

Aust J Crop Sci. 18(12):885-893 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p180
Karen Sabrina de Brito Sousa1, Ismael de Jesus Matos Viégas2, José Darlon Nascimento Alves3*, Heráclito Eugênio Oliveira da Conceição4, Bianca Cavalcante da Silva5, Diocléa Almeida Seabra Silva6, Jairo Neves de Oliveira7, Ricardo Shigueru Okumura8 and Dágila Melo Rodrigues9
1Engenheira Agrônoma formada pela UFRA Campus de Capanema – PA, Brazil
2Engenheiro Agrônomo, Doutor em Ciências Agrárias e Ex Pesquisador da EMBRAPA Amazônia Oriental – Belém - PA, Brazil
3Professor do IFPA, Campus Óbidos – PA, Brazil
4Professor da UFRA Campus de Capitão Poço – PA, Brasil
5Doutoranda em Agronomia (Produção Vegetal). FCAV/Unesp, Jaboticabal – SP, Brazil
6Professora da UFRA Campus de Capanema – PA, Brasil
7Doutorando em Agronomia (Ciência do Solo). FCAV/Unesp, Jaboticabal – SP, Brazil
8Professor da UFRA Campus de Parauapebas – PA, Brazil
9Doutorado em Engenharia Agrícola pela Universidade de Santa Maria - RS, Brazil
*Corresponding author: José Darlon Nascimento Alves

ORCID: 0000-0003-1290-5598
Abstract: The objective of this study was to assess the impact of nutrient omission and liming on the growth and nutritional status of crambe (Crambe abyssinica Hochst) plants in the eastern Amazon region of Brazil. The experiment was conducted in a greenhouse at the Federal Rural University of the Amazon, Campus Capitão Poço, Pará, Brazil. The experimental design was a completely randomized block with 11 treatments and five repetitions. The treatments were as follows: control without lime, control with lime, complete with lime, nitrogen (N) omission with lime, phosphorus (P) omission with lime, potassium (K) omission with lime, calcium (Ca) omission without lime, magnesium (Mg) omission without lime, sulfur (S) omission with lime, boron (B) omission with lime, and zinc (Zn) omission with lime. Following a 60-day period of cultivation, an evaluation was conducted on the biometric variables, biomass production, nutrient concentration, and the presence of symptoms indicative of nutrient deficiencies (N, P, K, Ca, Mg, S, B, and Zn). The experimental data indicated that the omission of nutrients had a deleterious effect on the biometric variables in comparison to the complete treatment with lime. The control treatments with and without the addition of lime, as well as the treatment with P omission with lime, exhibited the most restricted plant growth, with plant height measuring less than 7.0 cm and the number of leaves lower than 3.5. The plants exhibited deficiency symptoms indicative of deficiencies in their development. The most pronounced severe symptoms were observed in the treatments involving P omission with lime and in both controls with and without lime. These findings highlight the necessity of applying lime to the soil in crambe crops and underscore that P is the most limiting nutrient for this crop.
Keywords: mineral nutrition, oilseed, plant production.
Abbreviations: CEC_cation exchange capacity; Ca_calcium; Mg_magnesium; N_nitrogen; P_phosphorus; S_sulfur; K_potassium; PH_plant height; OBC_treatment of boron omission with lime; CC_complete treatment; Test.CC_ control treatment with lime; Test.SC_ control treatment without lime; OM_organic matter; pH_hydrogenionic potential; SD_stem diameter; Zn_zinc; ONC_ treatment of nitrogen omission with lime; OZnC_ treatment of zinc omission with lime; OPC_ treatment of phosphorus omission with lime; PL_ petiole length; LL_ leaf length; LW_ leaf width; Al_aluminum; H + Al_ potential acidity; H_hydrogen; OBC_ treatment of boron omission with lime; NL_number of leaves; OCaSC_ treatment of calcium omission without lime; OMgSC_ treatment of magnesium omission without lime; OSC_ treatment of sulphur omission with lime; ATP_ adenosine triphosphate; NADPH_ reduced form of nicotinamide adenine dinucleotide phosphate; DM_dry matter; OKC_ treatment of potassium omission with lime; LDM_leaf dry mass; StDM_ stem dry mass; BDM_Branches dry mass (BDM); B_boron; ROS_reactive oxygen species; RDM_root dry mass; TDM_total dry mass; RGLDM_relative growth of leaf dry mass; DAP_days after planting; PRNT_Relative Total Neutralizing Power; Na_sodium; RG_ relative growth; RNA_ribonucleic acid.
Introduction
The current concern about the impact of non-renewable energy sources on the intensification of climate change has prompted the search for sustainable alternatives, such as solar energy, wind energy, and biofuels. With regard to biofuels, Crambe abyssinica Hochst, or crambe, is a notable candidate for biodiesel production. A member of the Brassicaceae family, crambe is an annual oilseed with a high potential for oil production (35–45%), low production costs, and high resistance to droughts and low temperatures (Soratto et al., 2013; Bassegio et al., 2016; Rosmaninho et al., 2018; Mera et al., 2020; Jankowski et al., 2024).
Nevertheless, despite the growing interest in the crambe crop, there is still a paucity of studies examining its nutritional requirements and the effects of liming on crop yield, particularly within the Amazon region. Consequently, research has been conducted to elucidate the behavior of the crambe crop in edaphoclimatic conditions in the Brazilian Amazon. Amazonian soils are distinguished by low cation exchange capacity (CEC) and high acidity, a consequence of the elevated weathering degree and high rainfall volume, which collectively result in the limited availability of essential nutrients (Cunha et al., 2007; Souza et al., 2017; Olego et al., 2021).
These characteristics impede the full development of several crops, particularly those with a short cycle, as plants require larger quantities of nutrients for optimal growth (Siqueira et al., 2023). In this regard, liming has become a crucial practice for agricultural crops in the Amazon region. Liming not only supplies crops with the requisite calcium (Ca) and magnesium (Mg) of crops, but it also increases the soil pH, thereby improving the availability of macro- and micronutrients (Soratto et al., 2013; Santos et al., 2018a). Consequently, cultivation without liming can compromise plant growth and crop yield. In a study conducted by Silva et al. (2017), the impact of base saturation on the cultivation of crambe in an Oxisol was evaluated. The findings indicated that an increase in base saturation up to 70% resulted in a notable enhancement in crambe production.
Some studies have recommended specific doses of nutrients for crambe crops adapted to different soil conditions (Silva et al., 2012; Samarappuli et al., 2020, Alves et al., 2016). However, there are still knowledge gaps, particularly in relation to the crop response under the impact of the omission of macro- and micronutrients combined with liming in Amazonian soils (Mera et al., 2020). Mauad et al. (2019) investigated the impact of individual macronutrient omission on biomass production in crambe. Their findings revealed that nitrogen (N) had the greatest impact on biomass production, followed by calcium (Ca), phosphorous (P), magnesium (Mg), sulphur (S), and potassium (K) limitations. Notably, Ca omission led to the most pronounced restriction of plant growth. In light of these findings, the present study assessed the development and nutritional status of crambe plants under nutrient omission and liming in the eastern Amazon region in Brazil.
