lots, reaching 35.5% for IS and 76.17% for MZ after four days of germination. The reduction in germination capacity reached a percentage of 24.4% for IS and 41.67% for MZ after 8 days of germination. The MZ variety showed the lowest reduction in germination capacity compared to Isly. The number of germinated grains decreases with increasing germination time (Fig.1, B); Marzaq showed a slight decrease in germination speed from the first to the last day of germination.
Electrical conductivity
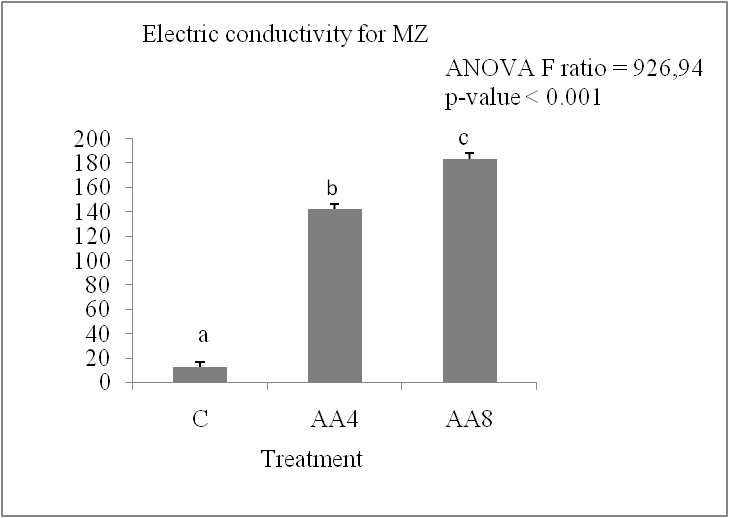
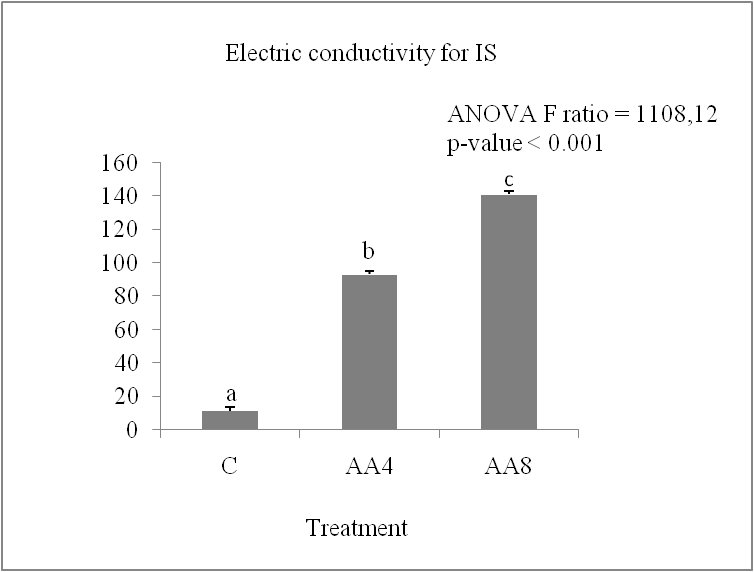
Accelerated ageing leads to a progressive increase in the release of electrolytes in the two durum wheat varieties studied. Figure 2 (A and B) shows that the electrolyte leakage, measured by the electrical conductivity in the grain imbibition medium over 24 hours, increased by 22% for IS and by about 13% for MZ. The MZ variety appears to have the least membrane damage after accelerated ageing treatment due to its low electrolyte leakage value. It is known that accelerated ageing at high temperature and 100% humidity reduces germination capacity (Gidrol et al., 1989; Bailly et al., 1998). In this work, we have shown that accelerated ageing affects the germination capacity and the speed of germination, which is visible from the second day of germination. These results are similar to those of (Calucci et al., 2004; Krishman et al., 2003) who observed a loss of germination after 8 days in soft wheat grains stored at 100% humidity and 45°C. Previous studies have shown that lipid peroxidation is the first event in the ageing process Ebone (2019), and there are also reports that lipid peroxidation and the destruction of cell membranes by free radicals are the main damages in the ageing process of seeds Yong-Bi (2015). The increase in electrolyte leakage in Isly and Marzak wheat varieties subjected to the ageing test confirms the idea of cell membrane damage. In sunflower seeds, the loss of vigour and viability during accelerated ageing is associated with a reduction in antioxidant enzyme potential, leading to lipid peroxidation (Bailly et al., 1996; De paula et al., 1996; Torres et al., 1997).
Phenol and carotenoid
During accelerated ageing of wheat grains for 4 and 8 days, significant changes in total phenolic content were observed (Table 2). After 4 days of accelerated ageing, IS and MZ varieties showed a decrease in total phenolic content by 5.23% and 7.27%, respectively. After 8 days of accelerated ageing, the reduction in phenolic content was still present, with IS and MZ durum varieties experiencing further decreases of 12.32% and 21.07%, respectively.
