

Aust J Crop Sci. 19(02):119-127 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.02.p121
Silicon mitigates cadmium toxicity and modulates positive anatomical changes in tree plants of African mahogany
Susana Silva Conceição1, Antonio Vinícius Correa Barbosa1, Carlos Alberto de Souza2 Edson Ugulino Lima3, Breno Ricardo Serrão da Silva1, Alba Lúcia Ferreira de Almeida Lins4, Jessivaldo Rodrigues Galvão1, Gilberto da Cruz e Silva Filho5, Jonas Pereira de Souza Júnior6, Renato de Mello Prado6, Cândido Ferreira de Oliveira Neto1, and Flávio José Rodrigues Cruz*7
1Federal Rural University of Amazonian, Belém - Pará - Brazil
2Federal Institute of Pará, Belém – Pará, Brazil
3Federal University of Pará, Belém – Pará, Brazil
4Emílio Goeldi Museum of Pará, Belém – Pará, Brazil
5Federal University of Pará, Ananindeua – Pará, Brazil
6São Paulo State University, Jaboticabal - São Paulo, Brazil
7Federal Institute of Education, Science and Technology of Amapá, Laranjal do Jari, AP, Brazil
Corresponding author: Flávio José Rodrigues Cruz (Cruz,
FJR) 
ORCID: https://orcid.org/0000-0001-6701-8748
Abstract: This study aimed to evaluate the effect of silicon mitigation (Si) on Khaya ivorensis exposed to cadmium (Cd) and anatomical changes promoted by Cd in roots and leaves tissues. O delineamento experimental foi inteiramente casualizado em esquema fatorial 4 x 4 com cinco repetições. Cd and Si treatments were 0, 25, 50, 75 mg L-1 and 0, 100, 150, and 300 mg L-1 respectively. At 25 mg L-1 mg, Cd has shown increases of 30%, 25%, and 33% in Epidermis thickness from adaxial (ETAd), spongy parenchyma thickness (SPT), and root cortex thickness (RCT). Also, 75 mg L-1 Cd decreased epidermis thickness from abaxial (ETAb) and root cortex thickness (RCT) by 70% and 81%. However, Si attenuated anatomical changes caused by Cd. It occurs especially in combination with treatments at 150 mg L-1 Si and 50 mg L-1 Cd. Under these conditions, ETAb increased by 33%. Both palisade parenchyma thickness (PPT) and spongy parenchyma thickness (SPT) were 48% and 55% thicker than tissues under 50 mg L-1 Cd. The K. ivorensis is tolerant to Cd up to 25 mg L-1 mg because it did not show considerable growth reduction. Si has shown modulation in tissue thickness. It has a positive impact on the vegetative growth of K. ivorensis . Therefore, this study indicates that K. ivorensis tolerates Cd toxicity up to 25 mg L-1 Cd and shows that Si induces anatomical modulations in leaves and roots.
Keywords: Si stress mitigation; Cd stress; mahogany; anatomical modifications; heavy metal.
Abbreviations: ETAd_Epidermis thickness from adaxial; ETAb_epidermis thickness from abaxial; PPT_palisade parenchyma thickness; SPT_spongy parenchyma thickness; PPT/SPT ratio_palisade parenchyma thickness/spongy parenchyma thickness ratio; RD_Root diameter; RCT_root cortex thickness; VCD_vascular cylinder diameter; VED_vessel element diameter.
Introduction
Cadmium (Cd) is a highly toxic heavy metal (HM) that affects plant growth in natural or artificial environments (Song et al., 2019). Cd is largely a pollutant, which is released into nature through anthropogenic action (e.g. power stations, heat systems, waste incinerators, urban traffic, cement factories, and phosphate fertilizers as by-products), but also weathering of rocks (Sanità di Toppi and Gabbrielli 1999; Andresen and Küpper 2013).
The Cd toxicity mechanisms in plants were studied and described in classic review articles (Das et al., 1997; Benavides et al., 2005). Plants affected by Cd have shown a reduction in their growth. It was caused by disorders such as physiological, nutritional, biochemical, and anatomical. For example, Cd hurts plants because it promotes reductions in gas exchange (Song et al., 2019). In addition, Cd reduces essential nutrient content (Gomes et al., 2013), induces oxidative stress (Pereira et al., 2018), affects nitrogen metabolism (Chaffei et al., 2004), reduces the size of endodermal cells and the promotes disintegration of root epidermis (Vitória et al., 2003; Liza et al., 2020).
Silicon (Si) is the second most abundant element in soils. It is not considered an essential element to plants (Epstein 1999; Marschner 2012), but it induces tolerance in plants, which was submitted to abiotic stresses such as HM toxicity (Ali et al., 2016; Pereira et al., 2018; Ali et al., 2019), water deficit (Saud et al., 2014; Safoora et al., 2018; Avila et al., 2020), salt stress (Liang 1999; Torabi et al., 2015; Raza et al., 2019). Furthermore, Si improves gas exchange, water use efficiency, and plant growth (Silva et al., 2015; Oliveira et al., 2019).
The mechanisms of promoting tolerance in plants to HM are diverse and modulated by Si. In vegetables, Si induces HM tolerance by reducing its absorption and promoting its immobilization in root apoplast. As a consequence, chelating HM, coprecipitating Si bound, reduces oxidative stress, improving gas exchange and increasing absorption of essential nutrients (Adrees et al., 2015).
The first organ to come into contact with HM is the root system which is present the in vegetal growth substrate. Also, the first to manifest morphological, anatomical and physiological results of HM toxicity (Pérez Chaca et al., 2014). On the other hand, leaves are organs that are exposed to the air environment, which becomes more sensitive and has plasticity characteristics to environmental changes compared to other organs (Vaculík et al., 2015). Thus, leaves are indicators organs that have shown how the surrounding environment of plants is, as well as morphological and structural characteristics that are ecological indicators of plant habitat (Farooq et al., 2016; Greger et al., 2016). In this context, HM toxicity is a stressful factor because promotes changes in the dimensions of leaf and root tissues, as well as on the surface of organs, such as stomata, epidermis and plant attachments (Li et al., 2007; Shi and Cai 2009; Cui et al., 2017; Pereira et al., 2017; Xu et al., 2017).
African mahogany or Khaya ivorensis A. Chev. (K. ivorensis ) is a tree species of african origin belonging to the Meliaceae family (Ribeiro et al., 2017). The species has great economic potential for commercialization because of its noble wood, which can be used in industry, naval and civil constructions, panels and laminates industries, among others (Pinheiro et al., 2011). K. ivorensis is a heliophile species, tolerant shade during its juvenile stage, which was classified as a pioneer and secondary species emerging (Budowski 1965; Denslow 1987; Foli 2000). In the literature, there is no record of the mitigating action of Si on the effects of toxic levels of Cd on the growth and anatomical characteristics of K. ivorensis plants since the species is not an accumulator of Si and Cd.
