

Aust J Crop Sci. 19(02):192-198 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.02.p276
Chlorophyll and respiration profiles of three different varieties of tobacco (Nicotiana tabacum. L). under waterlogging
Ismul Mauludin Al Habib*, Hanif Rafika Putri, Hasni Umul Hasanah
Biology Education Department, Argopuro PGRI University, Jember, East Java, Indonesia
*Correspondence author: Ismul Mauludin Al Habib 
Abstract: Evaluation of the chlorophyll content and respiration of tobacco plants under waterlogging conditions is rarely reported. This research aims to evaluate the response of tobacco in recovery of chlorophyll and respiration during and after waterlogging. This research is important for plant breeders to increase tolerance to waterlogging in tobacco. We used a factorial randomized block design with 3 replications, 2 factors (waterlogging treatment and tobacco genotypes that were tolerant, moderate and sensitive to waterlogging). Observations were made at 45 days after planting. Chlorophyll and respiration were studied at levels of 0%, 20%, 40%, and 60% of field capacity of waterlogging. Determination of chlorophyll content was carried out using acetone solvent. Observation of respiration was done using titration with 0.1 N KOH, using 1% PP indicator solution in 20 alcohol. The results showed that the amount of chlorophyll of genotype Bojonegoro 1 did not change in 20% waterlogging compared to the control (0%). Changes were observed in waterlogging of 40% and 60%. The amount of chlorophyll in the Benyak genotype looked unstable starting from 20%, 40% and 60% of waterlogging. A similar condition was also experienced by the GT genotype. The Bojonegoro 1 genotype had better respiratory stability, while the Benyak genotype and the GT genotype showed a higher respiration rate than the control. These conditions showed that waterlogging conditions influence the respiration levels of various genotypes. All tobacco genotypes showed a decrease in chlorophyll along with the increased percentage of waterlogging. The results indicate that each tobacco genotype has a special system for managing waterlogging stress.
Keywords: Moderate; Recovery; Sensitive; Tolerant.
Abbreviations: AOX_ Alternatif Oksidase; NO_Nitric Oxide; ROS_Reactive Oxygen Species.
Introduction
According to the Central Statistics Agency (BPS) report (2023), Indonesia's tobacco production was 238.8 thousand tonnes, an increase of 7% compared to 2022. The increase in production was due to the area of tobacco planting land which increased by 77.9% compared to 2022. In terms of quantity, production is not commensurate with the increase in planted area. One of the causes is the imbalance between production and planting area, which is influenced by weather anomalies and high rainfall (Myles et al., 2018; Secretariat et al., 2021). More than 99.6% of Indonesia's tobacco production comes from community plantations. The diversity of genotypes planted by farmers are thought to be one of the difficulties in identifying the causes of the inability of tobacco plants to withstand waterlogging stress. Al Habib et al. (2024), reported that there are three categories of tobacco plants in facing waterlogging stress, such as Bojonegoro 1 genotype in the tolerant category, the Benyak genotype in the moderate category and the GT genotype in the sensitive category.
Tobacco is very sensitive to the lack of water, so long periods of low-intensity rain can damage the quality of tobacco leaves and even result in crop failure. Plant waterlogging stress can occur as a result of excessive rain and waterlogging of the soil; thereby, inhibiting plant growth. The Chlorophyll plays an important role in light absorption, energy transfer, electron transfer and respiration in plants (Kato et al., 2020; Leles and Levine, 2023). Evaluation of chlorophyll recovery and respiration of tobacco plants under waterlogging are rarely reported.
The need to research and identify waterlogging stress tolerant genotypes is urgent (Perez-Jimenez et al., 2018). These efforts must begin by knowing the level of tolerance based on the time and level of waterlogging stress in tobacco plants to determine tolerant, moderate and sensitive genotypes (Al Habib et al., 2024). The level of tolerance based on the time and level of waterlogging stress in tobacco plants has been identified, but the chlorophyll response and resistance to waterlogging are not well-studied.
According to Mozo et al. (2021), after the end of the waterlogging stress period, plants can modify some traits to compensate for the loss of biomass during waterlogging stress. The lack of oxygen forces plants to perform anaerobic respiration (Jia et al., 2021). The response of tobacco plants can be different in response to waterlogging stress conditions. The response of chlorophyll and tobacco respiration in waterlogging conditions is very necessary to identify chlorophyll and respiration in assembling waterlogging tolerant tobacco in the future, especially in areas with high rainfall. This research aims to evaluate the response of tobacco in recovering chlorophyll and respiration under waterlogging and recovery waterlogging.