Results and discussion
Biometric variables
With regard to plant height (PH), the data indicates a more pronounced response in plants subjected to the treatment with boron (B) omission with lime (OBC) compared to the complete treatment (CC). The relative growth observed in the OBC was 9.78% higher than that observed in the CC treatment (Table 1). Conversely, the treatments with the least favorable outcomes were the controls with and without lime (Test.CC and Test.SC, respectively) (Table 1). The optimal response for plant height (PH) with the OBC treatment may have been the result of a 2.7-fold increase in organic matter (OM) content in the soil following liming. During the incubation period, the mineralization process of OM occurs, which represents the primary B source in the soil. This process may have supplied the B requirement for crambe plants. As Raij (2019) notes, the OM content, pH, soil mineralogical composition, and texture all influence the amount of B that a soil is capable of adsorbing.
The CC treatment yielded the greatest value of stem diameter (SD), with no notable distinction when compared to the treatments of N (ONC) and Zn (OZnC) omissions (Table 1). This may have occurred due to the high OM content in the soil, which facilitates the mineralization of nitrogen (N) and zinc (Zn) (Brady and Weil, 2013). Conversely, the phosphorus (P) omission treatment (OPC) exhibited a limitation in SD, with a relative growth of 19.07% and a reduction in SD by 80.93% (Table 1).
The omission of P also resulted in significant reduction in the number of leaves, leading to a relative growth rate of only 15.48% (Table 1). This decline can be attributed to the essential role of P in the formation of genetic material, the plasma membrane, and the ATP molecule within the plant (Prado, 2020).
With regard to petiole length (PL), the CC treatment yielded the most favorable outcomes, whereas the Test.SC treatment exhibited the most constrained PL, attributable to the inadequate provision of nutrients for plant growth (Table 1). This outcome may be attributed to the absence of soil correction, as lime is a crucial element in the development of crambe plants, as evidenced by Mera et al. (2020) in a medium-textured Oxisol.
With regard to leaf length (LL) and leaf width (LW), the OPC and Test.SC treatments exhibited the lowest mean values (Table 1). This indicates that phosphorous (P) is the nutrient that most constrains crambe growth (Table 1). In general, the absence of liming also had a detrimental impact on the growth of the crambe plant. This response may be related to the toxic effect of Al3+, which inhibits root system growth, thereby limiting water and nutrient absorption by the plant. Additionally, the elevated level of potential acidity (H + Al) (Table 4) resulted in a diminished effective CEC of the soil. This was due to the negative charges of the colloids being predominately occupied by H+ ions, which led to increased nutrient leaching (Marschner, 2022) and reduced P availability (Brady and Weil, 2013).
Figure 1a illustrates that the treatment involving the omission of B and subsequent application of lime (OBC) exhibited superior outcomes in terms of plant height compared to the CC treatment. In the other treatments, the reduction in responses may be attributed to a nutritional imbalance. As Marschner (2022) notes, an element can stimulate or inhibit the absorption of others, and interactions between nutrients can interfere with the mineral composition of plants. Conversely, for SD, the OZnC and ONC treatments exhibited superior performance relative to the CC treatment (Figure 1b). This outcome may be attributed to the beneficial impact of liming on the accessibility of microorganisms, which subsequently enhanced OM mineralization and Zn and N availability (Paradelo et al., 2015).
The number of leaves (NL) exhibited a positive response to the CC treatment, whereas the Test.SC treatment resulted in the pronounced reduction in NL. The treatments of OPC, calcium omission without lime (OCaSC), magnesium omission without lime (OMgSC), and Test.SC affected the number and width of leaves in crambe plants (Figures 1c and 1d). In general, treatments with OPC, OCaSC, OMgSC, sulfur omission with lime (OSC), Test.CC, and Test.SC resulted in lower plant growth when compared to the others. The results obtained in the treatment with P omission with lime can be attributed to the low formation of ATP and NADPH, reduced electron transport, and reduced photosynthesis by plants, which collectively led to a reduction in plant growth (Carstensen et al., 2018).
The OCaSC treatment exhibited a significant impact on plant growth, which is consistent with the findings of Mauad et al. (2013) in crambe plants. In that study, the researchers observed that the omission of calcium (Ca) led to a reduction in plant growth. A deficiency of Ca is characterized by a reduction in apical growth, which is typically observed in younger leaf tissues (Alves et al., 2008). Mauad et al. (2013) determined that Ca is the second most required element by the crambe plant, after N. Their findings indicate that Ca absence hinders crop development. Mauad et al. (2019) observed that Ca omission resulted in a more pronounced reduction in crambe development, with plants failing to reach the reproductive stage.
The absence of magnesium (Mg) in the absence of lime also resulted in a reduction in plant growth. Mg is an essential nutrient for photosynthesis, as it is a constituent of chlorophyll molecules (Prado, 2020). Approximately one-fifth of the Mg found in plant tissue originates from chlorophyll molecules. Moreover, Mg participates in the synthesis of oils and proteins, as well as in the activation of enzymes involved in energy metabolism (Brady and Weil, 2013).
Table 1. Plant height (PH), relative growth of plant height (RGPH), stem diameter (SD), relative growth of stem diameter (RGSD), petiole length (PL), relative growth of petiole length (RGPL), number of leaves (NL), relative growth of number of leaves (RGNL), leaf length (LL), relative growth of leaf length (RGLL), leaf width (LW), and relative growth of leaf width (RGLW) of crambe plants in function of the treatments.
| Treatments | PH (cm) | RGPH (%) | SD (cm) | RGSD (%) | NL | RGNL (%) | PL (cm) | RGPL (%) | LL (cm) | RGLL (%) | LW (cm) | RGLW (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | 32.95 b | 100.00 | 0.57 a | 100.00 | 21.15 a | 100 | 7.41 a | 100.00 | 12.38 a | 100.00 | 8.61 a | 100.00 |
| ONC | 31.54 b | 95.72 | 0.56 a | 98.02 | 12.55 b | 59.33 | 5.34 c | 72.10 | 7.96 c | 64.33 | 6.26 c | 72.73 |
| OPC | 6.83 f | 20.73 | 0.11 g | 19.07 | 3.27 e | 15.48 | 0.90 g | 12.21 | 2.05 f | 16.55 | 1.65 f | 19.15 |
| OKC | 21.92 d | 66.54 | 0.42 c | 73.39 | 12.70 b | 60.04 | 6.43 b | 86.81 | 7.74 c | 62.50 | 6.91 b | 80.25 |
| OCaSC | 12.74 e | 38.68 | 0.29 d | 50.29 | 3.59 e | 16.97 | 4.93 c | 66.50 | 5.40 d | 43.60 | 3.68 e | 42.76 |
| OMgSC | 14.56 e | 44.18 | 0.23 e | 41.21 | 5.53 d | 26.14 | 2.51 e | 33.94 | 4.2 e | 33.67 | 4.67 d | 54.28 |
| OSC | 14.35 e | 43.56 | 0.51 b | 87.96 | 12.60 b | 59.57 | 4.40 d | 59.43 | 6.88 c | 55.56 | 6.44 c | 74.84 |
| OBC | 36.17 a | 109.78 | 0.43 c | 75.32 | 12.52 b | 59.20 | 6.26 b | 84.51 | 9.52 b | 76.89 | 6.19 c | 71.88 |
| OZnC | 27.48 c | 83.42 | 0.54 a | 95.07 | 8.55 c | 40.44 | 6.09 b | 82.20 | 9.51 b | 76.79 | 7.51 b | 87.23 |
| Test.CC | 6.83 f | 20.73 | 0.18 f | 31.80 | 3.45 e | 16.31 | 1.57 f | 21.18 | 2.48 f | 20.07 | 2.13 f | 24.77 |
| Test. SC | 0.00 g | 0.00 | 0.00 h | 0.00 | 0.00 f | 0.00 | 0.00 h | 0.00 | 0.00 g | 0.00 | 0.00 g | 0.00 |
| CV (%) | 7.66 | 6.75 | 9.68 | 11.25 | 12.23 | 10.30 |
*Means followed by different letters in the columns differ significantly from each other by the Tukey’s test at 5% probability. Note: Test.SC: control without lime; Test.CC: control with lime; CC: complete with lime; ONC: nitrogen omission with lime; OPC: phosphorus omission with lime; OKC: potassium omission with lime; OSC: sulfur omission with lime; OBC: boron omission with lime; OZnC: zinc omission with lime; OCaSC: calcium omission without lime; and OMgSC: magnesium omission without lime.
