

Aust J Crop Sci. 18(12):837-846 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p124
Breeding new japonica TGMS lines and primary evaluation of its F1 combinations
Tran Thi Huyen2, Tran Van Quang*1, Phung Danh Huan3, Hoang Dang Dung4, Nguyen Van Muoi2, Nguyen Thi Tram2, Pham Thi Ngoc Yen2, Nguyen Thanh Tuan1, Tran Hoang Lan2
1Faculty of Agronomy, Vietnam National University of Agriculture, Vietnam
2Crop Research and Development Institute, Vietnam National University of Agriculture, Vietnam
3Department of Crop Production, Ministry of Agriculture and Rural Development, Vietnam
4Center for Experimental and Vocational training, Vietnam National University of Agriculture, Vietnam
*Corresponding author: Tran Van Quang 
https://orcid.org/0000-0002-3089-2092
Abstract: The breeding of hybrid rice (Oryza sativa L.) began in Vietnam in the 1990s and greatly increased rice yield. However, almost all combinations are indica hybrids. This study created 12 novel japonica TGMS lines by using phenotype selection and the molecular-assisted selection (MAS) method from indica TGMS and tropical japonica inbred cross. The results showed that all the novel japonica TGMS lines had good fertility-sterility alteration behavior, complete sterility in artificial and field conditions, and good agronomic traits. The growth duration of the japonica TGMS lines varied from 125 to 144 days and the plant height of the lines varied from 98.8 to 117.3 cm. The outcrossing rate (OCR) of the japonica TGMS lines was lower than its check (T6S), which varied from 24.1% (TJ12) to 64.8% (TJ9) and from 22.2% (TJ12) to 62.9% (TJ9) under bagged and natural conditions, respectively. The new TGMS were japonica genotype and phenotype based on the Chen index result. The result of the test crosses showed that the F1 combinations of the two japonica TGMS lines TJ6 and TJ12 reveal good heterosis. The F1 combinations yield were 7.9 and 7.8 tons/ha compared to 7.4 tons/ha of the check. The amylose content of these two F1 combinations was lower than the check 18.1% and 17.8%, respectively. In conclusion, the results of this study demonstrated that the using of japonica TGMS for japonica hybrid in Vietnam is promising although further studies are required to improve the OCR of japonica TGMS lines.
Keywords: japonica TGMS, hybrid japonica rice, two-line, heterosis.
Abbreviation: TGMS_thermo genic male sterility; PER_Panicle Exertion Rate; DM_Days to maturity; OCR_Outcrossing rate; PH_Plant height; PL_panicle length; NSPP_ number of spikelets per panicle; TPP_Productive tillers per plant; NSPP_number of spikelets per panicle; SE_Stigma exertion rate; PER_Panicle Exertion Rate; SSR_Seed Setting Rate of crosses; GW_1000 grain weight.
Introduction
Rice cultivation is widespread across the globe, with over 120 countries growing rice, primarily in Asia, Africa, and the Americas (Deng 2008; Hu 2010). Generally, cultivated rice can be broadly classified into two types within inbred or hybrid: japonica (Oryza sativa L. subsp. japonica) and indica (Oryza sativa L. subsp. indica) (Catudan and Arocena, 2003; Tripp et al., 2010, and Min et al., 2012). Indica and japonica rice are both ecologically adapted, although they differ significantly in terms of morphology (such as plant height and pubescence), agronomical traits, and physiological-biochemical properties. Japonica rice is typically grown in areas with cooler and temperate climates, while indica rice is grown in tropical, subtropical, and temperate zones. Japonica rice can also be grown in high-elevation mountainous terrain in various low-latitude rice-growing regions, including the Chinese provinces of Yunnan and Guizhou, Laos, Myanmar, and Vietnam, as well as many other Southeast Asian nations. In 2017, global japonica rice production was estimated at 71,255,000 tons and has been increasing by an average of 2.6% annually from 2010 to 2017. The global indica rice production was estimated at 417,349,000 tons in 2017, which is six times more than that of japonica. Its production has increased by 0.9% per annum from 2010 to 2017. In terms of consumption and trade, global japonica rice consumption was estimated at 69,286,000 tons in 2017, with a global trade of 2,329,000 tons, which increased by 2.7% per annum from 2010 to 2017. Overall, in 2017, japonica rice accounted for around 14.6% of global rice production, 14.4% of global rice consumption, and 4.8% of global rice trade.
Currently, the need for milled japonica rice in Asia is growing due to the increasing number of japonica rice consumers (Magno and Yanagida, 2000). In the Philippines, a majority of consumers of milled japonica rice are urban dwellers and immigrants from East Asia. The production of temperate japonica rice is very limited in tropical Asia, thereby demanding a higher price (Kang, 2010; Shim et al., 2015). To meet the increasing demand for temperate japonica rice in tropical regions, japonica rice materials intended for cultivation under tropical conditions have been developed. The developed materials are not only inbred lines but hybrid materials (male, female lines, maintainer) also in the breeding program due to their impressive yield and quality of hybrid rice from the first adoption of hybrid technology in 1966 by Yuan Long-ping. China was the first country to successfully employ heterosis for commercial rice production (Wang 2004). The major countries growing hybrid rice are China, India, Bangladesh, Pakistan, Indonesia, the Philippines, Myanmar, Vietnam, and the United States. Hybrid rice cultivation in the United States accounts for over half of its total rice production area. Similar to inbred varieties, there are two types of hybrids including indica hybrid and japonica hybrid.
Japan is the origin of research in hybrid japonica rice (Li and Wu., 1991). As of 2014, the area devoted to hybrid rice had reached 6.36 million hectares, with 5.91 million hectares in Asia and 0.45 million hectares in North and South America. In the 1980s, the area of japonica hybrid rice in China reached 133,000 hectares, accounting for 2% of the japonica rice area. In the 1990s, the area of japonica hybrid rice in China decreased to 80,000 hectares. However, in recent years, the area of japonica hybrid rice has increased again and is grown mainly in the provinces of Liaoning, Jiangzi, Shanghai, Zhejiang, and Yunan (Jiang et al., 2014; Kang 2013; Ma et al., 1998; Ni et al., 2001; Quan et al., 2000). The area of japonica hybrid rice has increased to 5% of the total area planted by japonica varieties (Pu et al., 2015). According to Gui et al. (2016), the hybrid advantage of yield between indica-japonica increased by 12.47% and japonica-japonica increased by 14.89%. This hybrid advantage was contributed mainly by increase of 32.0% in grain/panicle (indica-japonica) and 37.1% (japonica-japonica) and by 26.8% and 34.0% increase in firm seeds per panicle. However, the breeding and promotion of japonica hybrid rice are relatively limited compared to indica hybrid rice, partly because the yield potential of japonica rice is generally lower than that of indica rice (Xie and Peng, 2016).
In the 1991, in Vietnam, hybrid rice cultivars were tested crop on an area of 100 ha, where in 1991-1992 winter-spring crop, they were put into mass use and gradually expanded to 36 provinces representing different ecological regions, including mountainous areas, plains, northern midlands, central coast, Central Highlands and Mekong River Delta. The area planted for hybrid rice in Vietnam has grown at a fairly rapid rate, from 11,094 hectares (1992) to 435,508 hectares in 2000 and 613,117 hectares in 2012. To create a hybrid combination, CMS and TGMS lines development is massively important. To date, 9 tms genes have been identified by Chinese, Japanese, IRRI, Vietnamese, and Indian scientists. The gene tms1 is located on chromosome 8, tms2 on chromosome 7, tms3 on chromosome 6, tms4-1 on chromosome 2, tms5 on chromosome 2, tms1 on chromosome 10, ms-h on chromosome 9, tms6 on chromosome number 5 and tms8 on chromosome 11 (Lopez et al., 2003; Lee et al., 2005; Appibhai et al., 2012, Vu et al., 2015). The number of sterile lines (CMS, TGMS) generated in Vietnam was 26 lines but no hybrid japonica was developed (DCP, MARD, 2014). Therefore, developing the japonica TGMS lines are necessarily needed.