Result and discussion
Based on the results of the Duncan test, it is known that there are significant differences between waterlogging treatments in several plant genotypes for chlorophyll levels. This shows that waterlogging (Table 1) and after waterlogging (Table 2) of the 3 genotypes of tobacco plants greatly influence the chlorophyll levels in tobacco plants.
The chlorophyll analysis of waterlogging stress tolerant genotype (Figure 1) shows that Bojonegoro 1 genotype produced higher chlorophyll levels at 20% waterlogging condition, compared to other treatments either waterlogging and recovery conditions. Meanwhile, the smallest chlorophyll content with the waterlogging treatment was found in the 60% GT genotype treatment (Figure 3). The 20% waterlogging treatment in the
Table 1. Duncan test results of the interaction effect of treatment on waterlogging chlorophyll.
| Waterlogging | Genotype | ||
|---|---|---|---|
| Bojonegoro 1 | Benyak | GT | |
| 0% | 73,150 cdA | 71,324 abA | 73,818 dA |
| 20% | 73,050 aA | 75,390b aB | 73,090 aA |
| 40% | 75,930 bB | 70,444 aA | 75,751 bB |
| 60% | 75,245 bB | 74,248 abB | 73,366 aA |
Information: The numbers in the table show the average values of chlorophyll; Small letters next to the numbers indicate the results of the Duncan test interaction, compared horizontally/right-left (a, b, c, d, & e); The capital letters next to the numbers indicate the results of the Duncan test interaction, read vertically/up-down (A, B, & AB).
Table 2. Duncan test results of the interaction effect of treatment on chlorophyll recovery waterlogging.
| Waterlogging | Genotype | ||
|---|---|---|---|
| Bojonegoro 1 | Benyak | GT | |
| 0% | 73,400 C | 71,758 | 73,537 B |
| 120% | 73,298 C | 74,662 | 73,390 B |
| 140% | 70,898 A | 72,011 | 73,368 B |
| 160% | 72,293 B | 73,505 | 69,174 A |
Table 3. Duncan test results of the interaction effect of treatment on respiration during waterlogging.
| Waterlogging | Genotype | ||
|---|---|---|---|
| Bojonegoro 1 | Benyak | GT | |
| 0% | 0.090 aA | 0.095 abA | 0.090 aA |
| 120% | 0.108 cC | 0.112 dB | 0.100 bB |
| 140% | 0.105 bB | 0.105 bAB | 0.105 bB |
| 160% | 0.104 aB | 0.108 aB | 0.101 aB |
Information: The numbers in the table show the average values of respiration; Small letters next to the numbers indicate the results of the Duncan test interaction, compared horizontally/right-left (a, b, c, d, & e); The capital letters next to the numbers indicate the results of the Duncan test interaction, read vertically/up-down (A, B, & AB).
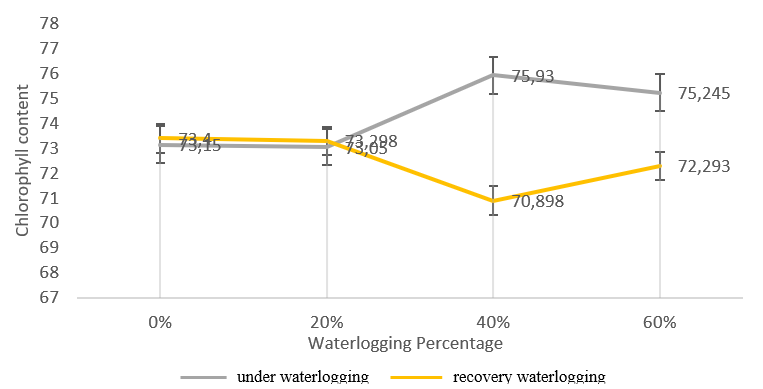
Figure 1. Chlorophyll content (mg/g) bojonegoro genotype 1 (waterlogging stress tolerant) during waterlogging and recovery waterlogging.
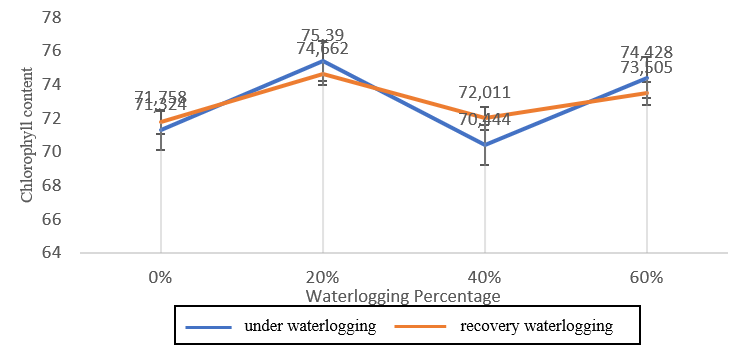
Figure 2. Chlorophyll content (mg/g) of benyak genotypes (moderate waterlogging stress) during waterlogging and recovery waterlogging.