Table 2. Leaf dry mass (LDM), stem dry mass (StDM), branch dry mass (BDM), root dry mass (RDM), total dry mass (TDM), relative growth of leaf dry mass (RGLDM), relative growth of stem dry mass (RGSDM), relative growth of branch dry mass (RGSDM), relative growth of root dry mass (RGRDM), and relative growth of total dry mass (RGTDM) in function of the treatments in crambe plants at 60 days after plating (DAP).
| Treatments | LDM (g plant1) |
StDM (g plant1) |
BDM (g plant-1) | RDM (g plant-1) | TDM (g plant-1) | RGLDM (%) | RGSDM (%) | RGBDM (%) | RGRDM (%) | RGTDM (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CC | 1.71 b* | 0.87 b | 1.39 a | 1.50 a | 5.20 a | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| ONC | 0.08 g | 0.33 e | 0.33 b | 0.14 f | 0.98 d | 4.66 | 38.08 | 23.91 | 9.81 | 4.66 |
| OPC | 0.00 g | 0.00 g | 0.00 c | 0.00 f | 0.00 e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OKC | 0.75 d | 0.22 f | 0.00 c | 0.42 e | 1.20 d | 44.05 | 26.06 | 0.00 | 28.20 | 23.06 |
| OCaSC | 1.89 a | 1.54 a | 0.37 b | 0.67 d | 4.49 b | 110.34 | 176.93 | 26.98 | 44.93 | 86.31 |
| OMgSC | 1.22 c | 0.77 c | 1.13 a | 0.44 e | 3.57 c | 71.87 | 88.04 | 81.61 | 29.50 | 68.66 |
| OSC | 0.66 e | 0.41 d | 1.07 a | 1.38 a | 3.53 c | 38.76 | 47.55 | 76.89 | 91.95 | 68.01 |
| OBC | 1.17 c | 0.86 b | 1.20 a | 0.92 c | 4.17 b | 68.66 | 98.46 | 86.40 | 61.71 | 80.19 |
| OZnC | 0.51 f | 0.72 c | 1.05 a | 1.09 b | 3.38 c | 30.26 | 82.87 | 75.47 | 72.72 | 65.09 |
| Test.CC | 0.00 g | 0.00 g | 0.00 c | 0.00 f | 0.00 e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Test.SC | 0.00 g | 0.00 g | 0.00 c | 0.00 f | 0.00 e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CV (%) | 12.42 | 11.15 | 33.82 | 23.85 | 12.71 | |||||
| *Means followed by different letters in the columns differ significantly from each other by the Tukey’s test at 5% probability. Note: Test.SC: control without lime; Test.CC: control with lime; CC: complete with lime; ONC: nitrogen omission with lime; OPC: phosphorus omission with lime; OKC: potassium omission with lime; OSC: sulfur omission with lime; OBC: boron omission with lime; OZnC: zinc omission with lime; OCaSC: calcium omission without lime; and OMgSC: magnesium omission without lime. | ||||||||||
Dry mass of leaves, stems, branches, roots, and total
The dry mass of leaves (LDM) and stems (StDM) produced by crambe plants was found to be higher in the treatment of Ca omission without lime (OCaSC), followed by the CC treatment (Table 2). The order of decreasing LDM production for crambe plants was as follows: OCaSC > CC > OMgSC = OBC > OKC > OSC > OZn > ONC = OPC = Test.CC = Test.SC (Table 2 and Figures 2a and 2b).
It is noteworthy that no dry matter (DM) production was observed in the OPC, Test.CC, and Test.SC treatments. This outcome was a consequence of inadequate plant material production, indicating that the growth of crambe plants was significantly impaired by the absence of soil acidity correction and the limited availability of nutrients, particularly phosphorous. Rogério et al. (2012) also observed a comparable outcome, noting that reducing P doses had a detrimental impact on the DM production of crambe plants. Oxisols are typically characterized by low natural fertility, with elevated concentrations of iron and aluminum oxides (Paz et al., 2016). As acidity increases, iron and aluminum oxides acquire H+ ions (protonation), becoming increasingly positively charged and enhancing the attraction force of H2PO4- ions, while simultaneously decreasing their availability to plants. This phenomenon explains the observed low performance of the Test. SC treatment. In contrast, when liming is conducted, the OH- groups consume H+ from the oxides, thereby reducing their attraction force for H2PO4, which increases P availability (Hue, 2022). However, when the soil labile P reserve is limited, low P
availability persists (Yadav et al., 2012), which elucidates the low biomass production observed in the OPC and Test. CC treatments.
Furthermore, the outcome obtained in Test. SC can also be explained by the negative effect of Al on plant roots (Castro et al., 2022). In addition, Al3+ has been reported to cause destruction to root epidermal cells, resulting in necrosis and preventing the absorption of water and nutrients. This ultimately leads to reduced plant growth (Hamim et al., 2018; Castro et al., 2022). In addition, a reduction in pH results in the unavailability of exchangeable cations (Ca, Mg, and K) due to an increase in soil reserve acidity (Neina, 2019).
The descending order of treatments for stem dry mass (StDM) was as follows: OCaSC > CC = OBC > OMgSC = OZnC > OSC > ONC > OKC > Test.CC = Test.SC = OP. The treatments with OPC, Test.SC, and Test.CC were the most limiting, as insufficient dry matter (DM) production precluded the possibility of conducting the analysis (see Table 2). The greatest impact on branch dry mass (BDM) was observed in response to K omission with lime (Table 2), followed by the treatments OPC, Test.CC, and Test.SC, when compared to the CC treatment. This was primarily due to the death of branches. Despite the emission of branches when potassium is withheld, the plant structure fails to develop due to the rise in reactive oxygen species (ROS), which results in leaf tissue necrosis as a consequence of putrescine production (Chen et al., 2016). With regard to BDM, the following descending order was observed: CC > OBC > OMgSC > OSC > OZnC > OCaSC > ONC > OPC = OKC = Test.CC = Test.SC (Table 2).