The hypothesis guiding this study was that increasing levels of Si improve the growth and anatomical parameters of K. ivorensis under toxic levels of Cd and that this species tolerates low levels of Cd. Research aimed to evaluate the mitigation effects of Si on K. ivorensis exposed to cadmium, as well as anatomical changes promoted by Cd in root and leaf tissues.
Results
Cadmium and silicon content
According to polynomial adjustment (ZCd leaf and ZCd root), Cd content in leaves (Figure 1B), and roots (Figure 1A) was high in Cd toxic treatments. Leaves and roots showed different Cd levels in response to Cd treatments. At 25 mg L-1 Cd treatment, Cd content followed decreased order of root (19.78 mg kg‑1 DM) > leaf (11.47 mg kg‑1 DM). However, as Cd treatment increased, there was an increase in Cd content in leaves. In the treatment of 75 mg L-1, there was a high accumulation of Cd in leaves, which reached a maximum point of 57 mg kg-1 Cd DM. Cd content was found at 45% higher than the root submitted to the same treatment and 87% higher than the control.
The Si supply reduced Cd levels in different evaluated organs of K. ivorensis . Canonical analysis showed 38 mg L-1 Cd and 141 mg L-1 Si interaction as the lowest Cd content obtained in leaves (Figure 2C and 2B). In these interactions, Cd levels were 60% less than levels found at 50 mg L-1 Cd. The minimum Cd content point was in the root system (Figure 2C), with the value of 4.8 mg kg-1 Cd DM which occurred in the presence of 150 mg L-1 Si and the absence of Cd. However, there was a reduction of 26% of Cd root content in the interaction between 50 mg L-1 Cd and 150 mg L-1 Si. The concentrations of 25 mg L-1 Cd and 50 mg L-1 Si did not affect Si content in all evaluated organs. The minimum levels of Si contents in leaves (0.012 mg kg-1 Si DM) and roots (0.18 mg kg-1 Si DM) were observed only at 75 mg L-1 Cd (Figure 2C, 2B and, 2A).
As Si concentration increased, also there was an increase in Si content in evaluated organs of K. ivorensis . The polynomial equation (ZSi leave and ZSi root) showed maximum points of Si contents at 150 mg L-1 Si concentration, which reached a mean of 4.9 and 7.7 mg kg -1 Si DM in leaves and roots respectively. Si accumulation in K. ivorensis was observed in root > leave (Figures 2A and 2B). According to the polynomial equation in plants treated with Cd, high Si content in leaves and roots was obtained between the interaction of Si and Cd at 27 mg L-1 Cd and 132 mg L-1Si; 30 mg L-1 Cd and 151 mg L-1 Si. These also resulted in the highest levels of Si in leaves and roots compared to plants at 50 mg L-1 Cd concentration.
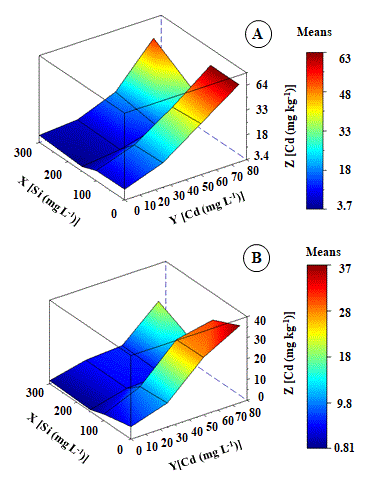
Fig 1. Cd content (mg kg-1 DM) in roots (A) and leaves (B) of Khaya ivorensis plants under silicon and cadmium treatments.
ZCd Leaf = 3.96858** + 8.51915*X - 0.62525nsY + 1.33539**X2 - 0.20243nsYX + 0.84597nsY2 R2 = 0.81; ZCd Root = 20.09769* - 11.15876*X - 1.12187nsY - 2.08924*X2 - 1.75749*YX - 0,4.17401nsY2 R2 = 0.89; * significant diferences at 5%; ** significant diferences at 1%; ns: no significant.
Growth parameters
Height (H), leaf area (LA), and root length (RL) get through similar variations in response to the increased value of Cd treatments. However, combined treatments between Cd and Si have shown that Si promoted improvements in the growth of K. ivorensis (Figure 3). The growth parameters H and LA were reduced by 49 and 60% in 75 mg L-1 Cd treatment compared to control, respectively (Figure 3A and 3B).
According to bivariate analysis, the highest means of H, LA, and RL were observed in the absence of Cd and the intermediate concentration of Si. Treatment with 150 mg L-1 of Si induced increases in all growth variables, with more significant effects on RL (Figure 3). The concentration of 150 mg L-1 of Si promoted a maximum point of 20 cm root-1. This average was 76% higher than that of plants treated with 50 mg L-1 of Cd.
The significant interaction between Cd and Si concentrations improved growth variables. Canonical analysis resulted in optimal growth levels occurring in 41 mg L-1 Cd and 145 mg L-1 Si interaction. In this interaction, there were increases of 33% in H and LA (Figure 3A and 3B). About RL, there was a 58% increase compared to 50 mg L-1 Cd (Figure 3C).
Leaf anatomical parameters
Concentration of 25 mg L-1 Cd increased the thickness of the abaxial (ETAb) and adaxial (ETAd) epidermis of K. ivorensis between 25% and 30% compared to control, respectively (Figure 4A and 4B). Treatment with 50 mg L-1 Cd causes the thickness the of vascular bundle sheath (Figur C). The concentration of 25 mg L-1 Cd increases spongy tissue thickness (SPT) (Figure 4D).