The TGMS lines being used in Vietnam all carry the tms5 gene, while only the 827S line carries two genes, tms4, and tms5 (Pham et al., 2015). This report is consistent with the announcement of Nguyen et al. (2015) adding that AT19 (T6S) line carries the tms5 gene. These are important sources of materials for making TGMS japonica strains. Although research on hybrid rice has made significant progress, but the existing hybrid rice varieties are all indica hybrids. Currently, none of the japonica hybrid rice varieties have been put into production in Vietnam. Therefore, this study was conducted to develop a new japonica TGMS for japonica hybrid breeding program in Vietnam.
Results and Discussion
Characterization of novel japonica TGMS lines
Characterization of 12 novel japonica TGMS lines was evaluated for traits like stem color, leaf color, leaf ear color, leaf neck color, leaf blade color, tilling pattern, flag leaf length (FLL), flag leaf width (FLW), plant height (PH), productive tillers per plant (TPP). The results showed that most of the novel japonica TGMS lines have green color stem and leaf except TJ2 and TJ10. Similarly, the leaf ear, leaf neck, and leaf blade color of the most novel japonica TGMS lines had white color except TJ2 and TJ10. In addition, all of the lines and T6S showed compact character for the tilling pattern. The flag leaf length and flag leaf width affect the outcrossing rate, whereas a long or wide flag leaf may reduce the outcrossing rate (OCR). In this study, most of the japonica TGMS lines had shorter flag leaf lengths except TJ1, compared to T6S, while none of the japonica TGMS lines had flag leaf width larger than T6S. It is proved that plant height affects the lodging resistance of the rice population. The result showed that most of the japonica TGMS had semi-draft plant types and were shorter than T6S except TJ1 and ranged from 101.20 cm (TJ3) to 116.9 cm (TJ6) (Table 3; Figure 5).
Yield-related traits and outcrossing rate (OCR) of japonica TGMS lines
We measured the mean values of floral traits including days to first flowering, days to 50% flowering, stigma exsertion rate (SE), productive tillers per plant (TPP), panicle length (PL) (cm), panicle excretion rate (PER) (%), seed setting rate (SSR) (%), sterility status (%). Success in the use of hybrid rice depends on the extent of heterosis and the efficiency of the seed production techniques. Growth duration and flowering behavior of novel japonica TGMS lines influence the F1 seed production. In northern Vietnam, the appropriate days for the first flowering of TGMS lines for F1 seed production are from 68 to 85 days. The results reveal that all 12 novel japonica TGMS lines had appropriate days to first flowering (68.5-84.3 days) and flowering concentration behavior (3-5 days from the date of first flowering to 50% flowering) for F1 seed production (Table 4).
The tiller per plant and the NSPP affect the yield of TGMS multiplication and F1 seed production. The results showed that TJ10 had the highest effective tillers per plant (11.20 tillers per plant), followed by TJ9 (10.9 tillers per plant) and TJ11 (9.93 tillers per plant). The number of spikelets per panicle (NSPP) of novel japonica TGMS lines varied from 136 ± 15 (TJ3) to 206 ± 12 (TJ4), while the NNPS of the check line T6S was 191 ± 125 spikelets.
Outcrossing potential in rice is one of the most important traits that depend on the floral characteristics of the female and male parents. The extent of outcrossing in the seed parent is influenced by floral traits such as the stigma exsertion rate (Virmani, 1994). The results showed that the stigma exsertion rate of all novel japonica TGMS lines was lower than the stigma exsertion rate of T6S. SE of novel japonica TGMS lines ranged from 62.2% to 72.5%, compared to 79.8% of T6S. Another feature of agronomic traits is panicle exertion which affects the quantity of grains and the number of spikelets. Therefore, this character affects the yield of F1 seed production and multiplication of novel japonica TGMS lines. The novel japonica TGMS lines with the highest panicle exsertion were TJ1 (80.7%), TJ5 (82.2%), and TJ10 (82.6%) and significantly longer than T6S but other novel japonica TGMS lines were more enclosed than T6S. Most of the novel Japanese TGMS lines had fare good panicle exsertion, which ranged from 65.7% in TJ7 to 82.5% in TJ10 (Table 4). The results indicated that the new novel japonica TGMS lines could be used in hybrid rice breeding.
Pollen Sterility- fertility pattern conversion of japonica TGMS lines under artificial climate
The pollen of the novel japonica TGMS lines was entirely sterile (pollen sterility more than 99.6%), but it became partially fertile under 24°C. Meanwhile, the new japonica TGMS lines had similar characteristics of fertility-sterility alteration to T6S. As a result, it will be safe to produce two-line hybrid seeds when the DMT is greater than 23°C. Nevertheless, under 23°C, japonica TGMS pollen sterility was less than 99.5%, whereas, in growing chambers with 24°C-25°C temperature, they were fully sterile (Table 5, Fig. 4). Thus, for a complete seed formation, the natural DMT (Daily mean temperature) should be higher than 24°C.
Sterility pollen ratio of japonica TGMS lines under field condition
The 12 novel japonica TGMS lines, which exhibited a high self-fertility rate (%) in the growth chamber and complete sterility, were further investigated. A consistent sterility duration of more than 30 days is required in Northen of Vietnam for a valid TGMS line. Pollen sterility in each line of the top five primary panicle spikelets from each line was dynamically examined under a microscope. The results showed that the pollen sterile of novel japonica TGMS varied from 94.6% (TJ5) to 100% (TJ1, TJ3, TJ4, TJ6, TJ7, TJ9, TJ10, TJ11). Other novel japonica TGMS lines (TJ2, TJ8, TJ9, TJ12) did not express complete sterility (Table 4).
Table 1. Names, sequences and binding temperatures of markers used in PCR reactions to detect tms genes.
| Gene | Marker | 3’-5’ sequence | 5’-3’ sequence | Annealing temp. | Reference |
|---|---|---|---|---|---|
| tms2 | RM11 | TCTCCTCTTCCCCCGATC | ATAGCGGGCGAGGCTTAG | 500C | Lopez, 2003 |
| tms3 | F18F/F18RM | TTCCCGGGTTCCACTAGGAT | GCGGACCGTGGAAGCTGGGG | 530C | Lang, 1999 |
| tms4 | RM257 | CCGTGCAACTTAAATCCAAACAGG | GGAATCCTATATGAGCCAGTGATGG | 520C | Reddy, 2000 |
| tms5 | C365-1 | ATTTTGGTTGCGCATTAGAGG | GAATATGCCAAGTACGGAGGAT | 520C | Yang, 2007 |
| tms6 | RM3351 | GTCGAAACGTAGCCAGGCAATGG | CCATGGAAGGAATGGAGGTGAGG | 550C | Lee, 2005 |
Table 2. Fourty-five insertion/deletion (InDel) molecular markers (based on Shen et al., 2004) were used for studying indica and japonica differentiation and identifying indica and japonica characteristics in rice varieties.