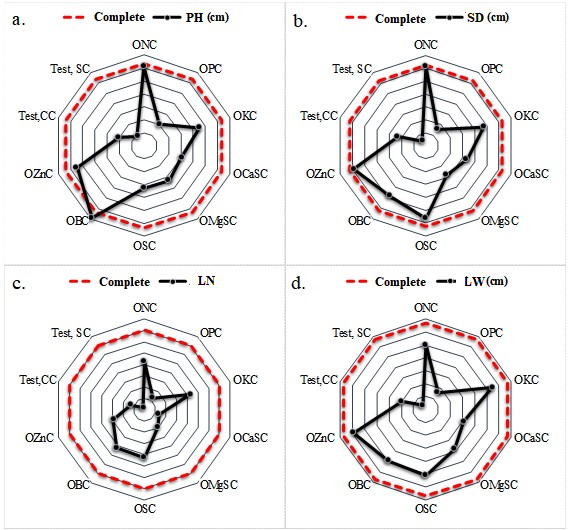
Figure 1. Graphic representation of plant height (PH), stem diameter (SD), number of leaves (LN), and leaf width (LW) in crambe plants subjected to different omissions of nutrients and liming in relation to the complete treatment.
With regard to root dry mass (RDM), the CC treatment yielded the greatest production, with exception of the OSC treatment. The treatments that exhibited the greatest limitations were ONC, OPC, Test.CC, and Test.SC (Table 2). This can be attributed to the low fertility of the Oxisol with medium texture, particularly with regard to phosphorous (Tables 4 and 5). The RDM exhibited the following descending sequence: CC > OSC > OZnC > OBC > OCaSC > OMgSC > OKC > ONC > OPC = Test.SC = Test.CC. Conversely, the CC treatment demonstrated the most favorable response with regard to total dry mass (TDM) (Table 2 and Figure 2c).
The most limiting factor in the OPC, Test.CC, and Test.SC treatments that did not produce biomass was the absence of phosphorous (P). A reduction in the P content of leaves results in impaired cell division, which in turn affects their expansion (Paz-Ares et al., 2022; Khan et al., 2023). A reduction in leaf area results in a decline in the photosynthetic rate and impairs the production of dry mass in plants (Lambers and Oliveira, 2019). Similar outcomes were observed by Colodetti et al. (2013), who reported a significant reduction in biomass production capacity, with a 96.53% decrease in the average DM production of crambe plants. This demonstrates the profound impact of P deficiency on plant growth.
The OCaSC treatment demonstrated a greater relative growth of leaf dry mass (RGLDM) (Figure 2d). Nevertheless, this treatment was inadequate for achieving the greatest production of TDM (Table 2), due to the fact that soil acidity restricts the availability of nutrients essential for vital metabolic processes, such as photosynthesis and cellular respiration. This ultimately results in a reduction in biomass accumulation (Taiz et al., 2017; Lambers and Oliveira, 2019; Gurmessa et al., 2020). Moreover, the absence of liming and Ca fertilization contributes to Al toxicity, which has a detrimental impact on root expansion and water relations, while inducing oxidative stress (Rosmaninho et al., 2018; Rahman and Upadhyaya, 2020). Rosmaninho et al. (2018) evaluated the effect of Al doses on crambe plants and concluded
that Al reduced root growth, biochemical activity, and biomass production.
Visual deficiency symptoms
The visual symptoms of macronutrient and micronutrient (B and Zn) deficiencies were observed as a consequence of the low natural chemical fertility of the medium-textured Oxisol and the nutrient requirements of the crambe plants.
The first indications of nitrogen (N) deficiency appeared at 31 days after planting (DAP), manifesting as a generalized yellowing of the older leaves. The plants that received a nutrient solution with N omission, in comparison to the complete (CC) treatment, exhibited restricted growth, both in terms of plant height and leaf size (Figure 3). This reduction in plant height and leaf size was attributed to the limited availability of this nutrient in the soil, which subsequently impeded the absorption, translocation, and incorporation of N-NH4+ into carbon skeletons in the aerial part of the plants, ultimately leading to a deficiency in protein formation (Singh et al, 2022).
The symptoms worsened with age, resulting in necrosis that began at the edges of older leaves and progressed toward the younger ones. This phenomenon is attributed to the mobility of nutrients, particularly nitrogen (N), which is broken down in plastids during the leaf senescence process. The resulting N source is then allocated by the plant to supplement the nutrition of young leaves (Masclaux-Daubresse et al., 2010).
Chlorosis is a typical symptom of N deficiency, as N directly affects plant growth. As reported by Carneiro et al. (2015), chlorosis is associated with the role of N in plant metabolism. This is due to the fact that it is closely linked to carbon metabolism and the photorespiratory process, which are both metabolic processes associated with photosynthesis. Moreover, N serves as a constituent of nucleic acids, chlorophyll, proteins, including enzymes, which regulate the majority of biological processes in plants, such as cell division, carbohydrate metabol-
Table 3. Leaf contents of the nutrients in crambe plants.
| Macronutrient | No deficiency (g kg-1) | With deficiency (g kg-1) |
|---|---|---|
| Nitrogen | 31.42 | 22.86 |
| Phosphorus | 2.17 | * |
| Potassium | 19.43 | 11.18 |
| Calcium | 24.98 | 16.58 |
| Magnesium | 10.18 | 1.86 |
| Sulphur | 2.33 | 1.20 |
| Micronutrient | No deficiency (mg kg-1) | With deficiency (mg kg-1) |
| Boron | 234.39 | 69.26 |
| Zinc | 71.00 | 66.50 |
| *Insufficient material for analysis. | ||
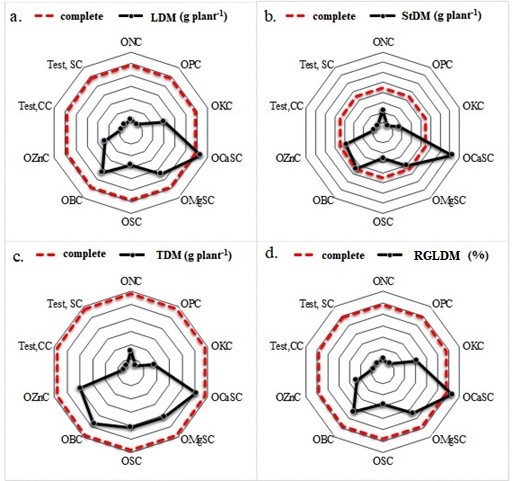
Figure 2. Graphical representation of leaf dry mass (LDM) (a), stem dry mass (StDM) (b), total (TDM) (c) and relative growth of leaf dry mass (RGLDM) (e) in crambe plants in subjected to different omissions of nutrients and liming in relation to the complete treatment.
ism, and the formation of new tissues (Brady and Weil, 2013; Maud et al., 2019; Fu et al., 2023; Zhu et al., 2023).
The plants exhibited typical development patterns until 15 days after planting (DAP). Subsequently, the plants ceased to grow, exhibiting only two or three leaves. A comparison of the leaves from the CC treatment with those from the treatments of P omission with lime and without lime revealed an abnormal growth pattern in the leaves due to a lack of phosphorous (Figure 3). This is due to the low phosphorous content of the soil utilized in the experiment, which is a medium-textured soil (Table 4) (Brasil et al., 2020), whereas the leaves in the CC treatment exhibited typical development. The visual deficiency symptoms observed in crambe can be attributed to the essential role of phosphorous (P) in various metabolic processes within plants, including photosynthesis, respiration, gene transfer, energy transfer, macromolecule synthesis, and active nutrient absorption (Carstensen et al., 2018; Kerbauy, 2019; Isidra-Arellano et al., 2021; Khan et al., 2023).