The carotenoid content decreased in both durum wheat varieties in the treated lots compared to the control (Table 3). This decrease became more pronounced with increasing duration of treatment. In particular, an accelerated treatment of 8 days led
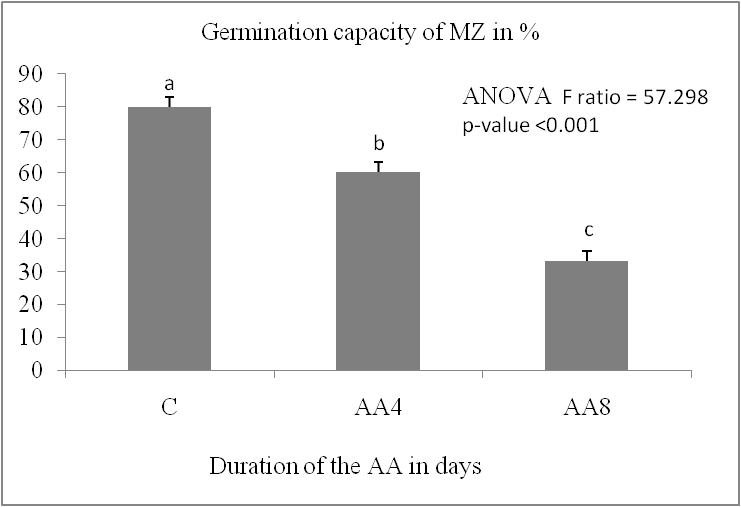
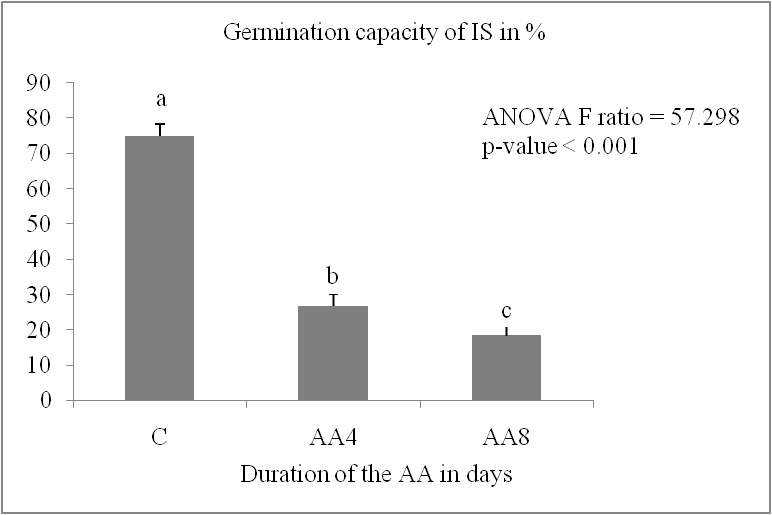
Fig 1. Germination capacity is expressed as a percentage of lots control C and accelerated ageing 8 days AA8 of two varieties, Marzaq (MZ) and Isly (IS). Vertical bars correspond to SEM. The means for 3 replicates followed by different letters are significantly different according to the Tukey test.
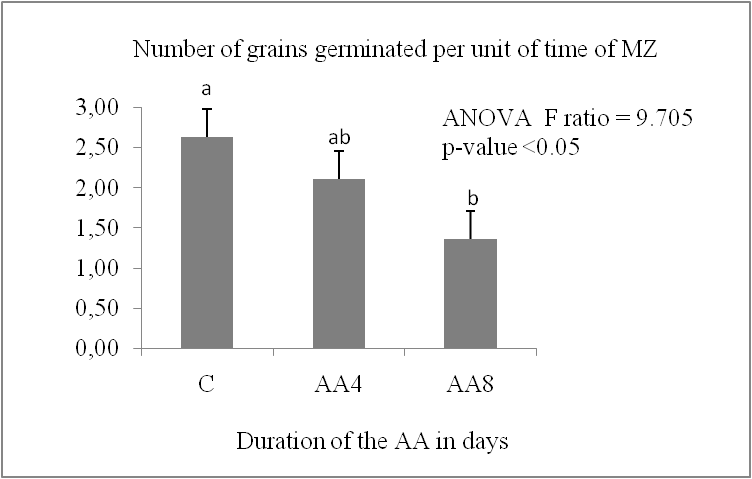
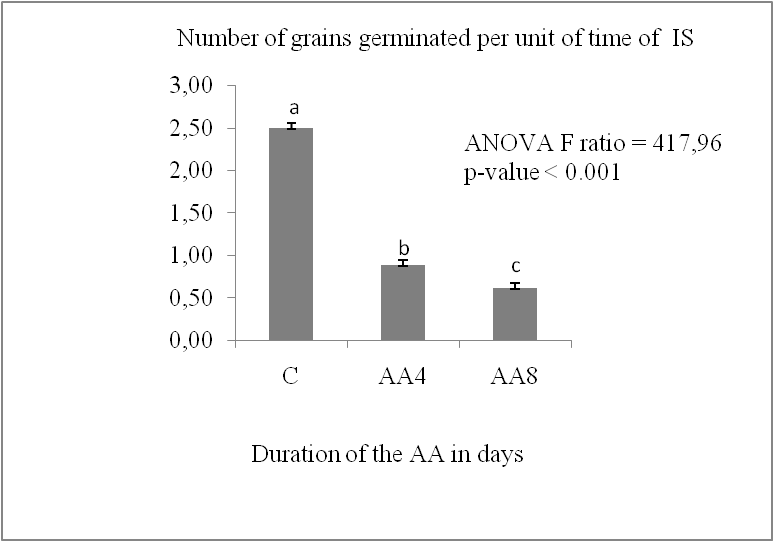
Fig 2. Germination speed of lots control C and accelerated ageing 8 days AA8 of two varieties: Marzaq (MZ) and Isly (IS). Vertical bars correspond to SEM. The Means for 3 replicates followed by different letters are significantly different according to the Tukey test.