| Locus | The DNA sequence of primer (5’-3’) | The DNA sequence of primer (3’-5’) | The difference in amplified DNA fragments between 93-11 and Nipponbare (bp) |
|---|---|---|---|
| R1M7 | ATTCCTGGTTCTACATTACTTA | CGCCTCACTAGAATATCGGA | 37 |
| R1M20 | TTGGAACAGGGAAGAAGC | AGGACATAGTTGTAATGGGTAG | 42 |
| R1M30 | AAGGGGCCCTAATTTATCTAG | TGTTTACTTTGTTCTTGGACTG | 49 |
| R1M37 | ATAGTTCGCCATCGTCAT | ACACGCCATAGCAAGGAA | 53 |
| R1M47 | AATAGAATTACTGATGAAACCTTA | GCCCGTTACCGCTTATGT | 51 |
| R2M10 | CCCAGTCTGCTGCCATCT | GAATGTATTTCAGTTCCAGTAAG | 48 |
| R2M24 | GGGCAACAACGGCTCTG | AGGGAATAAGGCGATACGG | 31 |
| R2M26 | GCAGCAAAGTGCGGAGTA | CAGGTGAATTGCCAATTT | 38 |
| R2M37 | ACTGTTACCCAAACGCTA | ACGTGCACCTACTACAGAAA | 65 |
| R2M50 | CCTGAAGGAAATGATAGCAATAG | GTTTTGTATGCTCTTCACTTGTC | 42 |
| R3M10 | CCGAGTACCATTGCTTTC | CTGCCATAGTTACTGCTCTGTT | 37 |
| R3M23 | TGCTTACAAGGGTCCAAT | GGAGGTGCCTACCAAGAG | 36 |
| R3M30 | AGGCTAAGTGAAGAAATAATAAG | CTCCGTATTCATTACTGGTTG | 24 |
| R3M37 | GCATTGAATTGTACTCTTATTATAT | ACGAATCAAAAGGAGACTAAAAT | 56 |
| R3M53 | ACACTGGCTACGGCAAAG | TTTGTTCGGGAATAATGATGC | 35 |
| R4M13 | TACACGGTAGACATCCAACA | ATGATTTAACCGTAGATTGG | 32 |
| R4M17 | AGTGCTCGGTTTTGTTTTC | GTCAGATATAATTGATGGATGTA | 51 |
| R4M30 | GCTTCTCCTGGTTGTATGC | AAAATAGGGAGGCAGATAGAC | 40 |
| R4M43 | CTTGAACCTGAGTGAGTGG | CGATGAAAATGATGTCTA | 34 |
| R4M50 | TTTTGTGAAACTTGACCCTC | GCGTCCATGTCTTTATTGTG | 33 |
| R5M13 | GAGAAAGAGTGGAAGGAG | AGTATCGTCAGGAGGGTC | 32 |
| R5M30 | CTCAATTTCACCCATCCC | CGCTCCGTCTCCAACCTC | 46 |
| R6M14 | AAATGTCCATGTGTTTGCTTC | CATGTGTGGAATGTGGTTG | 34 |
| R6M30 | CACAAGCCGTAGCAGAGC | TCACGAAAAAGACCCCAAG | 34 |
| R6M44 | TTAGGAATAAAGGCTGGATA | TTACCGTTAATAGGTGGAA | 34 |
| R7M7 | ACCTTCCCTCCCCTTTTGAT | AACTTGGTCTTCCTGTTTTATTG | 67 |
| R7M20 | GTTTTGTGCATTCCTTTAC | TTTATGACATTTTGACCG | 66 |
| R7M37 | CAGCCCTAAATCTAAATACCC | ACGTTGAGACAGGCGAGC | 36 |
| R8M23 | CCTATTCACTCTACCGACAT | GTTTAGTTCCCATTGCTTT | 36 |
| R8M33 | CGAAAGAGGAGAGGGGTAGT | CGAAAACGAGAAACAAATA | 38 |
| R8M46 | CAGCAGAGTCCAGAGAAGAT | GCATAAGATGGCGAGTGA | 30 |
| R9M10 | CTTTGGATTCAGGGGGA | AACTTGAAACGGAGGCAG | 43 |
| R9M20 | ACTGCTTTGATGGCTTGTG | CTCCCCAAACTGAATCC | 40 |
| R9M30 | CTCACCTACCTAAAACCCAAC | CCACCCAAATCTGATACTG | 32 |
| R9M42 | CTATAAGACCAAAACGAAAACT | GAAAACCATTGTGTCACTGTA | 48 |
| R10M10 | GAATACAACCCCCTAAAAAC | ATGGACCGTTGAGGAGAC | 38 |
| R10M17 | TGAACAATAAACCACAGAAGCA | CCCTTTATTCCCTCCTTTG | 31 |
| R10M30 | CCCTAAAAATAGAGCAACCT | ACCCATAATACTACCAATCAAC | 19 |
| R10M40 | GTCCCTAGGCCATCTCTTG | GCGAATAGGGGTGGACAG | 33 |
| R11M23 | AAGGTTGACAAGGACAGAAG | TCGCAGGAATGGATAAAA | 42 |
| R11M40 | AAGAAAAATATCTATTGAGGAGTG | GGAGGACCATAAATGACGG | 41 |
| R12M10 | ATCATTTCAGCCTGTGCC | AGCTTAATAGGGGGGACG | 47 |
| R12M27 | ATTTCATTGCCATCAGTT | GTAATCTTCTATCCGTTCA | 33 |
| R12M33 | TTGATGATAGTATTTGCTGATG | AGATAGTGTCGGCGGTGG | 42 |
| R12M43 | CCGCCGAGAAGAAACAAAG | CCCAAGAACAGGATTACA | 30 |
The 32 underlined InDel primer pairs in bold letters indicate the effective loci for indica and japonica identification.
These phenomena indicated that the sensitive stage to temperature was located 13 days before heading. The novel japonica TGMS lines had long growth duration and experienced the DMT decrease in late September (Data not show). Although the DMT declined in late September, the pollen of TGMS lines was altered slightly indicating their CTPs under natural conditions were similar to those in the growth chamber (Table 4 and Table 5). This result indicated that those japonica TGMS lines can be used for F1 seed production in the summer season in northern Vietnam.
Verify tms genes
According to Nguyen et al. (2015), T6S (tms gene donor) carries tms5 genes. In this study, the molecular markers that link to tms5 were used for screening and validation of the existence of two tms genes. The results showed that all the novel japonica TGMS lines possessed tms5 allen (Fig. 3). It is indicated that the tms5 genes were transferred successfully from T6S to novel japonica TGMS. Other tms genes were also validated but did not show the corresponding alleles (data not show).
Table 3. Morphological characteristics of novel japonica TGMS lines in the summer season 2022.
| Line | Stem color | Leaf color | Leaf ear color | Leaf neck color | Leaf blade color | Tilling pattern | Flag leaf length (cm) |
Flag leaf width (cm) |
Plant height (cm) |
|---|---|---|---|---|---|---|---|---|---|
| T6S | Green | Green | White | White | White | Compact | 40.2 | 1.5 | 112.70 |
| TJ1 | Green | Green | White | White | White | Compact | 41.1 | 1.6 | 114.70 |
| TJ2 | Green with purple stripes | Dark green | Light Purple | Light Purple | Light Purple | Compact | 39.3 | 1.6 | 101.70 |
| TJ3 | Green | Green | White | White | White | Compact | 42.6 | 1.7 | 101.20 |
| TJ4 | Green | Green | White | White | White | Compact | 40.7 | 1.7 | 115.60 |
| TJ5 | Green | Green | White | White | White | Compact | 38.9 | 1.5 | 114.70 |
| TJ6 | Green | Green | White | White | White | Compact | 35.5 | 1.5 | 116.90 |
| TJ7 | Green | Green | White | White | White | Compact | 38.2 | 1.6 | 102.80 |
| TJ8 | Green | Green | White | White | White | Compact | 32.6 | 1.7 | 105.90 |
| TJ9 | Green | Green | White | White | White | Compact | 33.8 | 1.5 | 103.70 |
| TJ10 | Blue with purple stripes | Dark green | Light Purple | Light Purple | Light Purple | Compact | 38.9 | 1.7 | 116.50 |
| TJ11 | Green | Green | White | White | White | Compact | 40.2 | 1.6 | 105.70 |
| TJ12 | Green | Green | White | White | White | Compact | 41.8 | 1.7 | 108.20 |
Table 4. Agronomic characteristics of novel japonica TGMS lines in the summer Season 2022.