Tabel 4. Duncan test results of interaction effect of treatment on respiration recovery waterlogging.
| Waterlogging | Genotype | ||
|---|---|---|---|
| Bojonegoro 1 | Benyak | GT | |
| 0% | 0.090 aC | 0.095 abA | 0.090 aB |
| 120% | 0.002 aA | 0.105 cA | 0.004 aA |
| 140% | 0.006 bcB | 0.099 dA | 0.005 abA |
| 160% | 0.006 aB | 0.117 cB | 0.005 aA |
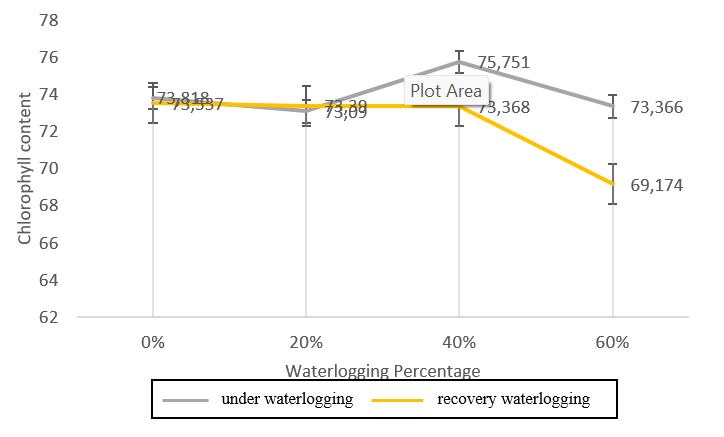
Figure 3. Chlorophyll content (mg/g) of genotype GT (waterlogging sensitive) during waterlogging and recovery waterlogging.
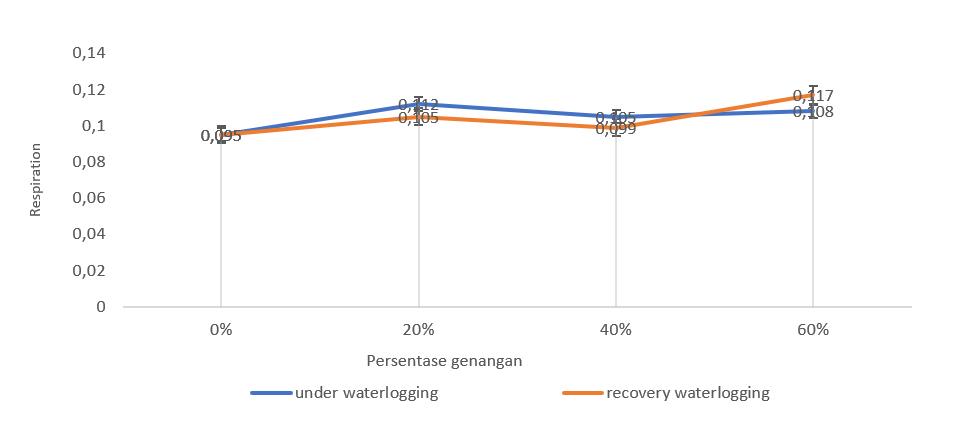
Figure 4. Respiration of genotype bojonegoro 1 (waterlogging tolerant) during waterlogging and recovery waterlogging.
Benyak genotype (Figure 2) showed higher chlorophyll levels than the other genotypes, in conditions exposed to waterlogging and after waterlogging. The Bojonegoro 1 genotype showed the lowest chlorophyll levels at 40% waterlogging waterlogging condition. The GT genotype had the lowest mean after waterlogging stress compared to other genotypes.
The GT genotype produces the lowest chlorophyll level at 60% treatment, compared to other genotypes including in control, waterlogging and recovery conditions. Meanwhile, in waterlogging conditions, the Benyak genotype showed the highest chlorophyll levels. The Bojonegoro 1 genotype had the highest chlorophyll levels during recovery waterlogging. Overall, the average amount of chlorophyll for all plant genotypes with various treatments under waterlogging stress and waterlogging recovery conditions showed both increase and decrease.
The Bojonegoro 1 genotype is not much different from the other genotypes, where the average results show an increase and decrease in chlorophyll levels in various treatments. In waterlogging conditions, the 20% treatment showed higher results than the 40% treatment. Meanwhile, when this genotype was freed from waterlogging, it showed the opposite result, where the highest average chlorophyll content was found in the 40% treatment and the lowest in the 20% treatment.