Table 1. Changes in germination capacity, germination rates and solute leakage in artificially aged hard wheat seeds of two Moroccan varieties: Isly and Marzak. Means followed by different letters are significantly different, according to the Tukey test.
| Varieties | |||||
|---|---|---|---|---|---|
| Isly | Marzak | ||||
| C | AA8 | C | AA8 | ||
| Germination capacity | 75 ± 2.89a | 18 ± 3.33b | 80 ± 5a | 33 ± 8b | |
| Germination rates | 2.5 ± 0.1a | 0.611 ± 0.11b | 2.66 ± 0.17a | 1.11± 0.29b | |
| Solute leaking (S/gDM) | 11 ± 1.53a | 141 ± 2.31b | 12.33 ± 0.87a | 183.67 ± 4.41b | |
| DBI or AGI/AGS (%) | 3.68 ± 0.07a | 3.13 ± 0.06a | 4.09 ± 0.02a | 4.12 ± 0.01a | |
AGI: Insaturated fatty acids; AGS: saturated fatty acids; DBI: Double-bond index; S: Siemens; DM: Dry matter; C: Control lot, AA2: Accelerated ageing 2 days, AA4: Accelerated ageing 2 days, AA6: Accelerated ageing 6 days, AA8: Accelerated ageing 8 days.
Table 2. Impact of seed treatment on total phenols in mg/g FM.
| Varieties (cultivars) | treatment | Total phenol |
|---|---|---|
| Marzaq (MZ) | C | 12.09 ± 1.110 ab |
| AA4 | 11.21 ± 0.085 ab | |
| AA8 | 10.60 ± 0.813 ab | |
| Isly (IS) | C | 13.11 ± 0.672 abc |
| AA4 | 13.87 ± 0.22 abc | |
| AA8 |
|
The lots Control (C), accelerated ageing 4 days (AA4) and accelerated ageing 8 days (AA8) for two durum wheat varieties. Values are average (n = 3) ± standard deviation. Significant differences within treatments are indicated by different letters (a, b and c) in each column (p <<0.05).
to a decrease in carotenoid content of 35.44 in IS and 67.40% in MZ. In MZ, the decrease was even more pronounced.
The accelerated ageing applied to wheat grains affects the phenolic content, which progressively decreases with increasing storage time (Table 2), which is consistent with the work of Alvarez et al. (2006), who showed that the least vigorous grains have the lowest phenolic content. This could be due to their antioxidant role against the free radicals produced during accelerated ageing (Reema et al., 2004). Fujii et al. (2007) have confirmed that the polyphenols contained in grape berries can prevent oxidative stress by reducing the production of free radicals. Zhou et al. (2004) showed a positive correlation between the content of ferulic acid - the predominant phenolic acid in wheat (around 57-77% of the phenolic acids present) - and anti-free radical activity. The treatment resulted in a decrease in the phenolic content in both varieties, which is in accordance with (Tonguç et al.¸ 2023).
Phenolic acids and other polyphenolic structures can interact and change their structure during various physiological processes, and they can also vary according to variety, grain size and other factors (Weidner et al.,1996; 1999). The reduction of free phenolic carboxylic acids depends on storage conditions such as humidity, temperature and oxygen (Beweley et al., 1985). Phenolic compounds are considered to be the most important contributors to the antioxidant activity of wheat. These compounds, which contain hydroxyl groups attached to aromatic rings, can react effectively and stabilize free radicals Di Silvestro (2017). Phenolic compounds are secondary plant metabolites that contain one or more aromatic rings attached to one or more hydroxyl groups Di Loreto (2018). Our study revealed that the storage time had a significant impact on the phenolic content of whole wheat flour. After 8 days of treatment, the phenolic content decreased in all varieties compared to the control. This can be attributed to the oxidation of polyphenols Srivastava (2007). Similar results were reported by Oliveira et al. (2021), who found a reduction in sorghum flour after 60 days of storage. It was expected that the oxidation of polyphenols leads to a lower antioxidant capacity, but the formation of new antioxidant polymers or degradation products of phenols also have antioxidant activity Kallithraka (2009). Zhou et al. (2014) found a reduction in the ferulic acid concentration of brown rice after 6 months of storage. The phenolic acid content during storage is influenced by dynamic metabolic processes, as reported by (Bolling et al., 2010).
The results in the table 3 show a decrease in carotenoid content in the two durum wheat varieties treated for 8 days. Several studies have already demonstrated such a decrease in wheat
 .
.
Fig 3. Electric conductivity in expressed in S/g DM of lots control (C), accelerated ageing 4 days (AA4) and accelerated ageing 8 days (AA8) of Marzaq and Isly varieties. Vertical bars correspond to SEM. The Means for 3 replicates followed by different letters are significantly different according to the Tukey test.
after natural ageing (Cappochi et al., 2000) and after accelerated ageing (Galleschi et al., 2002). Indeed, the work of Galleschi et al. (2002) showed that accelerated ageing in a durum wheat variety led to a significant reduction in carotenoid content, especially lutein. Trono (2019) cited that the losses of carotenoids in durum wheat grains were even higher after prolonged storage. The decrease of carotenoids in the two durum wheat varieties could
Table 3. Impact of seed treatment on total carotenoids in µg/gFM.
| Treatment | Varieties | |
|---|---|---|
| IS | MZ | |
| C | 0.618 ± 0.086 abcd | 0.798 ± 0.092 cd |
| AA4 | 0.643 ± 0.109 abcd | 0.400 ± 0.073 ab |
| AA8 | 0.454 ± 0.097 abc |
|
The lots Control (C), accelerated ageing 4 days (AA4) and accelerated ageing 8 days (AA8) for two durum wheat varieties. Values are average (n = 3) ± standard deviation. Significant differences within treatments are indicated by different letters (a, b and c) in each column (p <<0.05).