| Genotypes | Days to first flowering |
Days to 50% flowering | Productive tillers per plant | NSPP | SE | PER (%) |
SSR (%) |
Sterility status (%) |
|
|---|---|---|---|---|---|---|---|---|---|
Nature condition |
Bagged condition | ||||||||
| T6S (check) | 67.5 | 72.5 | 7.5 | 191\(\pm\)25 | 79.8 | 78.7 | 84.6 | 81.2 | 99.5 |
| TJ1 | 68.5 | 73.5 | 8.5 | 152\(\pm\)24 | 65.3 | 80.7 | 33.2 | 30.3 | 100 |
| TJ2 | 72.5 | 75.9 | 8.9 | 165\(\pm 2\)2 | 64.2 | 75.2 | 32.4 | 30.1 | 99.6 |
| TJ3 | 69.3 | 74.2 | 7.3 | 136\(\pm\)15 | 72.5 | 77.8 | 45.2 | 41.3 | 100 |
| T4 | 72.9 | 76.1 | 6.9 | 206\(\pm 1\)2 | 60.5 | 78.2 | 24.5 | 22.4 | 100 |
| TJ5 | 68.7 | 71.2 | 8.7 | 179\(\pm 2\)1 | 69.8 | 82.2 | 35.8 | 31.7 | 100 |
| TJ6 | 84.3 | 87.3 | 9.4 | 160\(\pm 3\)2 | 66.5 | 69.9 | 27.9 | 24.2 | 100 |
| TJ7 | 71.5 | 74.6 | 6.9 | 179\(\pm\)18 | 62.2 | 65.7 | 34.8 | 31.3 | 94.6 |
| TJ8 | 67.2 | 72.1 | 8.9 | 170\(\pm\)29 | 69.4 | 73.5 | 54.6 | 51.7 | 99.5 |
| TJ9 | 82.5 | 85.9 | 10.9 | 181\(\pm 2\)8 | 63.8 | 71.2 | 64.8 | 62.9 | 95.3 |
| TJ10 | 72.8 | 76.2 | 11.2 | 162\(\pm\)19 | 63.2 | 82.5 | 54.2 | 51.3 | 100 |
| TJ11 | 79.5 | 83.9 | 9.9 | 145\(\pm\)23 | 69.4 | 77.5 | 64.3 | 61.4 | 100 |
| TJ12 | 74.5 | 77.2 | 8.9 | 156\(\pm\)26 | 68.2 | 71.2 | 24.1 | 22.2 | 97.6 |
Note: NSPP: number of spikelets per panicle; SE: Stigma exertion rate; PER: Panicle Exertion Rate; SSR: Seed Setting Rate of crosses.
Determination of novel japonica TGMS lines for indica and japonica characteristics based on InDel molecular markers
By applying 32 selected InDel primer pairs that showed a strong association with indica-japonica rice differentiation, we calculated indica or japonica allele frequencies (Fi and Fj) of 12 TMGS lines with the banding pattern of 9311 and Nipponbare as reference using the established formulas (1 and 2). Upon comparing the results obtained from the two traditional InDel and Chen index methods using the same set of rice samples, we found that both independent methods yielded identical results. The frequencies (Fi and Fj) of specific indica or japonica alleles varied between 0.12 and 0.28 (Table 7). The results further confirmed that the new TMGS lines were all japonica types. These results indicate that InDel primer pairs play an important role in indica-japonica gene identification studies.
Characterization of F1 combinations under spring season
Characterization of 12 F1 combinations was done for traits like days to maturity, plant height (PH), productive tillers per plant (TPP), panicle length (PL), number of spikelets per panicle (NSPP), filled grain percentage, 1000 grain weight (GW), actual yield, and amylose content. The results showed that all the F1 combinations had medium growth duration and ranged from 125 days to 144 days. The shortest growth duration of the F1 combinations was TJ10 (125 days) which is similar to the check (127 days). The plant height of F1 combinations was measured and showed that all the F1 combinations had lower plant height compared to the check (113.0 cm) except TJ7 (117.3 cm) (Table 8) The yield-related traits of the F1 combinations were also
analyzed. All of the F1 combinations were found to be lower in productive tillers per plant (5.3 - 7.1 tiller), compared to the check (7.2 tillers). All the F1 combinations showed shorter panicle length than the check but this trait did not correspond to the NPPP which 3 combinations (TJ4, TJ11, TJ12) showed greater NNPP than the check. In addition, despite a significantly lower filling ratio of the F1 combinations compared to the check, the actual yield of two combinations TJ6 and TJ11 was higher than the check. This phenomenon can be explained by the 1,000 GW of the F1 combinations being higher than Laithom 6 (Table 8).
Discussion
TGMS line seeds are generally produced in cool season which favors pollen fertility at low temperatures (< 23°C) resulting in more fertility, while high temperatures (> 30°C) result in sterility irrespective of the photoperiod. These lines can also be easily multiplied in autumn because of the occurrence of low temperatures (Virmani et al. 2003). Evaluation of TGMS lines for flowering morphological and agronomic traits is a prerequisite to find commercially useable japonica TGMS lines (Virmani et al., 1997; Kalaiyarasi and Vaidyanathan, 2002; Hashim et al., 2021) because Vietnam experiences cool seasons with contrasting temperature regimes which can reverse the sterile TGMS lines into fertile ones.
Favorable environmental conditions with appropriate temperature regimes for TGMS lines coupled with the sufficient pollen load of the restorer line are necessary to have a maximum OCR, which may lead to high hybrid seed production. One of the keys to spread hybrid rice is to reduce the costs of seed production.
Table 5. Fertility-sterility alteration behavior of the newly developed TGMS lines and the recurrent parent under five different temperature regimes in growth chambers.
| Lines | Pollen sterility (%) | ||||
|---|---|---|---|---|---|
| 21°C | 22°C | 23°C | 24°C | 25°C | |
| T6S | 85.3 | 92.4 | 99.3 | 100 | 100 |
| TJ1 | 75.6 | 90.2 | 97.8 | 99.6 | 100 |
| TJ2 | 84.3 | 98.5 | 100 | 100 | 100 |
| TJ3 | 72.4 | 94.2 | 98.5 | 100 | 100 |
| TJ4 | 79.6 | 93.6 | 100 | 100 | 100 |
| TJ5 | 84.3 | 94.3 | 100 | 100 | 100 |
| TJ6 | 86.4 | 89.3 | 97.6 | 99.7 | 100 |
| TJ7 | 87.9 | 92.7 | 100 | 100 | 100 |
| TJ8 | 82.3 | 90.8 | 98.4 | 100 | 100 |
| TJ9 | 78.3 | 92.6 | 99.3 | 100 | 100 |
| TJ10 | 88.5 | 93.7 | 100 | 100 | 100 |
| TJ11 | 75.2 | 93.2 | 97.6 | 100 | 100 |
| TJ12 | 83.3 | 94.5 | 98.5 | 100 | 100 |
Table 6. Classification standard between typical indica and typical japonica rice based on the frequency of indica-specific alleles (Fi) or japonica-specific alleles (Fj) calculated from InDel amplified products.
| Allele frequency | Type of rice identified by the InDel index | |
|---|---|---|
| indica- specific (Fi) | japonica-specific (Fj) | |
| >0.9 | <0.10 | Typical indica |
| 0.75–0.89 | 0.11–0.25 | indica |
| 0.61–0.74 | 0.26–0.39 | Close to indica |
| 0.40–0.60 | 0.40–0.60 | Intermediate type |
| 0.26–0.39 | 0.61–0.74 | Close to japonica |
| 0.11–0.25 | 0.75–0.89 | japonica |
| <0.10 | >0.90 | Typical japonica |
Table 7. Classification of typical indica and japonica genotype using Chen’s index and insertion/deletion (InDel) molecular markers.
| Lines/varieties | Chen’s IDa | Indel IDb | Fic |
|---|---|---|---|
| 9311 | Indica | Typical indica | 1.00 |
| Nipponbare | japonica | Typical japonica | 1.00 |
| TJ1 | japonica | Typical japonica | 0.12 |
| TJ2 | japonica | Typical japonica | 0.16 |
| TJ3 | japonica | Typical japonica | 0.12 |
| T4 | japonica | Typical japonica | 0.16 |
| TJ5 | japonica | Typical japonica | 0.15 |
| TJ6 | japonica | Typical japonica | 0.19 |
| TJ7 | japonica | Typical japonica | 0.23 |
| TJ8 | japonica | Typical japonica | 0.15 |
| TJ9 | japonica | Typical japonica | 0.19 |
| TJ10 | japonica | Typical japonica | 0.23 |
| TJ11 | japonica | Typical japonica | 0.28 |
| TJ12 | japonica | Typical japonica | 0.15 |
aClassification of indica or japonica as identified by Chen’s index. bClassification of indica or japonica as indicated by the InDel molecular index.
cIndica-specific allen frequency.