Benyak genotype showed higher mean chlorophyll levels at 20% treatment, compared to other treatments in both waterlogging and recovery waterlogging conditions. Apart from that, the 20%
treatment also experienced an increase in chlorophyll levels which was the same as the 60% treatment which also experienced an increase. Meanwhile, there was a decrease in chlorophyll levels from waterlogging conditions to recovery waterlogging conditions at 40% treatment.
Several factors or mechanisms work independently or together to enable plants to cope with stress. The tolerance is manifested as a complex trait. These adaptation mechanisms induce plant anatomical, physiological, and biochemical adjustments, which are thought to be integrated responses of various organs, especially roots and leaves.
The amount of chlorophyll is one of the main components influenced by waterlogging stress. So, this character helps explain plant acclimatization to environmental conditions (Mishra et al., 2022; Ferreira-Neto et al., 2021). In marginata plants, flooding does not affect growth, chlorophyll content and dry mass or root-shoot ratio (Bender et al., 2016). Alternative chlorophyll oxidase (AOX) has a specific role under waterlogging stress, where AOX can stimulate nitric oxide (NO) production. This reaction drives chlorophyll and NO cycling to increase energy efficiency under stressful waterlogging conditions (Kumari et al., 2019).
Photosynthetic capacity, and the concentration of water-soluble carbohydrates are reduced due to waterlogging (Liu et al., 2017). The results of research (Luan et al., 2018) on barley plants showed that flooding treatment caused a greater reduction in biomass and photosynthetic performance in sensitive plants, compared to
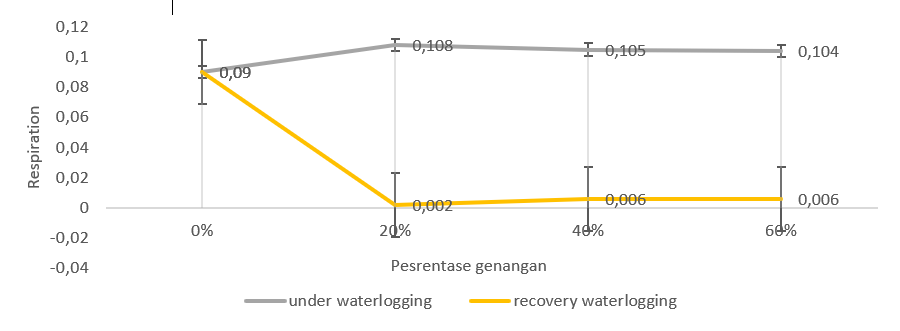
Figure 5. Respiration of Benyak genotype respiration (moderate waterlogging) during waterlogging and recovery waterlogging.
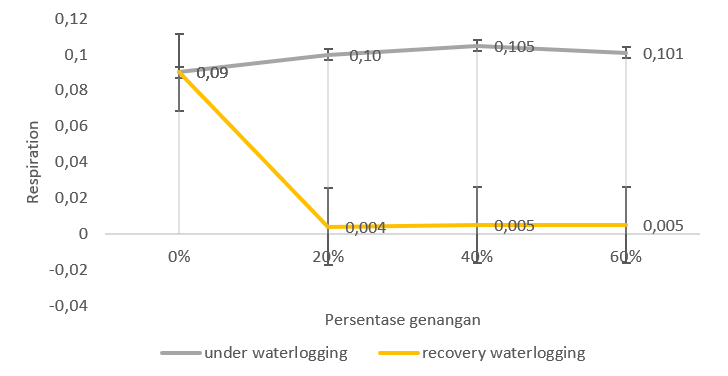
Figure 6. Respiration of GT genotype (waterlogging sensitive) during waterlogging and recovery waterlogging.
tolerant plants. A decrease in chlorophyll content in leaves increases reactive oxygen species (ROS) in roots and leaves during times of waterlogging stress (Fukudome et al., 2019).
Chlorophyll content can be influenced by waterlogging stress conditions (Park et al., 2020). The relative water and chlorophyll content decreases during flooding (Perez-Jimenez and Perez-Tornero, 2021). Increased waterlogging also degrades chlorophyll, stomata closure, plant aging (Kuai et al., 2014) and reduces crop yields by 31.68% (Zhen et al., 2024). The decrease in crop yields is related to the decrease in the amount of chlorophyll due to waterlongging.
Wen et al. 2019 reported that Triarrhena sacchariflora plants showed significant physiological changes in chlorophyll amount at flooded conditions, which automatically reduced photosynthesis. The photosynthetic pigment content decreased compared to the control after waterlogging, but the pigment content was higher at the end of the recovery phase. A decrease in the amount of chlorophyll was also reported by (Rao et al., 2021). A decrease in the amount of chlorophyll occurred at the beginning of waterlogging in Murbay plants. The photosynthetic pigment content in the waterlogging treatment showed an increasing pattern at the end of the recovery phase compared to the photosynthetic pigment content after waterlogging (Raras et al., 2021).