Table 4. Simple linear correlations (R2) among accelerated ageing (AA) test results, germination capacity (GC), solute leaking (SL), catalase activity (Cat) and peroxidase activity (Px).
| Varieties | Correlated parameters | Correlations | R2 |
|---|---|---|---|
| Isly | AA×GC | -0.88 | 0.77 |
| AA×SL | +0.81 | 0.66 | |
| AA×Cat | -0.99 | 0.98 | |
| AA×Px | -0.96 | 0.92 | |
| Marzak | AA×GC | -0.95 | 0.90 |
| AA×SL | +0.85 | 0.72 | |
| AA×Cat | -0.97 | 0.94 | |
| AA×Px | -0.95 | 0.90 |
be due to their destruction during exposure to the accelerated ageing test, which can be explained by the non-enzymatic destruction of carotenoids, whose conjugated structure with an electron-rich double bond can to react with electrophilic oxidising agents Schaub (2017). The stability of carotenoids is largely influenced by the oxygen present in the environment Britton (2009).
Antioxidant enzymes
The activities of the two antioxidant enzymes studied, peroxidase and catalase, decreased by 45.89% in Isly and by 43.43% in Marzak, while peroxidase activity decreased by 62.81% in Isly and by 50.42% in Marzak.
Detoxifying enzymes such as superoxide dismutase (SOD), peroxidase (POD), glutathione reductase (GR), catalase (CAT) and ascorbate peroxidase (APX) are often used as indicators of seed deterioration (Kim et al., 2010; Yan et al., 2016; Boniecka et al., 2019); they protect tissues against oxidation generated by exposure to stress (Sahu et al., 2017; Wang et al., 2018). In our work, seed performance represented by a decrease in germination capacity and germination speed may be related to a decrease in the antioxidant activity of the enzymes catalase and peroxidase when the treatment time is prolonged. Changes in the macromolecular structure of the enzyme can contribute to a decrease in germination efficiency (Ghasemi et al, 2014). A progressive decline in the antioxidant capacity of catalase has been observed in cabbage and lettuce seeds (Emmanuel Adetunji et al., 2021). High temperature and humidity are the cause of the decline in peroxidases observed in cultivars, as already cited by (Diao et al., 2019), or the enzymatic activity of peroxidases depends on several factors, the most important of which are pH and temperature. The latter has an optimal pH between 4.5 and 6 and an optimal temperature between 30 and 40°C.
According to (Raza et al., 2013; Kibinza et al., 2011), there is a relationship between the antioxidant activity of enzymes and the vigour of the seeds. Reactive oxygen species (ROS) induce lipid peroxidation, which negatively affects the function of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), glutathione reductase (GR), catalase (CAT) and ascorbate peroxidase (APX) (Kim et al., 2010; Yan et al., 2016; Boniecka et al., 2019). ROS species also cause oxidation of proteins and damage to DNA, RNA and carbohydrates in many plant tissues (Hawkins et al., 2009; Mittler, 2017), including seeds (Ferguson et al., 1990; Bailly et al., 2008). Damage caused by oxidative stress is the cause of degeneration of old seeds. Oxidation by free radicals, aldehydes and enzymatic dehydrogenation of proteins can affect seed quality (Ghassemi-Golezani et al., 2010). The activity of enzymes such as peroxidase, POD, CAT, APX and SOD decreases in aged cotton seeds Goel A (2003). These enzymes eliminate ROS species and reduce their
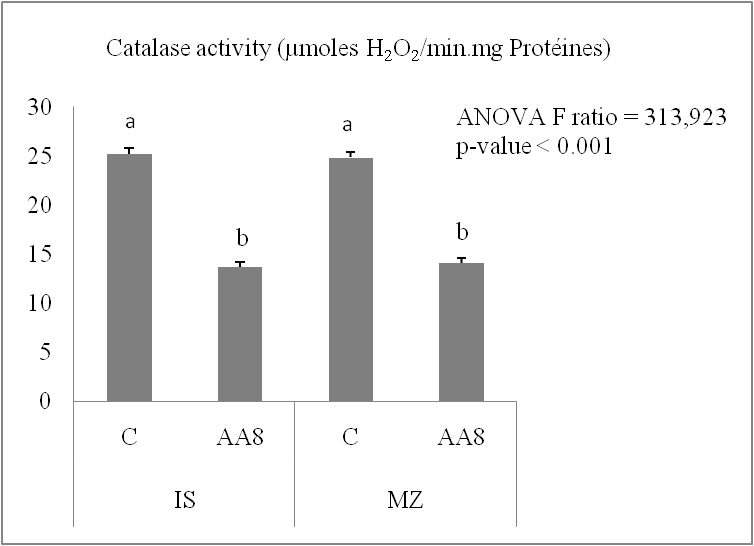
Fig 4. Catalase activity in the lots control (C) and accelerated ageing 8 days (AA8) of Marzaq and Isly varieties. Vertical bars correspond to SEM. The Means for 3 replicates followed by different letters are significantly different according to the Tukey test.