Therefore. it is necessary to improve the outcrossing rate (OCR). To achieve the highest OCR which could result in high hybrid seed production, favorable climatic conditions with suitable temperature regimes for TGMS lines are required. These conditions must also be combined with a sufficient pollen load of the restorer line. The outcrossing capacity of the parental lines affects the seed output in the development of hybrid rice seeds. The seed yield in hybrid rice seed production depends on the outcrossing potential of the parental lines. To succeed the hybrid seed production, a sufficient number of pollen grains must be deposited on the stigma lobes of the spikelets of male sterile parents (Virmani et al., 2003) and the higher stigma length increases the outcrossing ability of TGMS lines (Karpagam, 2011). In this study, the OCR of the novel japonica TGMS was quite low compared to T6S (Table 4).
Some constraints, such as grain-filling obstacles (Yang et al., 2002; Anis et al., 2019; Casco et al., 2021; Wang et al., 2023) and
sterility (Kubo and Yoshimura, 2005; Ouyang et al., 2010; Li et al., 2020; Zhang et al., 2020 Gou et al., 2023;) in the F1 generation, were considered generally to restrict the promotion of japonica/indica hybrids for large-scale production. In this study, three F1 combinations TJ4, TJ7, and TJ9 were expressed as grain-filling obstacles. The nine F1 combinations including TJ1, TJ2, TJ3, TJ5, TJ6, TJ8, TJ10, TJ11, and TJ12 showed satisfactory seed-set percentage (Table 8), while two combinations TJ6 and TJ11 showed a higher actual yield and acceptable amylose content and the other three combinations TJ1, TJ3, and TJ12 which showed acceptable actual yield. They should be further studied to select the best F1 combination performance and establish F1 seed production as well as multiplication of novel japonica TGMS lines. In addition, the quality analysis of selected japonica TGMS lines and F1 combination should be analyzed for the traits such as milling quality, protein content, and gel consistency the select the best combination for commercial.
Table 8. Agronomic characteristics of F1 combinations in the Spring Season 2023.
| Genotypes | DM (days) |
PH (cm) |
TPP | PL (cm) |
NSPP | Filled grain (%) | 1000 GW (g) |
Actual yield (tons/ha) |
Amylose content (%) |
|---|---|---|---|---|---|---|---|---|---|
| Laithom 6 (check) | 127\(\pm\) | 113.0 | 7.2 | 24.2 | 186.2\(\pm \ \)25 | 90.7 | 21.7 | 7.4 | 20.1 |
| TJ1 | 136 | 109.6 | 6.8 | 22.9 | 163.8\(\pm\)22 | 86.9 | 28.3 | 6.8 | 16.2 |
| TJ2 | 144 | 109.8 | 6.2 | 23.0 | 157.2\(\pm\)17 | 92.4 | 22.7 | 6.5 | 17.0 |
| TJ3 | 137 | 113.4 | 6.7 | 23.1 | 182.8\(\pm\)21 | 89.3 | 25.5 | 7.1 | 15.4 |
| T4 | 136 | 111.3 | 6.9 | 23.7 | 184.2\(\pm\)28 | 86.0 | 27.2 | 6.9 | 15.9 |
| TJ5 | 136 | 110.2 | 6.2 | 23.2 | 181.7\(\pm\)23 | 90.3 | 25.4 | 6.7 | 15.8 |
| TJ6 | 139 | 104.1 | 6.5 | 24.0 | 186.1\(\pm\)19 | 86.4 | 28.6 | 7.9 | 18.1 |
| TJ7 | 140 | 117.3 | 6.3 | 23.8 | 213.6\(\pm\)21 | 87.3 | 23.8 | 7.2 | 18.0 |
| TJ8 | 131 | 98.8 | 5.3 | 21.2 | 168.8\(\pm\)17 | 89.6 | 26.3 | 5.9 | 16.2 |
| TJ9 | 137 | 103.4 | 6.5 | 23.0 | 158.2\(\pm\)12 | 85.8 | 28.5 | 6.8 | 17.0 |
| TJ10 | 125 | 104.9 | 6.0 | 23.0 | 160.1\(\pm\)15 | 85.5 | 29.9 | 6.2 | 15.4 |
| TJ11 | 142 | 110.2 | 6.2 | 22.9 | 189.2\(\pm 13\) | 80.6 | 25.3 | 7.8 | 17.8 |
| TJ12 | 140 | 104.1 | 7.1 | 22.3 | 193.6\(\pm 12\) | 88.4 | 26.6 | 7.3 | 19.2 |
Note: DM: Days to maturity; PH; Plant height; TPP: Productive tillers per plant; NSPP: Number of spikelets per panicle; GW: grain weight.
Materials and methods
Plant materials
A population consisting of 12 novel japonica TGMS lines at BC3F5 generation was generated from a cross between an indica TGMS lines T6S, possessing the tms5 gene, with a tropical japonica inbred variety JH10 as parental lines (Figure 1). T6S was used as a check line for pollen sterility status and morphological traits.
9311 and Nipponbare were used as standard references for determining the indica or japonica genotype respectively, at a particular InDel locus and for indica or japonica phenotype.
Field experiment
The field experiments were conducted at the experiment field of Crop Research and Development Institute, Vietnam National University of Agriculture 210.01’07.6” N, 1050.9’30.1” E. The novel japonica TGMS lines (BC3F5 generation) and its F1 combination were transplanted using a randomized complete block design without replicates in 5 rows, each row 20 hills with a spacing of 20 cm between hills and 30 cm between rows then managed normally.
Lai thom 6 was used as a check for the F1 evaluation experiment and T6S was used as a check for the novel japonica TGMS evaluation experiment.
Characterization of novel japonica TGMS lines for fertility-sterility alteration in the growth chamber
Characterization of TGMS lines for fertility-sterility alteration under a growth chamber was followed according to Jiang et al., 2015. In the summer of 2022, five single plants per hill were transplanted in each plastic pot, using uniform, healthy rice seedlings that were at the 5-leaf stage (25 days after sowing). Each plant was labeled with a plastic tag to identify it. The plants in each allotment were carefully managed to ensure uniformity and healthy growth. One week before they were used for the studies, five growth chambers (Microclima 1000 Large Plant Growth Chamber Incubator Lab, Sneijder Scientific) were set up for dry runs. The DMT was set at 21°C, 22°C, 23°C, 24°C, and 25°C, respectively, while the light duration and relative humidity level were set to 14 hours and 75%, respectively, in all five growth chambers. The desired DMT and daylight conditions were attained by carefully planning the temperature and light duration to follow diurnal cycles. From 5 to 16 days after the primordial stage of panicle initiation, the plants were placed in growth chambers. After being exposed to the temperature for 12 days, the plants were removed from the growing chambers. The pollen grains were collected from the top five spikelets of each panicle per plant that headed during the 5 to 16 days after ending controlled growth chamber environment treatment and then visually under a microscope. The pollen sterility of each panicle
was recorded following the classification of pollen morphology upon IK-I staining of pollen grains (Virmani et al., 2003). The line was deemed fully sterile if the average pollen sterility was more than 99.5%.