Waterlogging causes several responses in plants, including wilting, leaf fall, development of aerenchyma in the root cortex, formation of adventitious roots, decreased chlorophyll content, inhibition of plant growth, changes in main physiological processes, decreased soil oxygen, and decreased wet weight and dry weight (Avivi et al., 2018; Toral-Juarez et al., 2021). Plants respond to waterlogging through adjustments in growth, accumulation and allocation of biomass, modification of anatomy and morphology in roots, stems and leaves (Fukushima
et al., 2020). Mozo et al. 2021 reported that chlorophyll increased again after a period of flooding in Willaw plants. The research results show a decrease in chlorophyll in line with an increase in the percentage of waterlogging.
For respiration levels, there were significant differences between waterlogging treatments (Table 3) and after waterlogging (Table 4) in the 3 genotypes based on Duncan test. This shows that waterlogging greatly influences the level of respiration in tobacco plants.
The lowest respiration with waterlogging was found in the 20% treatment in the Bojonegoro 1 genotype. The highest respiration was found in the Benyak genotype at 60% waterlogging. The 20% and 40% treatments on the Benyak genotype showed higher respiration levels compared to the other genotypes.
Overall, the waterlogging treatment of the Bojonegoro 1 genotype had better respiratory stability (Figure 4). However, the Benyak genotype (Figure 5) and the GT genotype (Figure 6) showed a higher respiration rate compared to the control. This shows that plants in waterlogging conditions generally have an influence on the level of respiration in plants in various genotypes. However, the three studied genotypes showed different responses during waterlogging.
The GT genotype showed an increase in respiration levels after waterlogging in various treatments. Increased respiration occurred at 20% waterlogging. Meanwhile, for waterlogging, 40% and 60% showed lower respiration than the control. Bojonegoro 1 genotype showed increased respiration after being released from waterlogging. The 20% waterlogging treatment had higher respiration than 40% and 60% treatments. Meanwhile, Benyak genotype indicate a decrease in respiration at 60% waterlogging. The Bojonegoro 1 genotype during waterlogging had a stable respiration rate compared to the control.

Figure 7. The waterlogging treatment condition. (a) Waterlogged conditions, (b).
Stabilizing respiration is one of the efforts of plants to survive during hypoxic stress conditions. According to Yeung et al. (2019), high respiration rates can be an important factor determining waterlogging tolerance and subsequent recovery. Plants are aerobic organisms that require oxygen for respiration. The availability of oxygen is a prerequisite for life in some living organisms. When oxygen supply is insufficient, most cellular functions are impaired, which can lead to death (Loreti et al., 2016). Plants use various adaptation strategies to keep oxygen levels always available.
Waterlogging causes hypoxia. Hypoxia occurs when oxygen levels limit aerobic respiration (usually between 1% and 5%) (Sasidharan et al., 2017). Oxygen is very important for the mitochondrial aerobic respiration process to supply the energy needs of plant cells (Nakayama et al., 2017). Hypoxia is a side effect of waterlogging, mainly due to limited gas diffusion underwater. Flooded plants experience dramatic variations in the availability of oxygen molecules (O2), ranging from partial O2 deficiency to total O2 depletion (anoxia, 0% O2) (Jethva et al., 2022). When oxygen is available externally, oxygen deficiency often occurs in large, dense or metabolically active tissues such as phloem, meristem, seeds and fruit (Nakayama et al., 2017).
The plants respond to waterlogging by making changes in energy metabolism, photosynthesis, respiration and biosynthesis/endogenous phytohormone signaling, because aerobic respiration is inhibited under stress. A further reduction in energy metabolism limits plant development. Consequently, to adapt under these unfavorable conditions, anaerobic respiration must be enhanced (Zhou et al., 2020).
Oxygen is important for cellular respiration in all eukaryotes, including plants, which often face oxygen-deficient conditions during their life cycles. The various responses result from the development of various sensing mechanisms and responses to oxygen availability (Loreti and Perata, 2020). Energy deficit at the root level causes a lack of aquaporin phosphorylation, resulting in greatly reduced root hydraulic conductivity (Tan et al., 2018). Herzog et al. (2016) stated that adventitious roots grow when O₂ at the root tip is still limited. However, because of the moderate porosity in soil and loss of O₂ into the rhizosphere and consumption during respiration, the growth is hampered or may even stop.
Materials and methods
This research was carried out in the Greenhouse at PGRI Argopuro University Jember from May to July 2024. The temperature was controlled in the range of 21°C - 30°C, humidity was in the range of 60% -65% and the photoperiod was in accordance with a tropical or long period climate. The soil type was inceptisol, which filled polybags measuring 50 x 50 cm, and buckets.