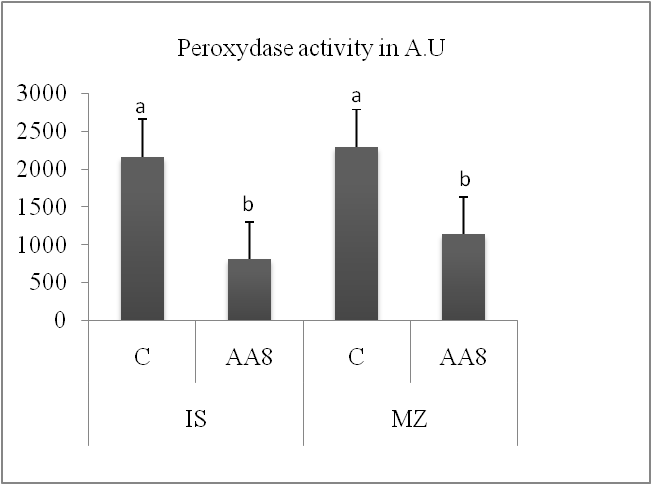
Fig 5. Peroxydase activity in the lots control (C) and accelerated ageing 8 days (AA8) of two varieties Marzaq and Isly. Vertical bars correspond to SEM. The Means for 3 replicates followed by different letters are significantly different according to the Tukey test. A.U: Arbitrary unit.
negative effects on the seeds (Kumar et al., 2015). Like peroxidase (POX), CAT is also primarily involved in the hydrolysis of hydrogen peroxide (H2O2), a highly toxic by-product of oxidative metabolism, into water and oxygen (Amjad et al., 2012; Kibinza et al., 2011; Sahu et al., 2017).
Correlation
The correlation between the measured parameters showed that accelerated ageing (AA) was positively correlated with the solute leakage content (r = + 0.81 for Isly and r = + 0.85 for Marzak) and negatively correlated with the antioxidant enzymes Cat and Px and CG (r = –0.99, r = -0.96, r = -0.88 for Isly and r = –0.97, r = -0.95, r = -0.95 for Marzak).
Materials and methods
Plant material
Two durum wheat varieties, Isly (IS) and Marzak (MZ), were the focus of the wheat varieties offered by the Provincial Directorate of Agriculture.
Accelerated ageing test
The wheat grains of the varieties (Marzak and Isly) are placed in the oven at a temperature of 40°C and a humidity of 100% according to the method of (Delouche and Baskin,1973) for a period of 2 to 8 days to obtain the following lots: Control lot (C): Accelerated ageing 0 days, Lot (AA2): Accelerated ageing 2 days, Lot (AA4): Accelerated ageing 4 days, Lot ( AA6): Accelerated ageing 6 days and lot (AA8): Accelerated ageing 8 days.
Germination study
Germination was measured in five lots: (C: Control, AA2: Accelerated ageing 2 days, AA4: Accelerated ageing 4 days, AA6: Accelerated ageing 6 days and AA8: Accelerated ageing 8 days), each containing 20 seeds. The seeds were disinfected with bleach for five minutes, rinsed three times with tap water and twice with distilled water and placed in Petri dishes with blotting paper soaked in distilled water. The Petri dishes are placed in an oven set at 25°C for a period of 2 to 7 days. Only germinated seeds are counted. Hard, fresh and rotten seeds - usually unstained seeds - need not require attention and are removed without being counted. For each box of 20 seeds, the percentage and germination speed are calculated.
Measure of electrical conductivity
To determine the electrical conductivity, 20 wheat grains from control lots AA2, AA4, AA6 and AA8 were placed in 50 ml distilled water after washing. The wheat grains were covered and incubated for 24 hours. The electrolyte release in the incubation medium was then measured using a thermo-orion conductivity meter. The conductivity of three replicates of each lot is expressed in S/gDM. (Parrish and Leopold, 1978; Serghini Caid et al., 2008).
Lipid extraction, separation and dosage
The lipids are extracted according to the method of Blight and Dyer (1959). 2 g of wheat grains are fixed in 10ml boiling water. Boiling is maintained for 3 to 5 minutes to stop any enzymatic reaction. The grains are then ground in a Turax first in 10 ml methanol and 5 ml chloroform, then in 5 ml chloroform and the remaining chloroform and 10 ml fixing water to which 1% NaCl has been added. The chloroform phase obtained after centrifugation is stored under nitrogen at -20°C. The Thin layer chromatography (Merck Silicagel G60 silica plates) is used to separate different lipid classes.
Determination of total phenols
The total phenols are determined using the Folin-Ciocalteu method (Hagerman et al., 2000). The unknown amount of phenols is determined by reference to a calibration range performed with coumaric acid. 1 to 2 g of flour is extracted in 50 ml of 1% hydrochloric acid in methanol for 24 hours. 10µl of the extract was diluted with 490µl of water, and then 0.25ml of Folin-Ciocalteu reagent and 1.25ml of a 20% aqueous sodium carbonate solution were added. At 725nm, the absorbance was measured.
Determination of Carotenoid
The determination of carotenoids was carried out according to the method of Jensen (1978). The seeds were ground in acetone at 4°C. The extraction was repeated several times until all the crushed material was used up. The optical density was measured at 480 nm after centrifugation.
Determination of anti-radical enzyme activity
Peroxidase activity determination
Wheat seed embryos are ground in a mortar at 4°C in the presence of an extraction buffer containing: KH2PO4 (1/15M), Na2HPO4 (1/15M) at pH= 6.5. After centrifugation, the collected supernatant is mixed with 3.5 ml buffer, 1ml 1% (v/v) guaiacol and 0.5ml 30% (v/v) H2O2 solution. The optical density will be measured at 470 nm every 30 seconds for 5 minutes after homogenization.