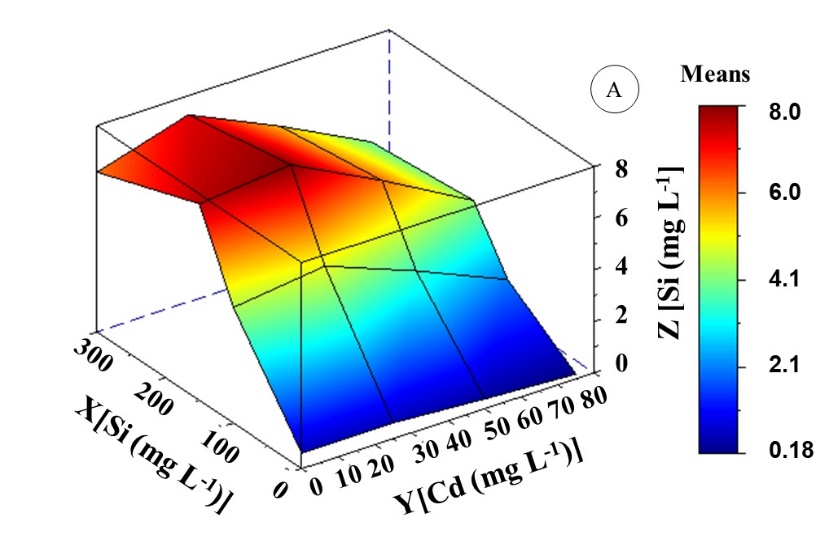
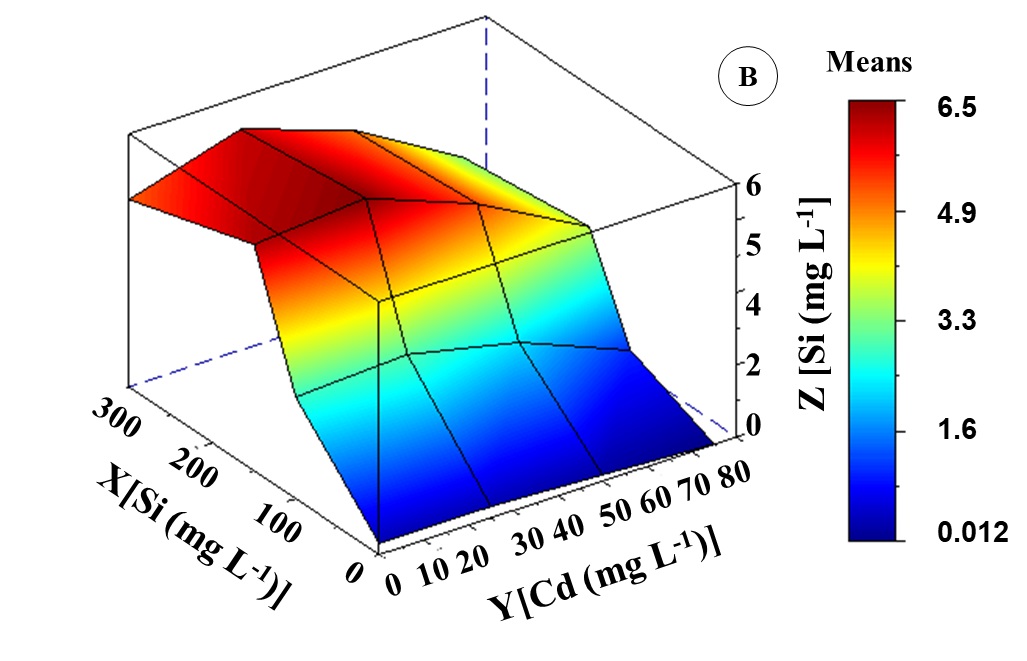
Fig 2. Si content (mg kg-1 DM) in roots (A) and leaves (B) of Khaya ivorensis plants under silicon and cadmium treatments. ZSi leaf = -7.60881** + 6.75170**X + 2.37499**Y - 0.85133**X2 - 0.27441**YX - 0.45728**Y2 R2 = 0.95; ZSi root = -7.55413** + 7.58789**X + 2.12173**Y + 1.02241**X2 - 0.24372**YX - 0.45438**Y2 R2 = 0.96; * significant diferences at 5%; ** significant diferences at 1%; ns: no significant.
Even though the magnitude of the increase was smaller than in the ETAb and ETAd, it was little significant in the thickness of spongy parenchyma (PPT). The lowest mean of the PPT/SPT ratio was observed at 25 mg L-1 Cd (Figure 4E). Above 25a mg L-1 Cd there was reduction in ETAp, ETAd, PPT and SPT of K. ivorensis lea,ves (Figure 4A, 4B, 4C and 4D). However, this effect was not observed in the PPT/SPT ratio, which increased with increasing Cd concentration (Figure 4E). The interaction between Cd and Si considerably increased STA,b, ETAd, and SPT (Figure 4A, 4B and 4D). These anatomical parameters have shown the best interaction between Cd and Si which was between 50 mg L-1 Cd and 150 mg L-1 Si.
Concerning cells from STAb, ETAd, and SPT tissues of plants treated with 75 mg L-1 Cd there was a loss of cell structure and reductions in intercellular spaces compared to the control plants (Figure 6E). Minimum thickness points of STAb, ETAd, and SPT were 4.5µm, 5.8µm and 17µm. These values were 73%, 70%, and 68% lower than control.
There was a compact arrangement of epidermal cells in the 150 mg L-1 Si and 50 mg L-1 Cd interaction. Furthermore, in this interaction, there was an increase in the epidermal thickness of leaf tissues (Figure 6D). Canonical analysis has shown optimal points between the interaction of Cd and Si, which occurred in combination with 45 mg L-1 Cd and 165 mg L-1 Si with an increase in the thickness of leaf tissues (Figure 6).
Root anatomical parameters
There were wide variations in root tissue thickness, as well as Cd concentrations increased in the culture medium (Figures 5 and 7). Even though K. ivorensis was treated with 25 mg L-1 Cd, they did not show anatomical changes compared to the control. Except for root cylinder thickness (RCT) (Figure 5B) and root vessel element diameter (VED) (Figure 5D). The concentration of 25 mg L-1 Cd increased by 33% and 28% RCT and VED. According to the fit of the regression equation, the maximum point of the RCT and VED were 120 and 55 µm (Figure 5B and D). According to surface analysis of response, RCT and VED at 75 mg L-1 Cd were reduced by 81% and 80%, respectively (Figure 5B and 5D). However, Si has been minimized to the toxic effect of Cd between the interaction of 40 mg L-1 Cd and 150 mg L-1 Si. These interactions, resulted in values of RD 250 µm and VED 58µm. These averages above of DR and VED represent increases of 53% and 56% compared to plants at 50 mg L-1 Cd, respectively.
Discussion
Increased Cd content in leaves and roots was observed in K. ivorensis (Figure 1). However, above 25 mg L-1 Cd high levels of Cd were observed in shoots and roots coinciding with a reduction in H, LA, and RL. Thus, indicates that even in this concentration K. ivorensis tolerates the toxicity of Cd. Cd content treatment in roots of K. ivorensis was 42% higher than in leaves. These results suggest the potential of K. ivorensis to accumulate Cd in the leaf (Cd content of 11.47 mg kg-1 DM) and root (Cd content of 19.78 mg kg-1 DM). These Cd concentrations in leaves and roots of K. ivorensis plants exceed the values of Cd concentrations observed in other plant species such as in Populus alba (Rafati et al., 2011) and Prosopis juliflora (Varun et al., 2011) which are phytoremediation potential plants. Cd is a non-essential element that negatively affects plant growth (Das et al., 1997; Benavides et al., 2005). Fan et al., (2011) in a study with plants of Swietenia macrophylla showed that this species accumulated 154 mg kg-1 Cd DM in the stem, with a reduction of 25% and 31% in the dry mass of the stem and the whole plant, respectively. Decreases in plant growth functionally connected to the negative impact of Cd on gas exchange parameters (López-Climent et al., 2011), chlorophyll fluorescence (Piršelová et al., 2016), chlorophyll content (Li et al., 201,2) and nutritional status of Cd treated plants (Jibril et al., 2017). However, exogenous Si supply minimizes the effects of Cd on growth because it reduces Cd uptake and transport to the aerial part of plants. This mechanism of action of Si improves gas exchange (Farooq et al., 2013), chlorophyll fluorescence (Howladar et al., 2018), chlorophyll contents (Ali et al., 20,19) and nutritional status (Alzahrani et al., 2018), which allows growth gains even under conditions of mild to moderate Cd toxicity in plants, as evidenced in these study.