Experimental design
This research used a factorial randomized block design with 3 replications, 2 factors, namely waterlogging and 3 different tobacco plant genotypes from the selection results (Al Habib, et.al 2024), namely the Bojonegoro 1 genotype (tolerant genotype), the Benyak genotype (moderate genotype), and GT genotype (sensitive genotype). Chlorophyll and respiration observations were investigated at levels of 0% (field capacity as control), 20%, 40%, and 60% under waterlogging and recovery waterlogging.
Waterlogging stress treatments
Seeds of 3 different tobacco genotypes were planted in pot trays. 40-day-old tobacco seedlings were transferred to polybags. Waterlogging was carried out 45 days after planting. There are 4 types of waterlogging regimes/treatments, namely 0%, 20%, 40% and 60% of field capacity. Field capacity is calculated by weighing 1 kg of soil and soaking it with 1 L of water for 24 hours until saturated. The soil media was allowed to stand until no water drips and weighed again. The difference in weight is 0.4 kg, and 0.4 kg/L is assumed to be 0% of field capacity. Land used for planting media is measured at 20 kg per polybag. so that waterlogging field capacity as control 0% = 0.4 kg/L * 20 kg soil = 8 L water. 20% waterlogging is a 20% increase in field capacity = 9.6 L of water. 40% waterlogging is a 40% increase in field capacity = 11.2 L of water. 60% waterlogging is a 60% increase in field capacity = 12.8 L of water.
Waterlogging is done by adding as much water as possible, whereas 8, 9.6, 11.2 and 12.8 liters of water corresponds to waterlogging of 0%, 20%, 40%, and 60%, respectively. Observations were carried out in 2 stages: 1) Providing waterlogging for 6 hours according to the specified percentage, after 6 hours. The samples were taken for chlorophyll and respiration analysis. 2) Removing water that causes waterlogging (freeing plants from waterlogging). After 6 hours of waterlogging recovery samples were taken for chlorophyll and respiration analysis.
Data collection and analysis
The steps taken in determining chlorophyll concentration are: sample extraction, measurement and calculation. The leaf sample taken was the third leaf from the tip, weighed 0.1 g, crushed with a mortar and pestle, added with 10 ml of 85% acetone then filtered with filter paper while pouring into a test tube, so that a clear extract was obtained. Measurements were carried out using a spectrophotometer (Spectronic 21 D, Milton Roy brand) at wavelengths of 645 nm and 663 nm. The following formula is used to calculate chlorophyll:
Total chlorophyll= 0.0202 (OD 645) – 0.00802 (OD 663)
Respiration measurements were carried out by preparing a 20 g sample in a 250 ml Erlenmeyer, adding 50 ml of 95% neutral alcohol, then heating for 10 minutes in a water bath while stirring. The solution was then titrated with 0.1 N KOH, using a 1% PP solution indicator in alcohol, until the pink colour was visible. Respiration rate was calculated as following:
| Respiration = | Ml KOH x N KOH x 58.1 |
|---|---|
| Sample weight (g) |
Conclusion
The amount of chlorophyll of the Bojonegoro 1 genotype did not change at 20% waterlogging but changes were seen at 40% and 60% waterlogging. The amount of chlorophyll of Benyak genotype looked unstable starting from 20%, 40% and 60% waterlogging. The same conditions, as the Benyak genotype, were also experienced by the GT genotype. All tobacco genotypes in observations with various waterlogging percentages showed a decrease in chlorophyll in line with an increase in the waterlogging percentage. The bojonegoro 1 genotype had better respiratory stability, the Benyak genotype and the GT genotype showed a higher respiration rate compared to the control. This condition shows that plants that are in waterlogging conditions generally have an influence on the level of respiration in plants of various genotypes. These changes indicate that each tobacco genotype has a specific system for managing waterlogging stress.
Acknowledgment
Research and community service institute at PGRI Argopuro University Jember, with research contract number 13/SP2H/PT.106/LPPM/2024.
References
Al Habib IM, Hasanah HU, Sukamto DS (2024) Selection of some important tobacco genotypes against waterlogging in Indonesia. Australian Journal of Crop Science. 18(3): 187–191. https://doi.org/10.21475/ajcs.24.18.02.PNE4105.
Allen M, Babiker M, Chen Y, de Coninck H, Connors S, van Diemen R, Dube OP, Ebi K, Engelbrecht F, Ferrat M (2018) Global Warming of 1 . 5 ° C an IPCC special report on the impacts of global. Ipcc, October 2018. https://www.ipcc.ch/sr15/chapter/summary-for-policy-makers/.