The initial indications of K deficiency manifested at 31 days after planting (DAP). In comparison to the plants subjected to the CC treatment, the plants exhibited diminished size, pronounced brittleness in the stems, mortality of the branches, and chlorosis
in the older leaves along the edges. Meurer (1981) notes that the initial symptom of K deficiency in plants is the emergence of chlorotic spots, predominantly on the leaf apices, leaf edges, and between veins. Plants exhibiting K deficiency display a cessation in the translocation of photoassimilates to the drains, resulting in the accumulation of carbohydrates within the leaf tissue (Zhao, 2001). Additionally, there is a reduction in photosynthetic efficiency, which is attributed to a decline in mesophyll conductance and a diminished capacity of CO2 fixation (Jin et al., 2011; Johnson et al., 2022; Ahammed et al., 2022).
The plants treated with Ca omission without lime (OCaSC) exhibited low development and the emission of few leaves. These leaves exhibited chlorosis at the edges progressing toward the center of the leaf, ultimately resulting in total necrosis. As the symptom intensified, the leaves became curled. No branches or petiole were observed to emerge. The leaves exhibited a rough texture and the stem displayed brittleness when compared to the plants in the CC treatment (Figure 3). Symptoms of Ca deficiency manifest more rapidly and severely in meristematic regions and young leaves, leading to tissue deformation or death (Epstein and Bloom, 2006). Calcium (Ca) in plant tissue is distinguished by its low mobility, with restricts redistribution in the phloem (Hawkesford et al., 2012). Plants exhibiting deficiencies in Ca demonstrate greater activity of the polygala-

Figure 3. Visual symptoms of nutrient deficiency in crambe leaves in relation to the complete treatment. a) CC (complete), b) nitrogen omission (-N), c) potassium omission (-K), d) zinc omission (-Zn), e) sulfur omission (-S), f) boron omission (-B), g) calcium omission without lime (-CaSC), h) control with lime (Test.CC), i) phosphorus omission (-P); j) magnesium omission without lime (-MgSC), and k) control without lime (Test.SC).
cturonase enzyme, which is responsible for the degradation of Ca pectates, a substance that plays a pivotal role in maintaining the stability of cell membranes and walls (Wang et al., 2023a; Viégas et al., 2024a).
The OMgSC treatment resulted in a reduction in growth, the absence of floral tassel emission, brittle stems, and older leaves exhibiting chlorosis throughout their entire length between the veins. As the deficiency intensified and evolved, the visual symptoms became necrotic, deformed facing upward, and with small thorns making the leaves rough (Figure 3). The chlorosis observed in the leaves is related to the function of the nutrient in question, as magnesium (Mg) constitutes the central atom of the chlorophyll molecule, participating in thylakoid membrane structures, which are essential components in the photosynthetic process and carbon metabolism (Wang et al., 2023b; Costa et al., 2024; Viégas et al., 2024b). Moreover, magnesium is a cofactor in numerous enzymes, including phosphatases, phosphate kinases, and ribulose diphosphate carboxylase. It is also involved in the formation of ATP (Huang et al., 2018; Ye et al., 2019; Wang et al., 2023b).
Sulfur
Some plants exhibited minimal growth, while others demonstrated typical development with sulfur (S) omission with lime. The emergence of new branches was observed, although some exhibited signs of withering, displaying a dark coloration. The youngest leaves exhibited chlorosis exclusively between the veins. As the symptoms progressed, the leaves and petioles assumed a purple hue, and the leaves themselves became rough with the emergence of small thorns (Figure 3). Epstein and Bloom (2006) have reported that S deficiency can cause chlorosis and reduce the size of younger leaves, promoting curling of leaf edges, necrosis, defoliation, and the formation of short internodes. These symptoms are associated to the function of sulfur in the synthesis of specific amino acids (cystine, cysteine, and methionine) and as a constituent of enzymes involved in N metabolism in plants (Viégas et al., 2023; Zayed et al., 2023).
Boron
The OBC treatment resulted in the development of crambe plants exhibiting aberrant morphology, including chlorotic leaves at the edges that were curved downward, twisted, and
deformed (Figure 3). These observations were attributed to the absence of B in the medial lamella and cell wall, as well as in pectic connections between two complexes called rhamnogalacturonan (Matoh et al., 1996). The petioles and leaves exhibited brittleness and a rough texture as a result of the B omission. The number of branches emitted was limited, and as the symptoms worsened, they perished. A comparison of plants subjected to B omission with those in the CC treatment revealed a clear distinction in their overall development. While plants in the CC treatment exhibited typical growth patterns, those in the B omission treatment displayed pronounced deformities. This deformation is associated with the function of boron in the synthesis of ribonucleic acid (RNA), the biosynthesis of macromolecules, the elasticity and porosity of the cell wall, and the transport of plant sugars (Shireen et al., 2018; Pereira et al., 2021; Vera-Maldonado et al., 2024).
Zinc
The youngest leaves of plants subjected to the OZnC treatment exhibited chlorosis and deformations between the veins of their leaves, as well as deformations (Figure 3). The influence of Zn has a direct influence on carbohydrate metabolism (Romheld and Marschner, 1991) and nucleic acid metabolism (Hassan et al., 2020) is well documented. Additionally, Zn plays a role in N metabolism, promoting the accumulation of amino acids in plants deficient in zinc (Mengel and Kirkby, 1987).
Nutrient concentration in crambe leaves
The omission of individual nutrients, including N, P, K, Ca, Mg, S, B, and Zn, resulted in a reduction in the leaf content of the respective nutrient, when compared to the CC treatment (Table 3). The greatest reduction of macronutrients (81%) was observed in the MgSC treatment. Conversely, the greatest reduction in micronutrients was noted with B omission (238%) (Table 3). As observed by Haag et al. (1983), a notable reduction in foliar levels of macronutrients and B was evident in rapeseed (Brassica napus) subjected to nutrient omission.
The pronounced reduction in leaf nutrient concentration, particularly of Mg and B, impeded the optimal growth of plants, as these two nutrients play a crucial role in cell division and elongation, plasma membrane formation, and the photosynthetic activity of the plant (Prado, 2020).
Table 4. Soil chemical analysis before implementing the experiment.
| Sample | Depth | OM | pH | P | K | Na | Ca | Ca+Mg | Al | H+Al | CTC | V | m | |||
| (cm) | g/kg | water | ------mg/dm3------- | -------------cmolc/dm3------------- | ----%---- | |||||||||||
| 0-20 | 7.86 | 4.9 | 3.0 | 13.0 | 8.0 | 0.6 | 0.8 | 0.8 | 3.96 | 4.82 | 17.8 | 48.2 | ||||
OM: organic matter; pH: hydrogen potential; P: phosphorus; K: potassium; Na: sodium; Ca: calcium; Mg: magnesium, and Al: Aluminum.