Until 25 mg L-1 mg Cd K. ivorensis presented thickness of evaluated tissues (ETad, ETAb, PPT, and SPT). The epidermis cell wall accumulates a considerable amount of negative charges from functional groups such as -OH, -COOH and -SH (Krzesłowska et al., 2011). The thickness of the epidermis can expand its function as a metal ion filter (Araújo and Silva 2013). However, high Cd treatments reduced the thickness of tissues evaluated (ETad, ETAb, P,PT and SPT), size of cells and induced loss of cell shape (Figure 6), in specific of treatment at 75 mg L-1 Cd. Toxic Cd affected the expansion of leaf tissues of tomatoes (Djebali et al., 2010). In bean, Cd reduced relative water contents and inhibited cell expansion, which indicates that HM negatively affects cell extensibility (Poschenrieder et al., 1989).
Anatomical modifications were induced by Cd, which implies decreases in leaf area and, consequently, a reduction in the photosynthetic capacity of plants (Chugh and Sawhney 1999; Di Cagno et al., 1999). Cotton plants have shown a reduction in the thickness of up and low epidermal and spongy parenchyma in response to 200 μM of Cd (Ozyigit et al., 2013). Similarly, Alternanthera tenella treated with 150 μM has shown a reduction
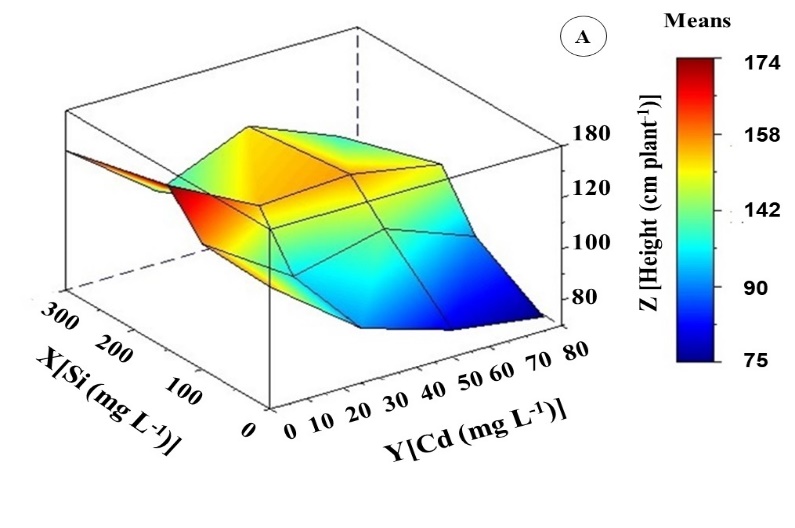

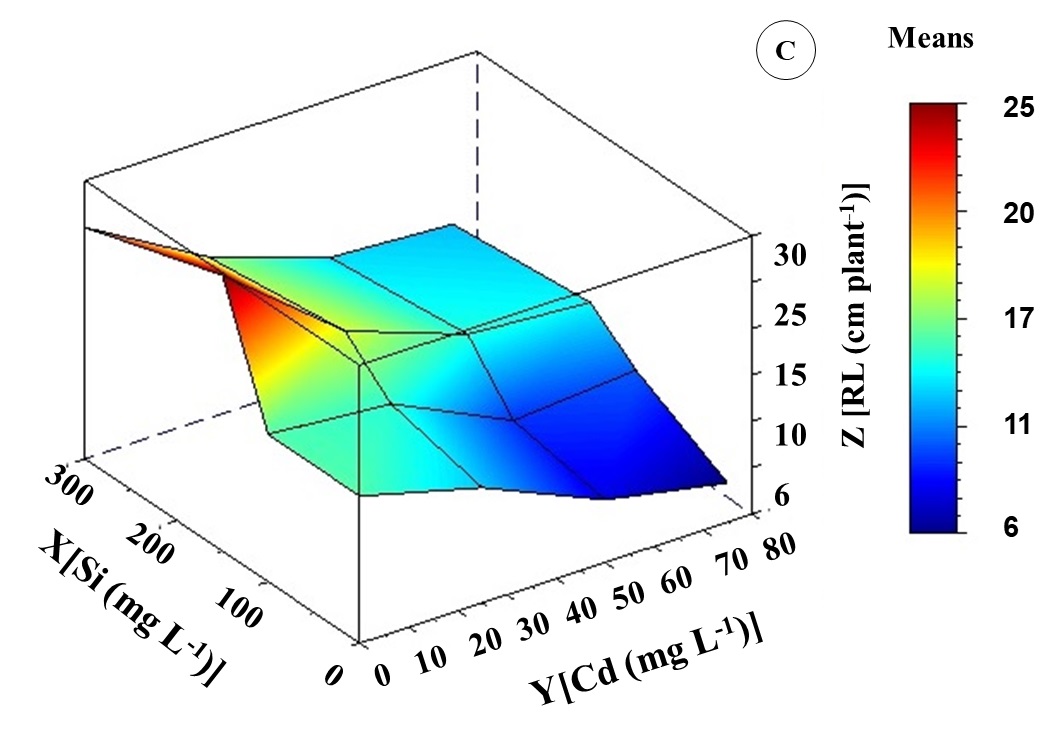
Fig 3. Height (A), leaf area (B), and root length (C) of Khaya ivorensis plants under silicon and cadmium treatments.
ZH = 110.27469**- 0.83716Y** + 0.21975X** + 0.00498Y2** + 0.00095XY** - 0.00062X2** R2 = 0.77; ZAF = 6189.17485** - 78.16847Y** + 6.58098X** + 0.47423Y2** + 0.05197XY** - 0.02076X2** R2 = 0.91; ZCR = 15.65691** - 0.26992Y** + 0.07626X** + 0.00169 Y2** - 0.00010XYns - 0.00015X2** R2 = 0.82; * significant diferences at 5%; ** significant diferences at 1%; ns: no significant.
in thickness of up and low epidermis, and palisade and spongy parenchyma (Rodrigues et al., 2017).
The 25 mg L-1 Cd treatment increased the thickness of VCD and VED. However, high Cd treatments reduced the thickness of these evaluated root tissues. In addition, there was great accumulation and transport of Cd from the root system to shoot in high Cd treatments. The great thickness of the epidermis and endodermis acts as a barrier to Cd transport to shoot. Due to the presence of negative charges on the cell wall of these tissues (Melo Marques et al., 2011; Krzesłowska et al., 2011). The epidermis and endoderm also represent an important apoplastic barrier for the radial transport of ions and water in the vascular system (Enstone et al.,, 2002; Enstone and Peterson, 2005). Root anatomy results found in these studies suggest that K. ivorensis is sensitive to concentrations greater than 25 mg L-1 Cd. Chickpea plants treated with Cd (250 - 1000 μM) have shown considerable reduction in root diameter and root tissue thickness (Liza et al., 2020), similar to results found in the present study. In rice crops, Cd was deformed and disarranged cells in the cortex (Fan et al., 2016). On the other hand, Brachiaria decumbens grown in soil contaminated with Cd (mixture of soil without Cd + soil containing 10.5 mg kg-1 Cd in proportions of 7.5 and 15%) have shown higher thickness of endoderm, exoderm, and cell wall of cortical cells (Gomes et al., 2011).