Avivi S, Syamsunihar A, Soeparjono S, Chozin DM (2018). Toleransi berbagai varietas tebu terhadap peng waterlogging pada fase bibit berdasarkan karakter morfologi dan anatomi. Jurnal Agronomi Indonesia (Indonesian Journal of Agronomy). 46(1):103. https://doi.org/10.24831/jai.v46i1.14081.
Bender B, Capellesso ES, Lottici ME, Sentkovski J, Mielniczki-Pereira AA, Rosa LMG, Sausen TL (2016) Growth responses and accumulation of soluble sugars in Inga marginata Wild. (Fabaceae) subjected to flooding under contrasting light conditions. Brazilian Journal of Biology. 77(2): 260–266. https://doi.org/10.1590/1519-6984.11315.
Ferreira-Neto JRC, Borges ANda C, da Silva MD, Morais DA de L, Bezerra-Neto JP, Bourque G, Kido EA, Benko-Iseppon AM (2021) The cowpea kinome: Genomic and transcriptomic analysis under biotic and abiotic stresses. Frontiers in Plant Science. 12(June). https://doi.org/10.3389/fpls.2021.667013.
Fukudome M, Watanabe E, Osuki KI, Uchi N, Uchiumi T (2019) Ectopic or over-expression of class 1 phytoglobin genes confers flooding tolerance to the root nodules of lotus japonicus by scavenging nitric oxide. Antioxidants. 8(7): 1–12. https://doi.org/10.3390/antiox8070206.
Fukushima A, Kuroha T, Nagai K, Hattori Y, Kobayashi M, Nishizawa T, Kojima M, Utsumi Y, Oikawa A, Seki, M, Sakakibara H, Saito K, Ashikari M, Kusano M (2020) Metabolite and phytohormone profiling illustrates metabolic reprogramming as an escape strategy of deepwater rice during partially submerged stress. Metabolites. 10(2). https://doi.org/10.3390/metabo10020068.
Herzog M, Striker GG, Colmer TD, Pedersen O (2016) Mechanisms of waterlogging tolerance in wheat - a review of root and shoot physiology. Plant Cell and Environment. 39(5): 1068–1086. https://doi.org/10.1111/pce.12676.
Jethva J, Schmidt RR, Sauter M, Selinski J (2022). Try or Die: Dynamics of plant respiration and how to survive low oxygen conditions. Plants. 11(2). https://doi.org/10.3390/plants11020205.
Jia W, Ma M, Chen J, Wu S (2021) Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. In International Journal of Molecular Sciences (Vol. 22, Nomor 3, hal. 1–24). MDPI AG. https://doi.org/10.3390/ijms22031088.
Kato K, Shinoda T, Nagao R, Akimoto S, Suzuki T, Dohmae N, Chen M, Allakhverdiev SI, Shen JR, Akita F, Miyazaki N, Tomo T (2020) Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nature Communications. 11(1): 1–10. https://doi.org/10.1038/s41467-019-13898-5
Kuai J, Liu Z, Wang Y, Meng Y, Chen B, Zhao W, Zhou Z, Oosterhuis DM (2014) Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Science. 223: 79–98. https://doi.org/10.1016/j.plantsci.2014.03.010.
Kumari A, Pathak PK, Bulle M, Igamberdiev AU, Gupta KJ (2019) Alternative oxidase is an important player in the regulation of nitric oxide levels under normoxic and hypoxic conditions in plants. Journal of Experimental Botany. 70(17): 4345–4354. https://doi.org/10.1093/jxb/erz160.
Leles SG, Levine NM (2023) Mechanistic constraints on the trade-off between photosynthesis and respiration in response to warming. Science Advances. 9(35): 1–15. https://doi.org/10.1126/sciadv.adh8043.
Liu M, Hulting A, Mallory-Smith C (2017) Comparison of growth and physiological characteristics between roughstalk bluegrass and tall fescue in response to simulated waterlogging. PLoS ONE. 12(7): 1–21. https://doi.org/10.1371/journal.pone.0182035
Loreti E, Perata P (2020) The Many Facets of Hypoxia in Plants. Plants. 9(6): 745. https://doi.org/10.3390/plants9060745.
Loreti E, van Veen H, Perata P (2016) Plant responses to flooding stress. Current Opinion in Plant Biology. 33: 64–71. https://doi.org/10.1016/j.pbi.2016.06.005.
Luan H, Shen H, Pan Y, Guo B, Lv C, Xu R (2018) Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Scientific Reports. 8(1): 1–13. https://doi.org/10.1038/s41598-018-27726-1.
Mishra S, Sahu G, Shaw BP (2022) Insight into the cellular and physiological regulatory modulations of Class-I TCP9 to enhance drought and salinity stress tolerance in cowpea. Physiologia Plantarum. 174(1): 1–15. https://doi.org/10.1111/ppl.13542.