Catalase activity determination
The catalase activity was estimated according to the method of Aebi (1984). The reaction mixture contained 0.6ml enzyme extract, 0.1ml 10mmol/l H2O2 and 2ml 30mmol/l potassium phosphate buffer (pH= 7.0). The absorbance is measured at 240 nm immediately after the addition of the enzyme extract at 15 second intervals for 2 minutes. The blank is without enzyme extract. The enzyme activity was expressed in mmol H2O2 decomposed (mg protein)-1min-1.
Statistical analysis
The statistical analysis was performed using the ANOVA test SPSS version 21. Analyses were carried out in triplicate. The mean values were subjected to Tukey's test to determine whether the mean values differed significantly (p <0.05).
Conclusion
The aim of this test was to identify the best-performing varieties that are most tolerant of stressful storage conditions in order to find more practical solutions to this problem and to obtain a more accurate idea of the technological quality and health status of the seed intended for milling. The seed vigour is reduced or lost during long-term storage, even if it is stored at low temperature and low humidity. Therefore, artificial ageing treatment, which leads to a rapid decline in seed vigour, is often used to study the mechanisms by which seed deteriorates physiologically (Rajjou et al., 2008). Our results show that most of the measured parameters are strongly influenced by the applied test, which increases the levels of solute leaking in comparison to the control and decreases the levels of germination capacity, Cat and PX. Oxidative stress appears to be a major factor in the loss of seed viability during the ageing of durum wheat. The Marzak variety showed the lowest levels of catalase and peroxidase activity. We conclude that the durum wheat variety Marzaq performed best as it was able to maintain germination capacity and germination speed. However, if the antioxidant defense system of wheat varieties is impaired, there is a risk that the wheat seeds will spoil and subsequently lose their viability. For this reason, we suggest that measuring the content of phenols, carotenoids and anti-free radical enzymes can be used as a good indicator of tolerance to storage conditions.
Acknowledgment
We thank all individuals or organizations who offered help or support throughout the research process.
Compliance with Ethical Standards:
The authors declare that they have no conflict of interest.
Source of Funding:
This study was financially supported by the Faculty of Science, Mohammed I University, Oujda.
References
Ademola EA, Sershenb Boby V, Norman P (2021) Effects of exogenous application of five antioxidants on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. S AfrJBota. 137: 85-97.
Aebi H (1984) Catalase in vitro. Methods enzymol. 105: 121-126.
Alvarez P, Alvarado C, Mathieu F, Jimenez L, De la Fuente M (2006) Diet supplementation for 5 weeks with polyphenol rich cereals improves several functions and the redox state of mouse leucocytes. EurJNutr. 45: 428–438.
Amjad H, Goher M, Nayyer I (2012) Heat stress-induced cell death, changes in antioxidants, lipid peroxidation, and protease activity in wheat leaves. J. Plant Growth Regul. 31: 283–291.
Bailly C, Benamar A, Corbineau F, Côme D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated ageing. Physiol plant. 104: 646-652.
Bailly C, Benamar A, Corbineau F, Côme D (1998) Free radical scavenging as affected by accelerated ageing and subsequent priming in sunflower seeds. Physiol Plant. 104:646-652.
Basra SMA, Ahmad N, Khan MM, Iqbal N, Cheema MA (2003) Assessment of cottonseed deterioration during accelerated ageing. Seed Sci. Technol. 31: 531–540.
Beweley JD, Black M (1985) Seeds: physiology of development and germination. Plenum Press, New York, p 70-73.
Blight EG, Dyer WJ (1959) A rapid method of total lipid extraction. Canad.J. Bioch. Physiol. 37: 911-917.
Bolling BW, Blumberg JB, Chen CYO (2010) The influence of roasting, pasteurization, and storage on polyphenols content and antioxidant capacity of California almond skins. Food Chem. 123: 1040–1047.
Boniecka J, Kotowicz K, Skrzypek E, Dziurka K, Rewers M, Jedrzejczyk I,Wilmowicz E, Berdychowska, JD ˛ abrowska GB (2019) Potential biochemical,genetic and molecular markers of deterioration advancement in seeds of oilseedrape (Brassica napus L.). Ind. Crops Prod. 130: 478–490.
Britton G, Khachik F (2009) Carotenoids in food. In Carotenoids Volume 5: Nutrition and Health; Britton G, Liaaen-Jensen S, Pfander H, Eds.; Birkhäuser Verlag: Basel, Switzerland; pp. 45–66.
Britton G, Liaaen-Jensen S, Pfander H (2009) Carotenoids. Volume 5: Nutrition and Health; Birkhäuser Verlag: Basel, Switzerland.
Calucci L, Capocchi A, Galleschi L, Ghiringhelli S, Pinzino C, Saviozzi F, Zandomeneghi M (2004) Antioxydants, Free radicals, storage proteins, puroindolines and proteolytic activities in Bread wheat (Triticum aestivum) seeds during accelerated ageing. J.Agric Food chem. 52: 4274-4281.
Capocchi A, Cinollo M, Galleschi L, Saviozzi F, Calucci L, Pinzino C, Zandomeneghi M (2000) Degradation of gluten from dry and germinating wheat (Triticum durum) seeds: an in vitro approach to storage proteins mobilization. JAgric Food. Chem.48: 6271-6279.