Morphological traits of the novel japonica TGMS lines
Ten plants were selected randomly from rows of each novel japonica TGMS plot in each set for recording the observations on, flag-leaf length, panicle length, panicle exertion, stigma exertion, days to first flowering days to 50% flowering, plant height (cm), productive tillers per plant, panicle length (cm), days to maturity (days), PER (%), OCR (%), sterility status (%), aromatic feature.
Morphological traits of the F1 combinations
Ten F1 plants from each combination were sampled at the maturity stage to measure the yield and yield-related traits and then observed based on SES (IRRI, 2002). Laithom 6 (using T6S as the female parent) was used as check lines.
DNA extraction and polymerase chain reaction (PCR) protocol
DNA was extracted using the modified CTAB method (Doyle and Doyle, 1990). About 0.5 g leaves were ground with 800 μl CTAB buffer by a mortar and pestle until a green solution appeared. The solution was transferred to an Eppendorf tube, and 56 μl of 10% SDS was added and mixed. Sample incubation at 65oC in a stable tank 60 in min then cool down at room temperature. An 800 μl of chloroform was added: isoamyl alcohol mixture (24:1), shaken gently until an emulsion is formed, centrifuge at 13,000 rpm, 5 min, 4°C. The supernatant then was transferred to a new eppendorf tube, add 800 μl of the chloroform: isoamyl alcohol mixture (24:1), and centrifuged at 13,000 rpm, for 5 minutes, 4°C. Collect the supernatant in an Eppendorf tube, and precipitate the DNA with isopropanol at a ratio of 1:1 (v/v). Place in the deep freezer for 1 hour. Centrifuge 13,000 rpm, 5 minutes, 4oC then wash the DNA precipitate with 70% ethanol. Dry the DNA at room temperature. Dissolve the DNA in double distilled water (approximately 200 μl). The extracted DNA was checked for integrity on a 1% agarose gel.
PCR reaction
Each 25 µl PCR reaction consisted of: 8.2 µl deionized double distilled water; 1.5 µl PCR buffer 10X + MgCl2 25 mM; 0.5 µl dNTPs 10 mM; 0.8 µl Taq DNA polymerase 1 U/µl; 3 µl 5 µM forward primer + 5 µM reverse primer; 1.0 µl DNA 10 ŋg/µl. PCR program on Bio-rad 9800: 95oC -5 minutes; 35 cycles (95oC-30s; 58oC-1min; 72oC-1.5 min); 72oC - 5 minutes; Keep the sample at 40C. The primer sequence for TGMS genes screening is listed in Table 1 (Nguyen et al., 2015; Pham et al., 2015). The primer sequences for japonica/indica identification are listed in Table 2 (Lu et al., 2009).
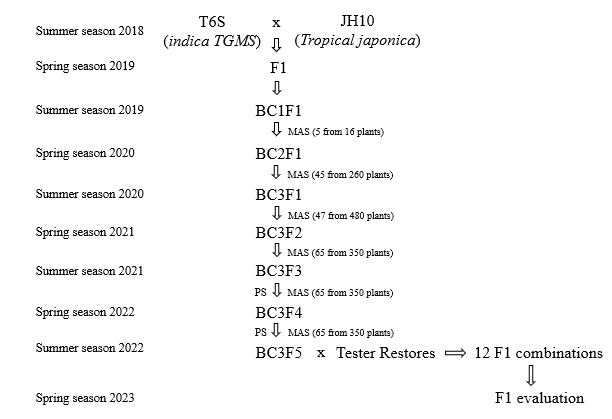
Figure 1. The breeding scheme shows the development of japonica TGMS lines by introgression of TGMS gene tms5 (donor parent T6S) into recipient parent tropical japonica JH10 through MAS approaches. MAS: Marker-assisted selection; PS: Phenotype selection
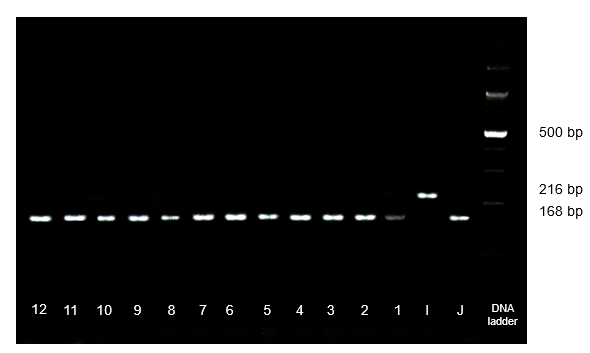
Figure 2. PCR amplification and electrophoresis of 12 novel japonica TGMS lines using the insertion/deletion (InDel) primer pair R1M37, showing the homozygous indica genotype (II), homozygous japonica genotype (JJ), Lanes J, I: Nipponbare and 93-11; lanes 1-12: newly TGMS line (homozygous japonica genotype).
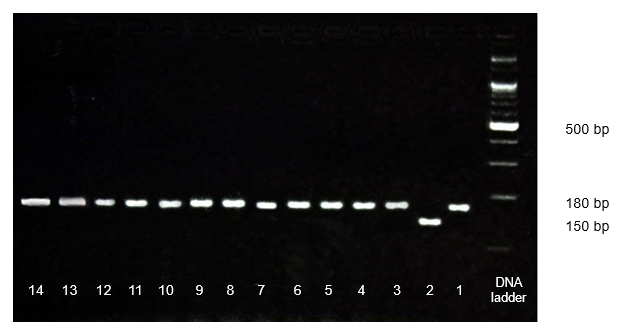
Figure 3. PCR amplication and electrophoresis of japonica TGMS lines using primer pair C365-1 for tms5 allen identification. Land 1: T6S; land 2: IR24; Lan 3-12: TJ1-TJ12.
PCR product electrophoresis
PCR products were separated on 4% agarose gel in 0.5 x TBE buffer and visualized under UV radiation using ethidium bromide (0.5 g/ml for 30 min).
Measurement of Cheng’s index
+ To classify lines/varieties as indica or japonica by phenotype, we use the Chen index (Lu, et al., 2009) including 6 criteria:
(1) Lemma hairiness: The subspecies japonica usually has more hair on the husk than the subspecies indica.
(2) Phenol response in rice grains: According to Walls (1965). Five seeds from each sample were taken twice and soaked in distilled water for 18 hours. Then add phenol (2%) and keep it at room temperature (28oC). After 24 hours, record the color change of rice grains according to positive (black color) and negative (no color change). The indica subspecies tended to have a positive reaction (discoloration) while the japonica subspecies sample had a negative reaction and remained unchanged.
(3) Inter-node length of panicle axes: The japonica form has shorter internodes than the indica form.
(4) Color of grain husks: Japonica granules have a brighter husk color than indica granules.
(5 Hairiness of leaf blades: The japonica form has more hair on the leaf blade than the indica form.
(6) Length/width ratio of grains: Twenty filled grains per line were sampled, the husks were separated to measure length and width, and then the length/width ratio (L/W) was calculated. Grain shapes were scored and classified according to SES (IRRI, 2002).
Genotype score and allele frequency calculation
Genotype score and allele frequency computation followed the Lu et al. 2009 approach. The banding patterns were evaluated as homozygous indica genotype (II), homozygous japonica genotype (JJ), or heterozygous indica-japonica genotype since the InDel molecular markers were co-dominant (IJ).
The electrophoretic banding patterns of the sequenced indica rice variety (93-11) and japonica rice variety (Nipponbare) were utilized as standard references. If the analyzed rice sample's banding pattern (band positions) matched those of 93-11, it was identified as having the homozygous indica genotype (II) at the specified InDel gene (Fig. 1, lanes 2 and 13, 14, 15).