Mozo I, Rodríguez ME, Monteoliva S, Luquez VMC (2021) Floodwater depth causes different physiological responses during post-flooding in willows. Frontiers in Plant Science. 12(May), 1–12. https://doi.org/10.3389/fpls.2021.575090.
Nakayama TJ, Rodrigues FA, Neumaier N, Marcolino-Gomes J, Molinari HBC, Santiago TR, Formighieri EF, Basso MF, Farias JRB, Emygdio, BM, De Oliveira ACB, Campos ÂD, Borém A, Harmon FG, Mertz-Henning LM, Nepomuceno AL (2017) Insights into soybean transcriptome reconfiguration under hypoxic stress: Functional, regulatory, structural, and compositional characterization. PLoS ONE. 12(11): 1–20. https://doi.org/10.1371/journal.pone.0187920.
Park SU, Lee CJ, Kim SE, Lim YH, Lee HU, Nam SS, Kim HS, Kwak SS (2020) Selection of flooding stress tolerant sweetpotato cultivars based on biochemical and phenotypic characterization. Plant Physiology and Biochemistry. 155: 243–251. https://doi.org/10.1016/j.plaphy.2020.07.039.
Pérez-Jiménez M, Pérez-Tornero O (2021) Short-term waterlogging in citrus rootstocks. Plants. 10(12). https://doi.org/10.3390/plants10122772.
Pérez-Jiménez M, Hernández-Munuera M, Piñero MC, López-Ortega G, del Amor FM (2018) Are commercial sweet cherry rootstocks adapted to climate change? Short-term waterlogging and CO2 effects on sweet cherry cv. ‘Burlat.’ Plant Cell and Environment. 41(5): 908–918. https://doi.org/10.1111/pce.12920.
Rao L, Li S, Cui X (2021) Leaf morphology and chlorophyll fluorescence characteristics of mulberry seedlings under waterlogging stress. Scientific Reports. 11(1): 1–11. https://doi.org/10.1038/s41598-021-92782-z.
Raras RP, Saptiningsih E, Haryanti S (2021) Respon Tanaman Cabai Rawit (Capsicum frutescens L.) Varietas Pelita F1 terhadap PengWaterlogging. Buletin Anatomi dan Fisiologi. 6(1): 56–65. https://doi.org/10.14710/baf.6.1.2021.56-65.
Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, Fukao T, Geigenberger P, Hebelstrup KH, Hill RD, Holdsworth MJ, Ismail AM, Licausi F, Mustroph A, Nakazono M, Pedersen O, Perata P, Sauter M, Shih MC, Voesenek LACJ (2017) Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytologist. 214(4): 1403–1407. https://doi.org/10.1111/nph.14519.
Secretariat IP, Gullino ML, Albajes R, Al-Jboory I, Angelotti F, Chakraborty S, Garrett KA, Hurley BP, Juroszek P, Makkouk K et al. (2021) Scientific review of the impact of climate change on plant pests. In Scientific review of the impact of climate change on plant pests. https://doi.org/10.4060/cb4769en.
Tan X, Xu H, Khan S, Equiza MA, Lee SH, Vaziriyeganeh M, Zwiazek JJ (2018) Plant water transport and aquaporins in oxygen-deprived environments. Journal of Plant Physiology, 227: 20–30. https://doi.org/10.1016/j.jplph.2018.05.003.
Toral-Juárez MA, Avila RT, Cardoso AA, Brito FAL, Machado KLG, Almeida WL, Souza RPB, Martins SCV, DaMatta FM (2021) Drought-tolerant coffee plants display increased tolerance to waterlogging and post-waterlogging reoxygenation. Environmental and Experimental Botany. 182: 104311. https://doi.org/10.1016/j.envexpbot.2020.104311.
Wen X, Wang J, Zhang D, Wang Y (2019) A gene regulatory network controlled by bperf2 and bpmyb102 in birch under drought conditions. International Journal of Molecular Sciences. 20(12). https://doi.org/10.3390/ijms20123071.
Yeung E, Bailey-Serres J, Sasidharan R (2019) After The Deluge: Plant Revival Post-Flooding. Trends in Plant Science. 24(5): 443–454. https://doi.org/10.1016/j.tplants.2019.02.007.
Zhen B, Zhou X, Lu H (2024) Effects of waterlogging on rice growth at jointing – booting stage. 1–12.
Zhou W, Chen F, Meng Y, Chandrasekaran U, Luo X, Yang W, Shu K (2020) Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiology and Biochemistry. 148(January): 228–236. https://doi.org/10.1016/j.plaphy.2020.01.020
After being released from waterlogging condition.