Treatments combined between Si with Cd improved shoot and root growth. This improvement was connected to positive changes in leaf anatomy caused by Si. Supply of Si reduced the negative impact of Cd on growth and anatomical variables, specifically in the treatment of 150 mg L-1 Si. Several studies report the role of Si in the mitigation of harmful effects of HMs on the growth of rice (Fan et al., 2016; Bari et al., 2020), corn (Cunha et al., 2009; Vakulík et al., 2012), cotton (Anwaar et al., 2015; Ali et al., 2016), wheat (Ali et al., 2019) and alfalfa (Wu et al., 2015). In wheat and rice, Si increases the suberization of ectoderm and endoderm. It also decreases Cd transport to shoot, which contributes to improving plant growth (Fleck et al., 2011; Wu et al., 2019). In addition, Si induces ion chelation through exudates released from the root or by decreases in the amount of free ions in vegetable organs. These two strategies reduce the transport of toxic ions to aerial parts of plants (Adrees et al., 2015). According to surface analysis, a concentration of 150 mg L-1 Si improved growth and anatomical parameters evaluated in leaves and roots. These results coincided with a great accumulation of Si and a reduction in the accumulation of Cd in K. ivorensis (shoot and root).
The study has shown that K. ivorensis tolerates Cd toxicity up to 25 mg L-1 Cd because plant growth was little affected compared to control. Also, this research has shown that a concentration of 150 mg L-1 Si improved the thickness of leaf tissues important to vegetative growth, specifically STAb, ETAb and SPT. These tissues are important to diffuse CO2 from the environment to the carboxylation site in chloroplasts (Ennajeh et al., 2010). The high STAb, ETAb favors less water loss because the epidermis is the layer that contributes to the efficiency of water use, which reduces its loss during the transpiration process (Javelle et al., 2011). Higher VED and VCD induced by Si in K. ivorensis indicate that the great thickness of these tissues can facilitate the transport of water and nutrients through symplast (Meyer et al., 2011).
Materials and methods
Plant material, growth conditions, and experimental design
The experiment was carried out in a greenhouse at the Federal Rural University of Amazon, Belém, Pará - Brazil (01° 28'03” S and 48° 29'18” W). During the experimental period, averages of temperature and relative humidity of air were recorded in the inside greenhouse at 30 ± 4 ° C and 90 ± 3%, respectively. Young plants of african mahogany, Khaya ivorensis A. Chev (K. ivorensis), 120 days old and average height of 60 cm were transferred to pots of 5 L, which contains a nutrient solution with 25% ionic strength (Sarruge, 1975). The changed nutrient solution was made in intervals of seven days. The pH of the solution was maintained at 5.8 ± 0.2. The plants remained under these conditions for 60 days. After that, the ionic strength of the nutrient solution was changed to 100% where Cd and Si treatments were applied for 60 days. The experimental design used was randomized blocks organized in a 4 x 4 factorial scheme (cadmium and silicon concentrations). Assessments were carried out when the plants reached 120 days old.
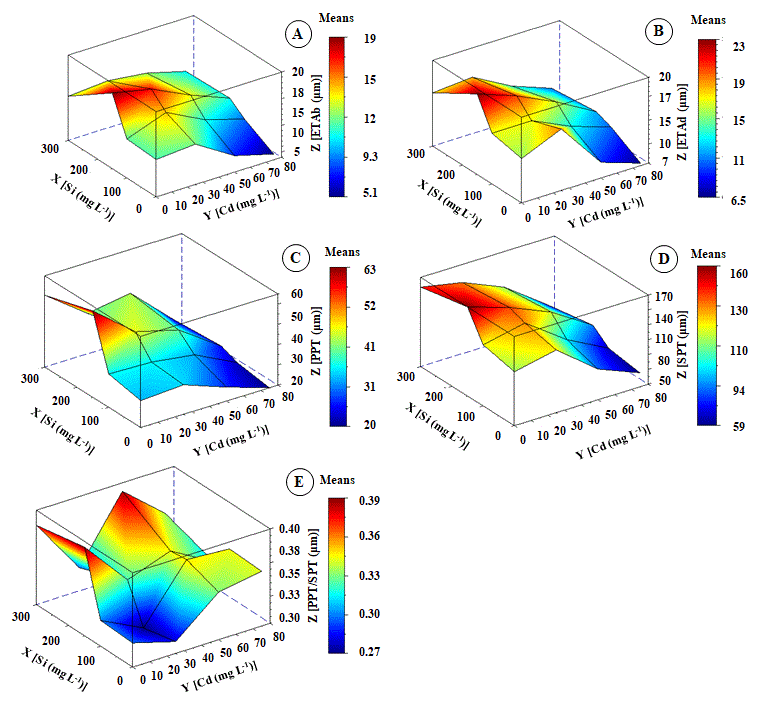
Fig 4. Change in epidermis thickness from abaxial - ETAb (A), epidermis thickness from adaxial - ETAd (B), palisade parenchyma thickness - PPT (C), spongy parenchyma thickness - SPT (D) and palisade parenchyma thickness and spongy parenchyma thickness ratio - PPT/SPT (E) of Khaya ivorensis plants under silicon and cadmium treatments. ZETAb = 11.16610** + 0.04756**X - 0.00891nsY - 0.00014**X2 + 0.00016**YX - 0.00113**Y2 R2 = 0.83; ZETAd = 16.44439** - 0.02913*X + 0.04162**Y - 0.00145* X2 + 0.00011*YX - 0.00012**Y2 R2 = 0.81
ZPPT = 34.11487** + 0.13758**X - 0.05983*Y - 0.00022**X2 - 0.00069**YX - 0.00193**Y2 R2 = 0.92; ZSPT = 115.78797** + 0.28610**X + 0.08478*Y - 0.00059**X2 - 0.00066**YX - 0.01216 **Y2 R2 = 0.90; ZPPT/SPT = 0.29183** + 0.00316**X - 0.00024*Y - 0.0000014*X2 - 0.0000367**YX - 0.0001189*Y2 R2 = 0.88; * significant diferences at 5%; ** significant diferences at 1%; ns: no significant.