Table 5. Soil chemical analysis after liming.
| Sample | Depth | OM | pH | P | K | Na | Ca | Ca+Mg | Al | H+Al | CTC | V | m | |||
| (cm) | g/kg | water | ------mg/dm3------- | -------------------cmolc/dm3--------- | ----%---- | |||||||||||
| 0-20 | 21.29 | 5.9 | 4.0 | 15.0 | 8.0 | 1.3 | 2.3 | 0.1 | 3.64 | 6.01 | 39.5 | 4.2 | ||||
Table 6. Sources and amounts of nutrients applied in the experiment.
| Source | Dose (mg kg-1) | Application time |
|---|---|---|
| CO(NH2)2 | 150 | 20 DAP* (50 mg kg-1) and 25 DAFA**(100 mg kg-1) |
| NaH2PO4H2O | 70 | Single dose at sowing |
| KCl | 100 | 20 DAP* (50 mg kg-1) and 25 DAFA**(100 mg kg-1) |
| CaSO42H2O | 50 | Single dose at sowing |
| SO4Mg2H2O | 30 | Single dose at sowing |
| NaSO4 | 25 | Single dose at sowing |
| Na2B4O7·10H2O | 0.4 | Single dose at sowing |
| ZnCl2 | 0.3 | Single dose at sowing |
| CuCl2.4H2O | 0.2 | Single dose at sowing |
| MnCl2.4H2O | 0.2 | Single dose at sowing |
| MoNa | 0.2 | Single dose at sowing |
*: application at 20 days after planting (DAP). **: application at 25 days after the first application (DAFA).
Materials and Methods
Study site
The experiment was carried out in a greenhouse at the Federal Rural University of Amazonia, Campus Capitão Poço, Pará State, Brazil (01° 44’ 42’ S, 47° 03’ 54’ W and 73 m a.s.l.). The site has an average air temperature of 26.1°C and an average annual rainfall of 2,256 mm (Pacheco and Bastos, 2001). The soil utilized for the experiment was an Oxisol, with medium texture (Santos et al., 2018b). Prior to the installation of the experiment, soil samples were collected at a depth of 0-20 cm for soil chemical analysis (Table 4).
The liming process was conducted in accordance with the findings of the soil chemical analysis, employing the base saturation method, with the saturation level increased to 50% (Broch and Roscoe, 2010). Dolomitic limestone was employed (32% CaO, 15% MgO, and Relative Total Neutralizing Power (PRNT) of 90%) and the soil was incubated for 21 days. Following this period, a further soil chemical analysis was conducted to ascertain the impact of liming on active acidity, exchangeable acidity, organic matter (OM), and the contents of P, K, Ca, Na, and Mg (Table 5).
Plant materials
Ten seeds of the cultivar FMS Brilhante of Crambe abyssinica Hochst were sown equidistantly at a depth of 2 cm in pots with a capacity of 4 kg. After 15 days, thinning was carried out, leaving only two plants per pot. Manual irrigation was employed to maintain the soil moisture content near that of field capacity.
Experimental design and treatments
The experimental design was a completely randomized block design with 11 treatments and five replications. The treatments applied were as follows: a control without lime (Test.SC), a control with lime (Test.CC), a complete treatment with lime (CC), a nitrogen omission treatment with lime (ONC), a phosphorus omission treatment with lime (OPC), a potassium omission treatment with lime (KC), a sulfur omission treatment with lime (OSC), a boron omission treatment with lime (OBC), a zinc omission treatment with lime (OZnC), a calcium omission treatment without lime (OCaSC), and a magnesium omission
treatment without lime (OMgSC). The applied amounts of nutrients are described in Table 6.
Biometric variables, biomass, and nutrient concentration
At 60 days after planting (DAP), a phytotechnical evaluation of the plants was conducted to measure various morphological traits, including plant height (from soil surface to plant apex), stem diameter at 5 cm from the ground, petiole length, leaf length, greater leaf width, number of leaves, and number of branches. During the same period, samples of the leaves, stems, branches, and roots were collected. The abovementioned parts were then separated and placed in identified kraft paper bags and subsequently subjected to a forced circulation oven at 70ºC until a constant mass was obtained, thus enabling the subsequent determination of the dry matter (DM) content. To determine the DM, the material was weighed on a precision scale and subsequently ground in a Willey mill to determine nutrient concentration. Nitrogen was determined by sulfuric acid digestion, while the contents of P, K, Ca, Mg, S and Zn were determined by nitroperchloric digestion. Boron, however, was measured by dry digestion. The analysis of nutrient contents was conducted at the IBRA laboratory (Instituto Brasileiro de Análises), Sumaré, São Paulo, Brazil.
To calculate the relative growth (RG) of biometric variables, equation 1 was used:
\(RG(\%) = \frac{PH.NO\ \ }{PH.\ CT}\) x100 (equation 1)
Where:
PH.NO = plant height obtained with each nutrient omission;
PH.CT = plant height obtained in the complete treatment.
Deficiency symptoms
The symptoms were identified and photographed in situ, and the leaf contents of N, P, K, Ca, Mg, S, B, and Zn were determined according to the procedures proposed by Malavolta et al. (1997).
Statistical analysis
The data obtained from the biometric measurements and DM production were subjected to the analysis of variance. When significant, the Tukey test was applied at a probability level of 5% to compare the means between treatments using the Agroestat software (Barbosa and Maldonado Júnior, 2015). The methodology proposed by Nunes et al. (2005) was employed to create the graphs in the Excel program.
Conclusion
Crambe plants have a high demand for nutrients, including phosphorous (P), nitrogen (N), and potassium (K). When liming is omitted, they exhibit reduced total dry mass production.
Crambe plants exhibit low tolerance to soil acidity, necessitating soil correction through liming. The presentation and identification of visual symptoms associated with deficiencies in treatments involving the omission of nutrients display a descending order, as follows: OPC > OMgSC > OZnC > ONC = OKC > OSC = OBC > OCaS.
Acknowledgements
The authors wish to thank the Federal Rural University of Amazonia, UFRA, Campus Capitão Poço, Pará, Brazil, for providing the greenhouse to carry out the research. Thanks to the National Council for Scientific and Technological Development (CNPq) for the scholarship granted to the first author. The authors would like to thank the Study Group on Plant Nutrition and Soil Fertility (GENFA) from the Federal Rural University of Amazonia (UFRA), Campus Capanema, for their collaboration.
References
Ahammed GJ, Chen Y, Liu C, Yang Y (2022) Light regulation of potassium in plants. Plant Physiol Bioch. 170: 316-324.
Alves AU, Prado RM, Gondim ARO, Fonseca IM, Cecílio Filho AB (2008) Desenvolvimento e estado nutricional da beterraba em função da omisão de nutrientes. Hortic Bras. 26: 292-295.
Alves JM, Leandro WM, Alves CCF, Carlos L, Ribon AA, Fernandes KL (2016) Crambe dry matter and yield under doses of phosphorus and base saturation in the Cerrado of Goiás. Rev Bras Eng Agr Amb. 20: 421-426.
Barbosa JC, Maldonado Junior W (2015) AgroEstat - sistema para análises estatísticas de ensaios agronômicos. 1st ed. Jaboticabal: FCAV/UNESP.
Bassegio D, Zanotto MD, Santos RF, Werncke I, Dias PP, Olivo M (2016) Oilseed crop crambe as a source of renewable energy in Brazil. Renew Sustain Energy Rev. 66: 311-321.