It was found that the sample under examination had the homozygous japonica genotype (JJ) at the specified InDel gene if its banding pattern matched that of Nipponbare (Fig. 1, lanes 1 and 4, 5, 6, 8, 9, 11, 16). For a certain InDel locus, if the investigated sample displayed both bands that were identical to 93-11 and Nipponbare, this locus was identified as heterozygous indica-japonica genotype (IJ) (Fig. 1, lane 3, 7, 10, 12). The average allelic frequency (F), derived from genotyping data, was used to identify whether a rice sample was indica or japonica. The following formula was used to determine the indica or japonica allelic frequency (Fi or Fj) of a specific rice sample at each of the InDel loci:
Frequency of indica alleles (Fi) (1)
\(Fi = = \frac{2\sum_{1}^{N}{Xii + \ \sum_{1}^{N}{Xij\ }\ }}{2N}\)
Frequency of japonica alleles (Fj) (2)
\[Fj = = \frac{2\sum_{1}^{N}{Xjj + \ \sum_{1}^{N}{Xij\ }\ }}{2N}\]
Where, Xii indicates the homozygous indica genotype (II) at a given InDel locus of a particular rice sample scored based on the electrophoresis banding pattern; Xjj indicates the homozygous japonica genotype (JJ) at a given InDel locus of a particular rice sample scored based on the electrophoresis banding pattern; Xij indicates the heterozygous indica– japonica genotype (IJ) at a given InDel locus of a particular rice sample scored based on the electrophoresis banding pattern; N indicates the total number of InDel loci examined.
Standards for indica and japonica rice determination
Following Lu et al. 2009, the average allelic frequency (F) of several InDel loci across the whole rice genome served as the foundation for the development of the InDel molecular index for recognizing indica and japonica rice varieties. This method's distinctive quality for identifying indica or japonica traits and examining the genetic links between indica and japonica rice varieties had a thorough judgment from numerous InDel loci. This InDel index approach may therefore determine the average frequency of alleles specific to indica (Fi) or japonica (Fj) at different loci across the entire genomes, which ensures the correct identification of indica and japonica rice. Using the established standard, distinct varieties of rice with varying degrees of indica and japonica differentiation in the analyzed samples could be precisely identified (Table 6). Based on the frequency of indica-or japonica-specific alleles at various InDel loci of the investigated rice samples, a standard was developed.
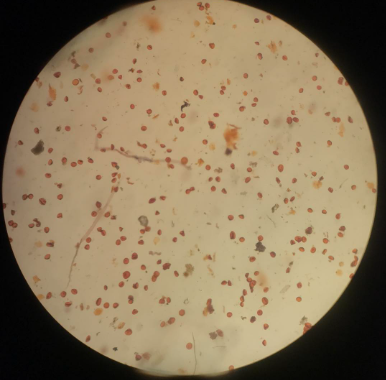
Figure 4. The abortive pollens of TJ6 with an irregular shape and unstainable in I2-KI solution.

Figure 5. Pheonotype of T6S and TJ6.
Observation of pollen fertility and sterility
The nuclear male sterility (TGMS) lines have a sterility temperature of 23°C-25°C at the susceptible stage. The daily ambient temperature (average), and the day and time of flowering in dates of the individuals were recorded. Before flowering, four anthers from the top, middle, and bottom spikelets of each plant were collected, and the pollen grains were checked by optical microscopy. Pollen grains were stained in 1% KI-I solution. The TGMS line has 2 types of sterility:
Type 1: Typical sterility, pollen grains are diamond-shaped, triangular, wrinkled shells;
Type 2: Sterile without pollen grains, empty anthers without pollen grains, when the rice sprouts, the anthers are white.
Accordingly, individuals with sensitization periods falling in the period with an average daily temperature lower than 23°C-25°C will have fertilized pollen grains, and vice versa will be selected because they have similar sexual metabolic characteristics with TGMS lines.
The pollen grains of the novel japonica TGMS lines and F1s were classified as follows based on the extent of pollen sterility (Virmani et al., 2003).
The following formulae were used to calculate pollen sterility (%).
\[Pollen\ sterility\ (\%) = \ \frac{No.of\ sterile\ pollens}{Total\ No\ of\ pollens}\ \ x\ 100\]
Testcross experiments
To assess the novel japonica TGMS line's maintenance-restoration connection, a test cross was conducted utilizing the 12 novel japonica TGMS lines as the female and a japonica inbred variety as the pollinator. Spikelet fertility of the testcross between the novel japonica TGMS and the identified restoration source was assessed by counting filled and unfilled grains from bagged panicles covered before flowering and panicles without bagging at harvest stage (about 25- 30 d after flowering) to assess the restorative potential of the source.
In the evening, novel japonica TGMS panicles were clipped, and the following morning, hand pollination was done by dusting pollen from 1 chosen japonica variety. The appropriate actions were taken to avoid unintended pollination. The crossed and clipped panicles were covered after being suitably tagged with white and brown sheets. Bagging was employed for 3–4 days after pollination to stop unintentional pollination.
Statistical analysis
The classification created by Govind and Virmani (1988) was applied as the basis for calculating the pollen and spikelet fertility percentage. Broad sense heritability was computed using Hanson et al. (1956), and data obtained on morphological and floral features were analyzed using the usual procedures of Panse and Sukhatme, (1984).
The NTSYSpc (Numerical Taxonomy and Multivariate Analysis System) software was used to analyze ADi, and correlation and regression analysis were conducted with the DPS (Data Program System) software.
Statistical analysis was conducted using SAS 9.0
Conclusion
The present study was the first study in japonica TGMS and japonica hybrid in Vietnam. This study developed 12 novel japonica TGMS lines using phenotype selection and molecular-assisted selection (MAS) methods. TJ6 and TJ12 showed good heterosis with the F1 combinations yield were 7.9 and 7.8 tons/ha compared to 7.4 tons/ha of the check. The amylose content of these two F1 combinations was lower than the check 18.1% and 17.8%, respectively and both belonged to the low amylose content group. The results demonstrated that utilizing japonica TGMS for japonica hybrid cultivation in Vietnam is promising, although further studies are needed to improve the OCR of japonica TGMS lines.
Acknowledgment
Parts of this work were supported by a grant from the Vietnam National University of Agriculture (Code T2021-41-14TĐ).
Statement of contributions
TVQ was responsible for the experimental design and write the paper. TTH was responsible for the experimental conducted and writing the paper. PDH was responsible for the test crossing and writing the materials and methods of the paper, and molecular works. HDD was responsible for measuring the traits. NVM was responsible for measuring the traits. NTT was responsible for writing the paper. PTTY was responsible for measuring the traits. THL was responsible for measuring the traits.
References
Anis G, Hassan H, El-Sherif A, Saneoka H, EL Sabagh, A (2019) Evaluation of new promising rice hybrid and its parental lines for floral, agronomic traits and genetic purity assessment. Pak. J. Agric. Sci. 56: 567–576.
Appibhai JH, Jauhar A, Ebrahimali AS, Vidya SG, Umesh KR & Prabhakar KR (2012) Mapping of tms8 gene for temperature-sensitive genic male sterility (TGMS) in rice (Oryza sativa L.). Plant Breeding. 131(1): 42–47.
Casco VV, Tapic RT, Undan JR, Latonio AMLS, Suralta RR, Manigbas NL (2021) Combining ability, floral biology, and seed producibility of promising cytoplasmic male-sterile (CMS) lines for hybrid rice development. CABI Agric. Biosci. 2(1): 1–10.
Catudan BM, & Arocena AC (2003) Determinants of the adoption of F1 hybrid rice in the Philippines. Philos. Rice Tech. Bull. 8: 64–69.
Deng H (2008) Japonica hybrid rice in China. China Agric Press. Beijing.
Department of Crop Production. Ministry of Agriculture and Rural Development (2014) Summary report of 2013 and implementing key tasks in 2014, January 25, 2104, Hanoi.
Doyle JJ & Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus. 12:11–5.