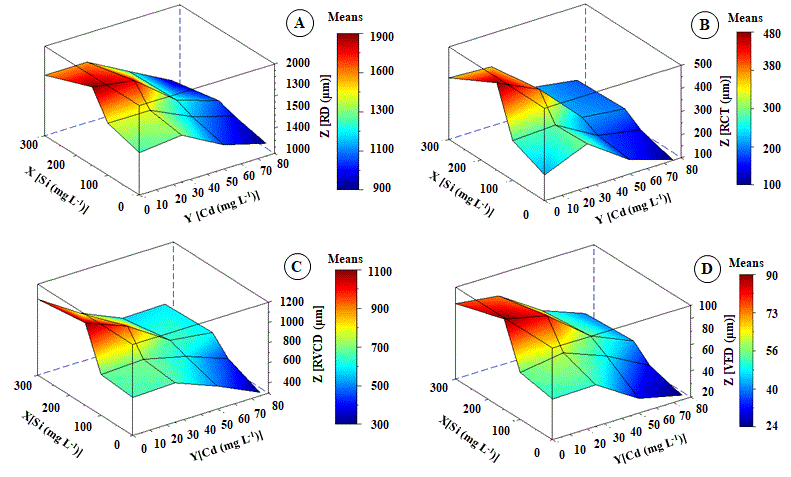
Fig 5. Change in root diameter - RD (A), root cortex thickening - RCT (B), vascular cylinder diameter - VCD (C) and vessel element diameter - VED (D) of Khaya ivorensis plants under silicon and cadmium treatments. ZRD = 1297.07468** + 3.49697**X - 0.76551*Y - 0.00783*X2 - 0.01056**YX - 0.08971**Y2 R2 = 0.76; ZRCT = 253.81416** + 1.14101**X - 1.16890*Y - 0.00219*X2 - 0.00391*YX - 0.01289*Y2 R2 = 0.80; ZVCD = 701.38552** + 2.52964**X - 4.42899**Y - 0.00505**X2 - 0.00619*YX + 0.724*Y2 R2 = 0.87; ZVED = 50.58553** + 0.25031**X - 0.07655*Y - 0.00050**X2 - 0.00056**YX - 0.00453*Y2 R2 = 0.89; * significant diferences at 5%; ** significant diferences at 1%; ns: no significant.
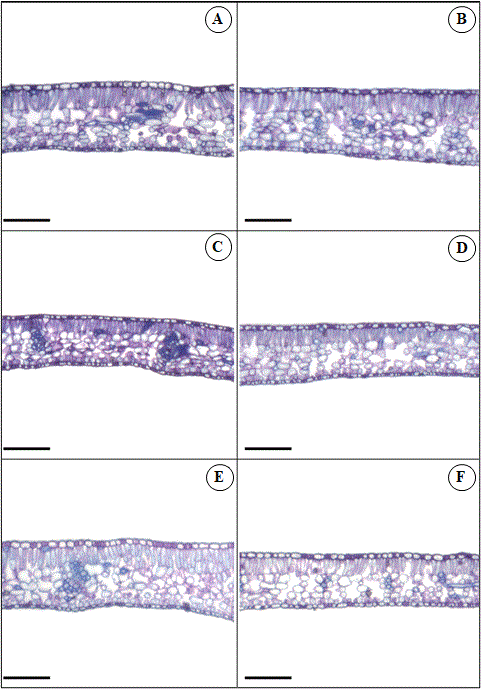
Fig 6. Leaf transversal sections of Khaya ivorensis plants under silicon and cadmium treatments. Capital letters represent Cd and Si treatments in mg L-1. A (Cd 0 x Si 0); B (Cd 0 x Si 150); C (Cd 50 x Si 0); D (Cd 50 x Si 150); E (Cd 75 x Si 0); F (Cd 75 x Si 150). Bars: 200 μm.
Cadmium and silicon treatments
During 60 days, plants were exposed to cadmium and silicon treatment interaction. For Cd (cadmium chloride) concentrations were 0, 25, 50, and 75 mg L-1. For Si were 0, 100, 150, and 300 mg L-1 (sodium metasilicate) with five repetitions.
Anatomical analyses
Samples were collected from the middle region of the leaf branch. Leaves were fully expanded from the third node and 5 cm of root apex. After that, all material botanicals collected were fixed in FAA 70 for 24 hours dehydrated in ethanol, and soaked in Historesin Leica TM (Leica, Nussloch, Germany). Cross sections with a thickness of 5 µm were obtained through rotative microtome (model Leica RM 2245, Leica Biosystems). Sections were stained with toluidine blue (O'Brien et al., 1964). Lamines were observed and photomicrographed under an optical microscope (Motic BA 310, Motic Group Co. LTD.) coupled to a digital camera (Motic 2500, Motic Group Co., LTD.). Previously
calibrated with micrometer lamine, images were analyzed with Moticplus 2.0. Anatomical parameters evaluated were: epidermis thickness from adaxial (ETAd), epidermis thickness from abaxial (ETAb), palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT) and PPT/SPT ratio. Root diameter (RD), root cortex thickness (RCT), vascular cylinder diameter (VCD) and, vessel element diameter (VED) were measured in root samples
Cadmium and silicon content
The Cd content was determined according to Miyazawa et al., (2009). Samples of 0.5g dry matter (leaf, stem or root) were digested in a digester tube with 8 mL of nitric acid and perchloric acid solution (3:1). The cadmium content was determined by atomic absorption spectrometry. The Si content was determined according to Kraska and Breitenbeck (2010) through wet digestion. In 0.1 g of dry matter (leaf, stem or root), 2 mL of 30% hydrogen peroxide and 0.1 M of sodium hydroxide were added.
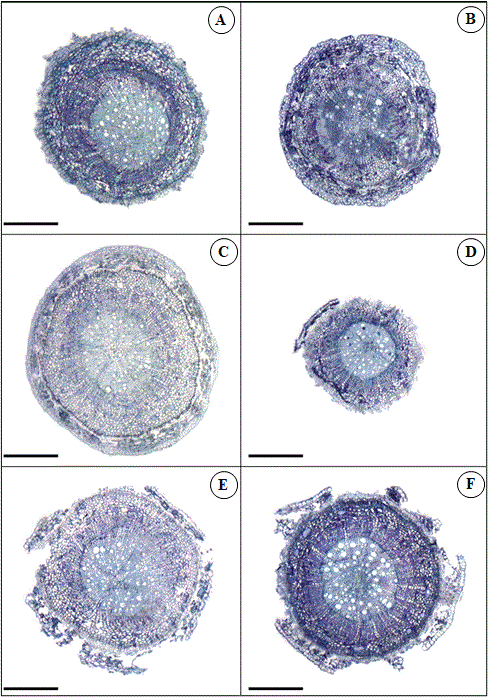
Fig 7. Root transversal sections of Khaya ivorensis plants under silicon and cadmium treatments. Capital letters represent Cd and Si treatments in mg L-1. A (Cd 0 x Si 0); B (Cd 0 x Si 150); C (Cd 50 x Si 0); D (Cd 50 x Si 150); E (Cd 75 x Si 0); F (Cd 75 x Si 150). Bars: 200 μm.