Carcea M , Marconi E (2023). In ICC Handbook of 21st Century Cereal Science and Technology.
Cooper DA (2004) Carotenoids in health and disease: Recent scientific evaluations, research recommendations and the consumer. J Nutr. 134: 221S–224S.
Delouche JC, Baskin CC (1973) Accelerated ageing techniques for predicting the relative storability of seed lots. Seed SciTechnol. 1:427-452.
De Oliveira KG, Queiroz VAV, De Almeida Carlos L, De Morais CardosoL, Pinheiro-Sant’Ana, HM, Anunciação PC, De Menezes CB, Da Silva EC, BarrosF (2017) Effect of the storage time and temperature on phenolic compounds of sorghum grain and flour. Food Chem. 216: 390–398.
De paula M, Pérez-Otaola M, Darder M, Torres M, Frutos G, Martinez-Honduvilla CJ (1996). Function of the ascorbate-glutathione cycle in aged sunflower seeds. Physiol plant 96: 543-550.
Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. AR S. 10:1818–1892.
De Oliveira KG, Queiroz VAV, De Almeida Carlos L, De Morais Cardoso L, Pinheiro-Sant’Ana HM, Anunciação PC, De Menezes CB, Da Silva EC, Barros F (2017) Effect of the storage time and temperature on phenolic compounds of sorghum grain and flour. Food Chem. 216 : 390–398.
Diao M, Dembele RH, Konate K, Dicko Mamoudou H (2019) Etude comparative des peroxydases de dix (10) plantes supérieures couramment rencontrées au Burkina Faso. Int J Biol. Chem Sci. 13(6): 2533-2545.
Di Loreto A, Bosi S, Montero L, Bregola V, Marotti I, Sferrazza RE, Dinelli G, Herrero M, Cifuentes A (2018) Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis. 39: 2001–2010.
Di Silvestro R, Di Loreto A, Bosi S, Bregola V, Marotti I, Benedettelli S, Segura-Carretero, A, Dinelli G. (2017) Environment and genotype effects on antioxidant properties of organically grown wheat varieties: A 3-year study. J Sci Food Agric. 97: 641–649.
Ebone LA, Caverzan A, Chavarria, G (2019) Physiologic alterations in orthodox seeds due to deterioration processes. Plant PhysiolBiochem. 145: 34–42.
FAO (2022) Impact of the Ukraine-Russia Conflict on Global Food Security and Related Matters under the Mandate of the Food and Agriculture Organization of the United Nations.
Ferguson JM, Tekrony DM, Egli DE (1990) Changes during early seed and axes deterioration: Lipids. Crop Sci. 30. 179–182.
Ficco DBM, Mastrangelo AM, Trono D, Borrelli GM, De Vita P, Fares C, Beleggia R, Platani C, Papa R (2014) The colors of durum wheat: A review. Crop Pasture Sci. 65: 1–15.
Fujii H, Nakagawa, T, Nishioka H, Sato E, Hiros A, UenoY, Sun B, Yokozawa T, Nonaka G (2007) Preparation, characterization and antioxidative effects of oligomeric proanthocyanidin-l-cysteine complexes. J. Agric and Food Chem. 55: 1525–1531.
Galleschi, L, Capocchi A, Chiringhelli S, Soviozzi F, Callucci L, PinzinocZandomeneghi M (2002) Antioxydants, free radicals, storage proteins and proteolytic activities in wheat (Triticum durum) seeds during accelerated ageing. J Agric Food Chem. 50:5450-5457.
Ghasemi E, Goodarzian Ghahfarokhi M, Darvishi B, Heidari Kazafi Z (2014) The effect of hydro-priming on germination characteristics, seedlinggrowth and antioxidant activity of accelerated ageing wheat seeds.
Ghassemi-Golezani K, Khomari S, Dalil B, Hosseinzadeh-Mahootchy A, Chadordooz-Jeddi A (2010) Effects of seed ageing on field performance of winter oilseed rape. J Food. Agric. Environ. 8:175-178.
Gidrol X, Serghini H, Noubhani A, Mocquot B, Mazliak P (1989) Biochemical changes induced by accelerated ageing in sunflower seeds. I. Lipid peroxidation and membrane damage. Physiol Plant. 76: 591-597.
Goel A, Sheoran, IS (2003) Lipid peroxidation and peroxide-scavenging enzymes in cotton seeds under natural ageing. Biol. Plant. 46: 429–434.
Hagerman A, Harvey-Mueller I, Makkar, HPS (2000) Quantification of 325 tannins in Tree foliage-A. Laboratory Manual. FAO/IAEA Vienna. 4-7.
Hawkins CL, Morgan PE, Davies MJ (2009) Quantification of proteinmodification by oxidants. Free Radic. Biol. Med. 46: 965–988.
Jensen A (1978) In: Hand book of physiological methods. physiological and methods Hellobust JA, Coigio JS, eds. Cambridge Universitypress. 69-70.
Kallithraka S, Salacha MI, Tzourou I (2009) Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 113: 500–505.
Kapoor N, Arya A, Siddqui A, Kumar H, Amir A (2011) Physiological and Biochemical Changes during Seed Deterioration in Aged Seeds of Rice (Oryza sativa L.). AmJ Plant Physiol. 6 : 28–35.
Kibinza S, Bazin J, Bailly C, Farrant JM, Corbineau F, El-Maarouf-Bouteau H (2011) Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 181 : 309–315.