Brady NC, Weil RR (2013) Elementos da natureza e propriedades dos solos. 3rd ed. Porto Alegre: Bookman Editora LTDA.
Brasil EC, Cravo MS, Viégas IJM (Eds.) (2020) Recomendações de calagem e adubação para o estado do Pará. 2nd ed. Brasília: Embrapa.
Broch DL, Roscoe R (2010) Fertilidade do solo, adubação e nutrição do crambe. In: Fundaçâo MS. Tecnologia e produção: crambe 2010. Maracajú: Fundação MS.
Carneiro MMLC, Gomes MP, Santos HRB, Reis MV, Mendonça AMC, Oliveira LEM (2015) Fotorrespiração e metabolismo antioxidante em plantas jovens de seringueira cultivadas sob diferentes fontes de nitrogênio (NO3- e NH4+). Rev Bras Ci Agr. 10: 66-73.
Carstensen A, Herdean A, Schmidt SB, Sharma A, Spetea C, Pribil M, Husted S (2018) The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 177: 271–284.
Castro LMR, Vinson CC, Gordo SMC, Williams TCR, Cury NF, Souza MC, Pereira LAR (2022) Molecular and physiological aspects of plant responses to aluminum: what do we know about Cerrado plants? Braz J Bot. 45: 545–562.
Chen D, Cao B, Qi L, Yin L, Wang S. Deng X (2016) Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann Bot. 118: 305-315.
Colodetti TV, Rodrigus WN, Christo LF, Martins LD, Tomaz MA (2013) Perda de biomassa causada pela deficiência de macronutrientes em Crambe abyssinica. Enc Bio. 9: 2031- 2034.
Costa MG, Viégas IJM, Cordeiro RAM (2024) Dynamics, Requirements, and Use Efficiency of Magnesium Throughout the Life Cycle of Acai Palm Plants. Agr Res. 13: 1-11.
Cunha TJF, Madari BE, Benites VM, Canellas LP, Novotny EH, Moutta RO, Trompowsky PM, Santos GA (2007) Fracionamento químico da matéria orgânica e características de ácidos húmicos de solos com horizonte A antrópico da Amazônia (Terra Preta). Acta Amazon. 37: 91-98.
Epstein E, Bloom AJ (2006) Nutrição mineral de plantas: princípios e perspectivas. 2nd ed. Londrina: Editora Planta.
Fu Y, Mason AS, Song M, Ni X, Liu L, Shi J, Wang T, Xiao M, Zhang Y, Fu D, Yu H (2023) Multi-omics strategies uncover the molecular mechanisms of nitrogen, phosphorus and potassium deficiency responses in Brassica napus. Cell Mol Biol Lett. 28: 63.
Gurmessa B (2021) Soil acidity challenges and the significance of liming and organic amendments in tropical agricultural lands with reference to Ethiopia. Environ Dev Sustain. 23: 77-99.
Haag HP, Casarini MAG, Dechen AR (1983) Nutrição mineral da colza (Brassica napus L.): I. Carências nutricionais. Anais Esc Sup Agric Luiz de Queiroz. 40: 87-94.
Hamim H, Miftahudin M, Setyaningsih L (2018) Cellular and Ultrastructure Alteration of Plant Roots in Response to Metal Stress. In: Ratnadewi D, Hamim, D (Ed.). Plant Growth and Regulation - Alterations to Sustain Unfavorable Conditions. 1st ed. London: IntechOpen.
Hassan MU, Aamer M, Chattha MU, Haiying T, Shahzad B, Barbanti L, Nawaz M, Rasheed A, Afzal A, Liu Y, Guoqin H (2020) The critical role of zinc in plants facing the drought stress. Agriculture. 10: 396.
Hawkesford M, Horst W, Klchey T, Lambers H, Schjoerring J, Moller IS, White P (2012) Functions of Macronutrients. In: Marschner P (Ed.). Mineral Nutrition of Higher Plants. 3rd ed. London: Academic Press.
Huang JH, Xu J, Ye X, Luo TY, Ren LH, Fan GC, Qi YP, Li Q, Ferrarezi RS, Chen LS (2018) Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees, 33: 171-182.
Hue N (2022) Soil Acidity: Development, Impacts, and Management. In: Giri B, Kapoor R., Wu QS, Varma A (Ed). Structure and Functions of Pedosphere. Cingapura: Springer.
Isidra-Arellano MC, Delaux PM, Valds-Lpez O (2021) The Phosphate Starvation Response System: Its Role in the Regulation of Plant-Microbe Interactions. Plant Cell Physiol. 62: 392–400.
Jankowski KJ, Szatkowski A, Okorski A (2024) The effect of sowing date and sowing density on the yield and quality of crambe seeds. A case study in north-eastern Poland. Eur J Agron. 152: 127010.
Jin SH, Huang JQ, Li XQ, Zheng BS, Wu JS, Wang ZJ, Liu GH, Chen M (2011) Effects of Potassium Supply on Limitations of Photosynthesis by Mesophyll Diffusion Conductance in Carya cathayensis. Tree Physiol. 31: 1142-1151.
Johnson R, Vishwakarma K, Hossen MS, Kumar V, Shackira AM, Puthur JT, Abdi G, Sarraf M, Hasanuzzaman M (2022) Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol Bioch. 172: 56-69.
Kerbauy GB (2019) Fisiologia vegetal. 3rd ed. Rio de Janeiro: Guanabara Koogan.
Khan F, Siddique AB, Shabala S, Zhou M, Zhao C (2023) Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants. 12: 2861.
Kwiatkowski J, Krzyżaniak M, Załuski D, Stolarski MJ, Tworkowski J (2020) The physical properties of fruits and the physiological quality of seeds of selected crambe genotypes. Ind Crop Prod. 145: 111977.
Lambers H, Oliveira RS (2019) Plant Physiological Ecology. 3rd ed. NewYork: Springer.
Malavolta E, Vitti GC, Oliveira SA (1997) Avaliação do estado nutricional das plantas: princípios e aplicações. 2nd ed. Piracicaba: Potafos.
Marschner P (2022) Marchner’s mineral nutrition of higher plants. 4th ed. London:
Academic Press.
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 105: 1141-1157.
Matoh T, Kawaguchi S, Kobayashi M (1996) Ubiquity of a Borate Rhamnogalacturonan II Complex in the Cell Walls of Higher Plants. Plant Cell Physiol. 37: 636-640.
Mauad M, Garcia RA, Vitorino ACT, Silva RMMF, Garbiate MV, Coelho LCF (2013) Matéria seca e acúmulo de macronutrientes na parte aérea das plantas de Crambe. Ciência Rural, 43: 771-778.
Mauad M, Santana RS, Silva HHM, Altomar PH, Silva RMMF, Vitorino ACT (2019) Macronutrient deficiency and anatomic modifications in crambe leaves. J Plant Nutr. 42: 2363-2372.
Mengel K Kirkby EA (1987) Principles of Plant Nutrition. Switzerland: International Potash Institute.
Mera WYWL Viégas IJM, Galvão J.R, Yakuwa TKM, Silva AO (2020) Effects of Liming on the Growth and Nutritional Status of Crambe (Crambe abyssinica Hochst). J Agric Stud. 8: 590-603.