The reaction was incubated the in oven at 95 °C for four hours. Ammonium fluoride (NH4F) was added at 5 mM in samples to facilitate the formation of monosilicic acid. Absorbances were determined using a spectrophotometer at 630nm (Hallmark et al., 1982).
Growth parameters
Plant height (H) and root length (RL) were measured with a millimeter ruler. The leaf area was determined after scanning and processing the images obtained using the ImageJ Software.
Statistical analysis
The statistical package “Statical Analysis System” (SAS Institute, 1999) was used for data analysis. The regression equation model Y (X1, X2) = Β0 + Β1X1 + Β2 X12 + Β3 X2 + Β4X22 + Β5X1 * X2 was generated by procedure PROC Rs reg. The F test was performed to significance (p<0.05) of the interaction between cadmium and silicon concentrations. Then, response surface analysis was performed.
Conclusion
This study presents unedited evidence about the effect of cadmium and silicon on Khaya ivorensis because were founds
toleration of cadmium toxicity up to 25 mg L-1 Cd without considerable growth reduction. Furthermore, silicon has shown a positive modulation in tissue thickness, which is important to water use efficiency and CO2 carboxylation with positive impacts on the vegetative growth of Khaya ivorensis. Therefore, this study indicates that Khaya ivorensis tolerates cadmium toxicity up to 25 mg L-1 Cd and positively responds to anatomical modulations induced by silicon.
Acknowledgment
This work was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq/Brasil). In addition, we thank the Museu Paraense Emílio Goeldi (MPEG/Brazil) for the use of infrastructure for anatomical analysis.
References
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, KashifIrshad M (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotox Environ Safe. 119:186-197.
Ali S, Rizwan M, Hussain A, ur Rehman MZ, Ali B, Yousaf B, Wijaya L, Alyemeni MN, Ahmad P (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Bioch. 140:1-8.
Ali S, Rizwan M, Ullah N, Bharwana AS, Waseem M, Farooq MA, Abbasi GH, Mujahid Farid (2016) Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol Plant. 38:262.
Alzahrani Y, Kuşvuran A, Alharby HF, Kuşvuran S, Rady MM (2018) The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotox Environ Safe. 154:187-196.
Andresen E; Küpper H (2013) Cadmium toxicity in plants. Met Ions Life Sci. 11: 395-413.
Arcanjo and Silva S, Techio VH, Castro EM, Faria MR, Palmieri MJ (2013) Reproductive, Cellular, and Anatomical Alterations in Pistia stratiotes L. Plants Exposed to Cadmium. Water Air Soil Pollut. 224:1454.
Avila RG, Magalhães PC, Silva EM, Júnior CCG, Lana UGP, Alvarenga AA, Souza TC (2020) Silicon supplementation improves tolerance to water deficiency in sorghum plants by increasing root system growth and improving photosynthesis. Silicon. 12: 2545-255.
Bari MA, Prity SA, Das U, Akther MS, Sajib SA, Reza MA, Kabir AH (2020) Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol 22:472-479
Benavides, MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol. 17:21-34
Budowski G (1965) Distribution of tropical American rainforest species in light of successional processes. Turrialba. 15:40-43.
Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Masclaux-Daubresse C (2004) Cadmium Toxicity Induced Changes in Nitrogen Management in Lycopersicon esculentum Leading to a Metabolic Safeguard Through an Amino Acid Storage Strategy. Plant Cell Physiol. 45:1681-1693.
Chugh LK, Sawhney SK (1999) Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant Physiol Bioch. 37:297-303.
Covre WP, Pereira WVS, Gonçalves DAM, Teixeira OMM, do Amarante CB, Fernandes AR (2020) Phytoremediation potential of Khaya ivorensis and Cedrela fissilis in copper contaminated soil. J. Environ. Manage. 268:110733.
Cui J, Liu T, Li F, Yi J, Liu C, Yu H (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ Pollut. 228:363-369
Cunha LFS, Oliveira VP, Nascimento AWS, Silva BRS, Batista BL, Alsahli AA, Lobato AKS (2009) Leaf application of 24-epibrassinolide mitigates cadmium toxicity in young Eucalyptus urophylla plants by modulating leaf anatomy and gas Exchange. Physiol Plantarum. 173:67-87.
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut. 98:29-36.
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Ver Ecol Syst. 18:431-451.
Di Cagno R, Guidi L, Stefani A, Soldatini G (1999) Effects of cadmium on growth of Helianthus annuus seedlings: Physiological aspects. New Phytol. 144:65-71.
Di Sanità Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot. 41:105-130.
Djebali W, Hédiji H, Abbes Z, Barhoumi Z, Yaakoubi H, Zoghlami Lb, Chaïbi W (2010) Aspects on growth and anatomy of internodes and leaves of cadmium-treated Solanum lycopersicum L. plants. J Biol Res-Thessalon. 13:75-84.
Ennajeh M, Vadel AM, Cochard H, Khemira H (2010) Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotech. 85:289-294.
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul. 21:335-351.
Enstone DE, Peterson CA (2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ. 28:444-455.
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol. 50:641-664.
Fan X, Wen X, Huang F, Cai Y, Cai K (2016) Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38:197.
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf. 96:242-9.
Farooq MA, Islam F, Ali B, Najeeb U, Mao B, Gill RA, Yan G, Siddique KHM, Weijun Z (2016) Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot 132:42-52.
Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot. 62:2001-2011.
Foli EG, Alder D, Miller HG, Swaine MD (2003) Modelling growth space requirements for some tropical forest tree species. Forest Ecol Manag. 173:79-88.
Gomes MP, Marques TCLLSM, Nogueira MOG, Castro EM, Soares AM (2011) Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Sci Agric. 68:566-573.
Gomes MP, Marques TCLLSM, Soares AM (2013) Cadmium effects on mineral nutrition of the Cd-hyperaccumulator Pfaffia glomerata. Biologia. 68:223-230.
Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ Pollut. 211:90-99
Hallmark CT, Wilding LP, Smeck NE (1982) Silicon. In: “Methods of Soil Analysis” No. 9, Part 2. (Page AL, Miller RH, Keeney DR, editors). American Society of Agronomy, Incorporation, Soil Science Society of America, Inc. Publisher, Madison, USA, p.263-273.
Howladar SM, Al-Robai SA, Al-Zahrani FS, Howladar MM, Aldhebiani AY (2018) Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotox Environ Safe. 159:143-152.
Javelle M, Vernoud V, Rogowsky PM, Ingram GC (2011) New Phytol. 189:17-39.
Jibril SA, Hassan SA, Ishak CF, Wahab PEM (2017) Cadmium Toxicity Affects Phytochemicals and Nutrient Elements Composition of Lettuce (Lactuca sativa L.). Adv Agr. 2017:Article ID 1236830.
Kraska JE, Breitenbeck GA (2010) Simple, robust method for quantifying silicon in plant tissue. Commun Soil Sci Plan. 41:2075-2085.