Kim DH, Han SH, Lee JC (2010) Germination and biochemical changes in acceleated aged and osmoprimed seeds. J Korean Soc. 99: 244–250.
Krishman P, Nagarajam S, Dadlani M, Moharir AV (2003) Characterization of wheat (Triticum aestivum) and soybean (Glycine max) seeds under accelerated ageing conditions by proton nuclear magnetic spectroscopy. Seed Sci Technol. 31:541-550.
Kumar JSP, Prasad S, Banerjee R, Thammineni C (2015) Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann. Bot. 116: 663–668.
Machado Neto N, Custodio C,Takaki M (2001) Evaluation of naturally and artificially aged seeds of Phaseolus vulgaris L. Seed Sci. Technol. 29 : 137–149.
Marcos F (2015) Seed vigor testing: an overview of the past, present and future perspective.JScien Agric. 72: 363-374.
Mittler R (2017) ROS Are Good. Trends Plant Sci.: 22: 11–19.
Moraes CE, Lopes JC, Farias CCM, Maciel KS (2016) Qualidadefisiológica de sementes de Tabernaemontanafuchsiaefolia A. DC emfunção do teste de envelhecimentoacelerado. CiencFlorest. 26:213-223.
Parrish DJ, Leopold AC (1978) On the mechanism of ageing in soybean seeds, Plant physiol. 61:365-368.
Rajjou L, Lovigny Y, Groot SP, Belghazi M, Job C, Job D (2008) Proteome-wide characterization of seed ageing in Arabidopsis. A comparison between artificial and natural ageing protocols. Plant Physiol. 148: 620-641.
Rao RGS, Singh PM, Rai M (2006) Storability of onion seeds and effects of packageing and storage conditions on viability and vigor. Sci Horticulturae 110: 1-6.
Raza SH, Shafiq F, Chaudhary M, Khan I (2013) Seed invigoration with water, ascorbic and salicylic acid stimulates development and biochemical characters of okra (ablemoschus esculentus) under normal and saline conditions. Int J Agric Biol. 15:486–492.
Reema HCK, Sadana B (2004) Nutritional evaluation of supplementary foods prepared from germinated cereals and legumes. JFood Sci.Tech. 41: 627–629.
Sahu B, Sahu AK, Thomas V, Naithani SC (2017) Reactive oxygen species, lipid peroxidation, protein oxidation and antioxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. S Afr J Bot. 112:383–390.
Schaub P, Wüst F, Koschmieder J, YuQ, Virk P, Tohme J, Beyer P (2017) Nonenzymatic Carotene degradation in provitamin A-biofortified crop plants. JAgric. Food Chem. 65:6588–6598.
Serghini Caid, H, Ecchemmakh T, Elamrani A, Khalid A, BoukrouteA, Mihamou A, Demandre C (2008) Altérations accompagnant le vieillissement accéléré de blé tendre. Cahiers Agricultures. 17(1) : 39-44.
Srivastava A, Akoh CC, Yi W, Fischer J, Krewer G (2007) Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles.J Agric Food Chem. 55 : 2705–2713.
Tidiane SallA, Chiari T, Legesse W, Seid-Ahmed K, Ortiz R, Van Ginkel M, & Bassi F M (2019) Durum wheat (Triticum durum Desf.): Origin, cultivation and potential expansion in Sub-Saharan Africa. Agronomy. 9(5): 263.
Tonguç M, Gûler M, ônder S (2023) Germination, reserve metabolism and antioxidant enzyme activities in safflower as affected by seed treatments after accelerated ageing. S Afr J Bot. 153: 209-218.
Torres M, De Paula M, Pérez-Otala M, Darder M, Frutos G, Martinez-Honduvilla CJ (1997) Ageing-induced changes in gluthatione system of sunflower seeds. Physiol plant 101: 807-814.
Trono D (2019) Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants. 8 : 551.
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2:1231–1246.
WangW, He A, Peng S, HuangJ, Cui K, Nie L (2018) The effect of storage condition and duration on the deterioration of primed rice seeds. Front Plant Sci. 9:1–17.
Weidner S, Paprocka J (1996) Phenolic acids and dormancy in oat (Avena sativa L.) and rye (Secale cereale L.) caryopses. Acta PhysiolPlant. 18: 277–286.
Weidner S, Amarowicz R, Karamac M, Dabrowski G (1999) Phenolic acids in two cultivars of wheat rye and triticale caryopses that display different resistance to preharvest sprouting. Eur Food Res Technol. 210: 109–113.
Yan, H F, Mao, P S, Sun Y, & Li, M L (2016) Impacts of ascorbic acid on germination, antioxidant enzymes and ultrastructure of embryo cells of aged Elymus sibiricus seeds with different moisture contents..In J Agri Biol .18 (1): 176–183.
Yong-Bi Fu, Zaheer A, Axel D (2015) Towards a better monitoring of seed ageing under ex situ seed conservation. ConservPhysiol. 26 : 3.
Zhang Y, Truzzi F, D’Amen E, Dinelli G (2021) Effect of Storage Conditions and Time on the Polyphenol Content of Wheat Flours. Processes,9,248.
Zhou K, Laux J J, Yu L(2004) Comparison of swiss red wheat grain and fractions for their antioxidant properties.J Agric Food Chem. 52: 1118-1123.
Zhou Z, Chen X, Zhang M, Blanchard C (2014) Phenolics, flavonoids, proanthocyanidin and antioxidant activity of brown rice with different pericarp colors following storage. J. Stored Prod. Res. 59:120–125.