Govind RK, Virmani SS (1988) Genetics of fertility restoration of WA cytoplasmic male sterility in rice. Crop Sci. 28:787–792.
Guo J, Zhou X, Chen K, Ye C, Liu J, Sun K, Tang G, Wang S, Zhang G, Chen Y, et al. (2023) Genetic analysis of S5 regulating the hybrid sterility between indica and japonica subspecies in Rice. Agronomy. 13: 1094.
Hanson FH, Robinson HF, Comstock RE (1956) Biometrical studies of yield in segregating populations of Korean Lespedeza. Agron J. 48:267–282.
Hashim S, Ding P, Ismail MF, Ramli A (2021) Floral traits and flowering behaviors of Malaysian rice cytoplasmic male sterile and maintainer lines and its relationship with out-crossing performance. Aust. J. Crop Sci. 15: 180–186.
Hu, P. (2010) Development and technological innovation of hybrid rice. Industry J of Agric Sci Tech. 12:17–23.
Jiang Y, Zhang H, Zhao K, et al. (2014) Difference in yield and its components characteristics of different type rice cultivars in the lower reaches of the Yangtze River. Chin J Rice Sci. 28:621–630.
Kalaiyarasi R, Vaidyanathan P (2002) Cytological differentiation of TGMS gene expression in rice indica/japonica derivatives. In: Proceedings of the 4th International Symposium on Hybrid Rice. 14-17 May. 2002. Hanoi. Vietnam. Los Banos. Philippines: International Rice Research Institute: 357–371.
Kang KH (2010) Made for the Tropics. Rice Today. 9:34–35.
Kang Z (2013) The development report of "Yongyou" series hybrid rice is a great innovation in the history of rice development in China. Ningbo Communication. 1:47–48.
Karpagam V (2011) Development of two-line hybrids through exploitation of thermo-sensitive genic male sterility system in rice (Oryza sativa L.). MS Thesis. Coimbatore. India: Tamil Nadu Agricultural University.
Kubo T, Yoshimura A (2005) Epistasis underlying female sterility detected in hybrid breakdown in a japonica-indica cross of rice (Oryza sativa L.). Theor. Appl. Genet. 110:346–355.
Lee DS, Chen LJ, Suh HS (2005) Genetic characterization and fine mapping of a novel thermo-sensitive genic male- sterile gene tms6 in rice (Oryza sativa L.). Theor. Appl. Genet. 111:271–277.
Li J, Zhou J, Zhang Y, Yang Y, Pu Q, Tao D (2020) New insights into the nature of interspecific hybrid sterility in rice. Front Plant Sci. 11: 555572.
Li Z, Wu J (1991) The present situation and prospect of three-line hybrid japonica rice breeding in China. Hybrid Rice. 15:13–16.
Lopez MT, Toojinda T, Vanavichit A, Tragoonrung S(2003) Microsatellite makers flanking the tms2 gene facilitated tropical TGMS rice line development. Crop sci. 43(6):2267–2271.
Lu BR, Cai X, Jin X (2009) Efficient indica and japonica rice identification based on the InDel molecular method: Its implication in rice breeding and evolutionary research. Prog. Nat. Sci. 19:1241–1252.
Ma Z, Liu X, Sun L, et al. (1998) Effect of several main problems on developing japonica hybrid rice in Tianjin. J Tianjin Agric Sci. 4:55–56.
Magno RC, & Yanagida JF. (2000) Effect of trade liberalization in the short‐grain japonica rice market: A spatial‐temporal equilibrium analysis. J. Philipp. Dev. 27:71–99.
Min J, Zhu ZW & Chen, N (2012) Study on the quality and the percentages meeting fine quality standards in Chinese indica conventional rice. China Rice. 18:4–7.
Nguyen, MV, Yen PTN, Quang TV, Tram NT (2015) Development of aromatic thermo-sensitive genic male sterile (TGMS) lines, J. Sci. & Devel. 13(8):1360–1371.
Ni L, Yuan Q, Cao L, Wu Y (2001) Genetic analysis of rice quality characters of hybrid japonica varieties in Shanghai area. Acta Agric Shanghai. 17:22–26.
Oka H (1974) Analysis of genes controlling f(1) sterility in rice by the use of isogenic lines. Genetics. 77:521–534.
Ouyang YD, Liu YG, Zhang QF (2010) Hybrid sterility in plant: Stories from rice. Curr. Opin. Plant Biol. 13: 186–192.
Panse VG. & Sukhatme PV. (1984) Statistical methods for agricultural research workers. ICAR. New Delhi. 145–152.
Pham VT, Hung, DV., Trung NQ., Quan, TV, Doanh LQ (2015) Evaluation of agronomic traits and thermo-sensitive gene(s) of some TGMS lines. J. Sci. & Devel. 13(1):1–22.
Pu H, Zhou Z, Xu D (2015) Development history and problems of three-line hybrid japonica rice. J Jiangsu Agric Sci. 43:74–77.
Quan L, Shi B, Gao W, Yuan Q (2000) An approach to japonica hybrid rice quality breeding in Shanghai region. Acta Agric Shanghai. 16:21–23.
Shen YJ, Jiang H, Jin JP, et al. (2004) Development of genome-wide DNA polymorphism database for map-based cloning of rice genes. Plant Physiol. 135:1198-205.
Shim J, Torollo G, Angeles-Shim RB, Cabunagan RC, Choi IR, Yeo US & Ha WG (2015) Rice tungro spherical virus resistance into photoperiod‐insensitive japonica rice by marker‐assisted selection. Breeding Sci. 65:345–351.
Standard Evaluation System for Rice (SES) (2002) International Rice Research Institute. 66p.
Tripp R, R Hu & S Pal (2010) “Rice seed provision and the evolution of seed markets. In Pandey, Byerlee D, Dawe D, Dobermann A, Mohanty S, Rozelle S, et al. (Eds.) Rice in the Global Economy: Strategic research and policy issues for food security. Los Banos, Philippines: International Rice Research Institute (IRRI). 213–230.
Virmani S. S., Sun, Z. X., Mou, T. M., Jauhr, Ali, A., Mao, C. X. (2003) Two-line hybrid rice breeding manual. Manila. Philippines: International Rice Research Institute.
Virmani SS, Viraktamath BC, Lopez MT (1997) Nucleus and breeder seed production of thermosensitive genic male sterile line. Int Rice Res Notes. 22:26–27.
Virmani, SS (1994) Heterosis and hybrid rice breeding. In: Frankel R, Grossman BM, Linskens WHF, Maliga NP, Riley PR (Eds). Monographs on theoretical and applied genetics, vol 22. Springer-Verlag, Berlin Heidelberg Germany.
Vu TTH & Atsushi Y (2015) Identifying map location and markers linked to thermosensitive genic male sterility gene in 103S line. J. Sci. & Devel. 13(3):331–336.
Walls WE (1965) A standardized phenol method for testing wheat seed for varietal purity. Contribution no. 28 to the Handbook on Seed Testing. The Assoc. of Official Seed Analysts, Lincoln, NE.
Wang CL (2004) Status and prospects of hybrid rice breeding in Jiangsu. China Rice Sci. 12:219–225.
Wang S, Wu H, Lu Z, Liu W, Wang X, Fang Z, He X (2023) Combining ability analysis of yield-related traits of two elite rice restorer lines in Chinese hybrid rice. Int J Mol Sci. 24(15):12395.
Xie F & Peng S (2016) History and prospects of hybrid rice development outside of China. Chinese Sci Bulletin. 61:3858–3868.
Yang JC, Zhang JH, Liu LJ, Wang ZQ, Zhu QS (2002) Carbon remobilization and grain filling in japonica/indica hybrid rice subjected to postanthesis water deficits. Agron. J. 94:102–109.
Zhang GQ (2020) Prospects of utilization of inter-subspecific heterosis between indica and japonica rice. J. Integr. Agric. 19: 1–10.