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant. 33:35-51.
Li Q, Yu L, Deng Y, Li W, Li M, Cao J (2007) Leaf epidermal characters of Lonicera japonicaandLonicera confuseand their ecology adaptation. J For Res.18:103-108.
Li X, Zhao M, Guo L, Huang L (2012) Effect of cadmium on photosynthetic pigments, lipid peroxidation, antioxidants, and artemisinin in hydroponically grown Artemisia annua. J. Environ. Sci. 24:1511-8.
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil. 209:217-224.
Liza SJ, Shethi KJ, Rashid P (2020) Effects of cadmium on the anatomical structures of vegetative organs of chickpea (Cicer arientinum L.). Dhaka Univ J Biol Sci. 29:45-52.
López-Climent MF, Arbona V, Pérez-Clemente RM, Gómez-Cadenas A (2011) Effects of cadmium on gas exchange and phytohormone contents in citrus. Biol Plantarum. 55:187-190.
Marschner H (2012) Mineral nutrition of higher plants. 3.ed London: Elsevier, 643p.
Melo Marques TCLLS, Soares AM, Gomes MP, Martins G (2011) Respostas fisiológicas e anatômicas de plantas jovens de eucalipto expostas ao cádmio. Rev Arvore. 5:997-1006.
Meyer CJ, Peterson CA, Steudle E (2011) Permeability of Iris germanica’s multiseriate exodermis to water, NaCl, and etanol. J Exp Bot. 62:1911-1926.
Miyazawa M, Pavan MA, Muraoka T, Carmo CA, Melo WJD (2009) Análise química de tecido vegetal. Manual de análises químicas de solos, plantas e fertilizantes. Brasília: Embrapa Informações Tecnológicas.
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 59:368-373.
Oliveira RLL, Mello Prado R, Felisberto G, Cruz FJR (2019) Different Sources of Silicon by Foliar Spraying on the Growth and Gas Exchange in Sorghum. J Soil Sci Plant Nutr. 19:948-953.
Ozyigit II, Vardar F, Yasar U, Akinci S (2013) Commun. Soil Sci. Plan. 44:3076-3091.
Pereira FJ, Castro EM, Pires MF, Oliveira C, Pasqual M (2017) Anatomical and physiological modifications in water hyacinth under cadmium contamination. J Appl Bot Food Qual. 90:10-17.
Pereira TS, Pereira TS, Souza CLFC, Lima EJA, Batista BL, Lobato AKS (2018) Silicon deposition in roots minimizes the cadmium accumulation and oxidative stress in leaves of cowpea plants. Physiol Mol Biol Plants. 24:99-114.
Pérez Chaca MV, Vigliocco A, Reinoso AM, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant. 36:2815-2826.
Pinheiro AL, Couto L, Pinheiro DT, Brunetta JMFC (2011) Ecologia, silvicultura e tecnologia de utilizações dos mognos-africanos (Khaya ssp.). Viçosa: Sociedade Brasileira de Agrossilvicultura.
Piršelová B, Boleček P, Gálusová T (2016) Effect of cadmium and arsenic on chlorophyll fluorescence of selected soybean cultivars. Russ J Plant Physiol. 63:469-473.
Poschenrieder C, Gunse B, Barcelo J (1989) Influence of cadmium on water relations, stomatal resistance, and abscisic acid content in expanding bean leaves. Plant Physiol. 90:1365-1371.
Rafati M, Khorasani N, Moattar F, Shirvany A, Moraghebi F, Hosseinzadeh S (2011) Phytoremediation potential of populus alba and Morus alba for cadmium, chromuim and nickel uptake from polluted soil. Int J Environ Res. 5:961-970.
Raza MM, Ullah S, Aziz T, Abbas T, Yousaf MM, Altay V, Ozturk M (2019) Alleviation of Salinity Stress in Maize Using Silicon Nutrition. Not Bot Horti Agrobo. 47:1340-1347.
Ribeiro A, Filho ACF, Scolforo JRS (2017) O Cultivo do Mogno Africano (Khaya spp.) e o Crescimento da Atividade no Brasil. Floresta e Ambiente. 24:e00076814.
Rodrigues LCA, Martins JPR, Júnior AO, Guilherme LRG, Pasqual M, Castro EM (2017) Tolerance and potential for bioaccumulation of Alternanthera tenella Colla to cadmium under in vitro conditions. Plant Cell Tiss Organ Cult. 130:507-519.
Safoora D, Cyrus G, Bahram B, Mahdi G, Siamak S (2014) Effect of Silicon on Growth and Development of Strawberry under Water Deficit Conditions. Hortic Plant J. 4:226-232.
Sarruge JR (1975) Soluções nutritivas. Summa Phytopathol. 1:231-233.
SAS (1999) SAS Software. Version 9.1. Cary, North Carolina: SAS Institute Inc.
Saud S, Li X, Chen Y, Zhang L, Fahad S, Hussain S, Sadiq A, Chen Y (2014) Silicon Application Increases Drought Tolerance of Kentucky Bluegrass by Improving Plant Water Relations and Morphophysiological Functions. Scient World J. 2014:1-10.
Shi G, Cai Q (2009) Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ Exp Bot. 67:112-117.
Silva ES, Prado RM, Santos DMM, Cruz FJR, Almeida HJ, Campos CNS (2015) Nitrogen components, growth and gas exchange in spring wheat plants grown under interaction of silicon (Si) and nitrogen (N). Aust J Crop Sci. 9:790-798.
Song X, Yue X, Chen W, Jiang H, Han Y, Li X (2019) Detection of Cadmium Risk to the Photosynthetic Performance of Hybrid Pennisetum. Front Plant Sci. 10:798.
Torabi F, Majd A, Enteshari S (2015) The effect of silicon on alleviation of salt stress in borage (Borago officinalis L.). Soil Sci. Plant Nutr. 61:788-798.
Vaculí M, Landberg T, Greger M, Luxová M, Stoláriková M, Alexander Lux (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot. 110:433-443.
Vaculík M, Pavlovič A, Lux A (2015) Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath's cell chloroplasts ultrastructure in maize. Ecotox Environ Safe. 120:66-73.
Varun M, D’Souza R, Pratas J, Paul MS (2011. Phytoextraction potential of Prosopis juliflora (Sw.) DC. with specific reference to lead and cadmium. Bull Environ Contam Toxicol. 87:45-49.
Vitória AP, Rodriguez APM, Cunha M, Lea PJ, Azevedo RA (2003) Structural changes in radish seedlings exposed to cadmium. Biol Plantarum. 4:561-568.
Wu J, Guo J, Hu Y, Gong H (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci. 6:453.
Wu J, Mockd HP, Giehl RFH, Pitann B, Muhling KH (2019) Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard Mater. 364:581-590.
Xu Q, Wang C, Li S, Li B, Li Q, Chen G, Chen W, Wang F (2017) Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (Oryza sativa L.). Environ Sci Pollut Res Int. 24:11319-11330.