Meurer EJ, Wang GM, Wang SR (1981) Funções dos nutrientes e sintomas de deficiências. In: Miyasaka S, Medina JC (Eds.). A soja no Brasil. Campinas: Instituto Tecnológico de Alimentos.
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl environ soil sci. 2019: 5794869.
Nunes JAR, Ramalho MAP, Abreu AFB (2005) Graphical method in studies of adaptability and stability of cultivars. Annu Rep Bean Improv Coop. 48: 182-183.
Olego MÁ, Quiroga MJ, López R, Garzón-Jimeno E (2021) The importance of liming with an appropriate liming material: Long-term experience with a typic palexerult. Plants, 10: 2605.
Pacheco NA, Bastos TX (2001) Caracterização climática do município de Capitão Poço-PA. Belém: Embrapa Amazônia Oriental.
Paradelo R, Virto I, Chenu C (2015) Net effect of liming on soil organic carbon stocks: a review. Agric Ecosyst Environ. 202: 98-107.
Paz CG, Rodríguez TT, Behan‐Pelletier VM, Hill SB, Vidal‐Torrado P, Cooper M (2008) Ferralsols. In: Chesworth W (Ed). Encyclopedia of Soil Science. Encyclopedia of Earth Sciences Series. Dordrecht: Springer.
Paz-Ares J, Puga MI, Rojas-Triana M, Martinez-Hevia I, Diaz S, Poza-Carrión C, Miñambres M, Leyva A (2022) Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol Plant 15: 104–124.
Pereira GL, Siqueira JA, Batista-Silva W, Cardoso FB, Nunes-Nesi A, Araújo WL (2021) Boron: More than an essential element for land plants? Front. Plant Sci. 11: 610307
Prado RM (2020) Nutrição de Plantas. 2nd ed. Jaboticabal: Editora UNESP.
Rahman R, Upadhyaya H (2021) Aluminium toxicity and its tolerance in plant: A review. J Plant Biol. 64: 101-121.
Raij Bvon (2019) Fertilidade do solo e manejo de nutrientes. 2nd ed. Piracicaba: NPCT.
Rogério F, Santos JI, Silva TRB, Migliavacca RA, Gouveia B, Barbosa MC (2012) Efeito de doses de fósforo no desenvolvimento da cultura do crambe. Bioscience J. 28: 251-255.
Romheld V, Marschner H (1991) Function of Micronutrients in Plants. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM (Eds.). Micronutrients in Agriculture. 4th ed. Madison: SSSA.
Rosmaninho CLB, Dias AS, Silva MF, Vasconcelos A, Santos WO, Perez CEA, Vergutz L, Cardoso LG (2019) Performance of Crambe submitted to aluminum stress: an important oilseed. plant. J Agric Sci. 11: 454.
Samarappuli D, Zanetti F, Berzuini S, Berti MT (2020) Crambe (Crambe abyssinica Hochst): A non-food oilseed crop with great potential: A review. Agronomy, 10: 1380. Santos DR, Tiecher T, Gonzatto R, Santanna MA, Brunetto G, Silva LS (2018a) Long-term effect of surface and incorporated liming in the conversion of natural grassland to no-till system for grain production in a highly acidic sandy-loam Ultisol from South Brazilian Campos. Soil Till Res. 180: 222-231.
Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Araújo Filho JC, Oliveira JB, Cunha TJF (2018b) Sistema Brasileiro de Classificação de Solos. 5th ed. Brasilia: Embrapa.
Shireen F, Nawaz MA, Chen C, Zhang Q, Zheng Z, Sohail H, Sun J, Cao H, Huang Y, Bie Z (2018) Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 19: 1856.
Silva TRB, Reis ACS, Maciel CDG (2012) Relationship between chlorophyll meter readings and total N in crambe leaves as affected by nitrogen topdressing. Ind Crop Prod. 39: 135-138.
Silva TRB, Carraro TV, Frigo P, Barbosa NA, Tiburcio MGG, Secco D, Santos RF, Alves CZ (2017) Crambe development under lime application in sandy soil. Acta Iguazu. 6: 59-63.
Singh P, Kumar K, Jha AK, Yadava P, Pal M, Rakshit S, Singh I (2022). Global gene expression profiling under nitrogen stress identifies key genes involved in nitrogen stress adaptation in maize (Zea mays L.). Sci Rep. 12: 4211.
Siqueira JA, Zsögön A, Fernie AR, Nunes-Nesi A, Araújo WL (2023) Does day length matter for nutrient responsiveness? Trends Plant Sci. 18: 1113-1123.
Soratto RP, Souza-Schlick GD, Fernandes AM, Souza EFC (2013) Effect of fertilization at sowing on nutrition and yield of crambe in second season. Rev Bras Ci Solo, 37: 658-666.
Souza RS, Chaves LHG (2017) Crescimento e produção do crambe submetido a doses de nitrogênio e fósforo. Rev Espacios, 38: 1-16.
Taiz L, Zeiger E, Møller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal. 6th ed. Porto Alegre: Artmed.
Vera-Maldonado P, Aquea F, Reyes-Díaz M, Cárcamo-Fincheira P, Soto-Cerda B, Nunes-Nesi A, Inostroza-Blancheteau C (2024) Role of boron and its interaction with other elements in plants. Front Plant Sci. 15: 1332459.
Viégas IJM, Silva WDS, Ferreira EVO, Costa MG, Conceição HEO, Barata HS, Brito AEA, Oliveira Neto CF (2022) Cultivation age of oil palm plants alters the dynamics of immobilization, recycling and export of sulfur and increases its use efficiency. Intl J Agric Biol. 29: 74-82.
Viégas IJM, Souza LC, Ferreira EVO, Costa MG, Nogueira GAS, Nascimento VR, Oliveira Neto CF (2024a) Changes in calcium accumulation and utilization efficiency and their impact on recycling, immobilization, and export across the oil palm cycle. Oil Crop Sci. 9: 143-150.
Viégas IJM, Souza AES, Costa MG, Ferreira EVO, Ferreira LGN, Silva DAS, Oliveira Neto CF (2024b) Age-Related Changes in Magnesium Status within Oil Palm Cultivation in Eastern Amazon. Intl J Agric Biol. 31: 437-446.
Wang T, Chen X, Ju C, Wang C (2023a) Calcium signaling in plant mineral nutrition: from uptake to transport. Plant Commun. 4: 100678.
Wang Y, Zhang X, Zhang W, Peng M, Tan G, Qaseem MF, Li H, Wu A-M (2023b) Physiological and transcriptomic responses to magnesium deficiency in Neolamarckia Cadamba. Plant Physiol Biochem. 197: 107645.
Yadav RS, Meena SC, Patel SI, Patel KI, Akhtar MS, Yadav BK, Panwar J (2012) Biodisponibilidade de P do solo para nutrição de plantas. In: Lichtfouse E (Ed.) Farming for Food and Water Security. Sustainable Agriculture Reviews. 1st ed. Dordrecht: Springer.
Ye X, Chen XF, Deng CL, Yang LT, Lai NW, Guo JX, Chen LS (2019) Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants. 8: 389.
Zayed O, Hewedy OA, Abdelmoteleb A, Ali M, Youssef MS, Roumia AF, Seymour D, Yuan ZC (2023) Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomol. 13: 1443.