

Aust J Crop Sci. 18(12):801-810 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p3836
Investigating abiotic and biotic parameters associated with gradually declining Valencia citrus trees in South Africa
Mathys C. Pretorius*1, N. Labuschagne2, M. Daneel3, P. Raath1, P. Cadet4, A. McLeod5
1Citrus Research International, P. O. Box 28, Nelspruit, 1200, South Africa
2Department of Plant and Soil Sciences, University of Pretoria, Pretoria, 0002, South Africa
3Agricultural Research Council - Tropical and Subtropical Crops, Private Bag X11208, Nelspruit, 1200, South Africa
4Moulin de Lespinasse, 473, Route de Lespinasse, F-42640 St Forgeux, Lespinasse, France
5Department of Plant Pathology, Private Bag X1, University of Stellenbosch, 7601, South Africa
*Corresponding author: Mathys C. Pretorius 
Abstract: The current study determined if a combination of biotic and abiotic parameters could differentiate Citrus sinensis trees (three categories based on visual tree canopy status) with root disease-related decline symptoms in two citrus orchards. Two experimental sites, containing a range of healthy and different stages of decline trees, were randomly selected. Three decline category trees were selected with abiotic and biotic parameters associated with decline measured. Principal component analyses of 41 soil, leaf, root and trunk associated parameters ordered the category 1 to 3 trees chronologically on the factorial plan for both orchards. However, the parameters only described a low level of the variability. Only the healthy (category 1) and more diseased (category 3) trees were studied further. Soil electrical conductivity and leaf %N, were the only parameters differing significantly between the two categories in both orchards, but both parameters were significantly higher in category 3 trees in orchard 1 whereas the opposite was true for orchard 2 with significantly higher values in category 1 trees. In the more severely declining MP1 orchard, parameters differing significantly between the two categories included soil Na, cation exchange capacity and leaf Fe, B, Mo and Zn. In orchard MP2, parameters were soil %C, soil citrus nematodes, leaf size and leaf %Na and %Mg. Discriminant analysis indicated that leaf parameters were more specifically associated with decline. Leaf nutrient status can possible be an additional process to indicate decline.
Keywords: Citrus sinensis, tree decline, South Africa, multivariate analysis, discriminant analysis.
Abbreviations: CB_Citrus Blight; DA_Discriminant analysis; HLB_Huanglongbing; PCA_Principal Component Analysis.
Introduction
The phenomenon in citrus trees of reduced growth, occasional twig die-back and yield reductions over a period of time within an orchard has been studied for decades and is generally referred to as ‘slow decline’ or tree decline of citrus. A typically declining tree will have a yellowing tree canopy, discoloured and distorted leaves, sparse foliage, deadwood in its canopy, reduced growth, and a reduction in yield and fruit size (DuCharme, 1971). During very dry or wet periods, declining trees have a wilted appearance compared to adjoining healthy trees. This suggests that the trees have a damaged root- or vascular system. Within an orchard, the declining trees all show the same general symptoms and are spread randomly throughout the orchard. This disease distribution is unlike that of trees affected by a biological factor where decline gradually spreads from the affected trees to the adjacent trees (Suit and DuCharme, 1947; Suit and Knorr, 1949).
Several biotic factors (insects, fungi, fungal-like organisms, nematodes, phytoplasms, bacteria and viruses) are well-known for causing damage and decline in citrus trees. Insects that can indirectly contribute to tree decline include certain species of the brown citrus aphid (Toxoptera aurantii Boyer de Fonscolombe, 1841) and citrus psyllids (Diaphorina citri Kuwayama, 1908) which are vectors for the Citrus Tristeza Virus (Gottwald et al. 1998) and the Ca. Liberibacter asiaticus (HLB) bacterial pathogen (Hall et al., 2015) respectively. Soil microbes that can incite fibrous root rot, collar rot, stem canker, and eventually tree decline, include oomycete pathogens within the genera Phytophthora (Graham, 1995) and possibly Pythium and Phytopythium (Maseko and Coutinho, 2002; Benfradj et al., 2017). The citrus nematode (Tylenchulus semipenetrans Cobb, 1913) also contributes toward feeder root rot and poor root development in citrus orchards (Pretorius and Le Roux, 2017). All the aforementioned biotic factors have been extensively studied, mainly on their own, whereas the influence of a combination of several factors has rarely been investigated. Exceptions included studies on the interaction between the citrus nematode and Phytophthora spp. (El-Borai et al., 2002), and the synergistic effect between the citrus nematode and Fusarium spp. (Safdar et al., 2013).
Symptoms of Citrus blight (CB) do not readily distinguish it from the various other citrus decline diseases. Although there is no consensus as to the cause of CB, diagnostic tools are available. Affected trees frequently show zinc (Zn) deficiencies in leaves, indicating interference with its translocation in the tree. Although foliar Zn deficiency symptoms are not diagnostic for CB, a significantly higher Zn content is present in the wood and bark of blighted trees relative to healthy trees. A decrease in water movement in the inner wood also occurs (Derrick and Timmer, 2000; Young et al., 1980), of which the extent can be measured using a syringe water uptake test (Lee et al., 1984). The restricted water movement in CB trees contributes to the characteristically drought-associated wilting symptoms of trees with CB. Fusarium solani has been implicated as causing CB either independently or in conjunction with certain soil factors (Burnett et al., 1982; Janse van Rensburg et al., 2001). The fungus is a ubiquitous soilborne microbe that readily colonizes citrus roots, and it has been isolated from the rhizosphere of symptomless and CB trees (Nemec et al., 1989). Under certain conditions, F. solani can cause a dry root rot of citrus, although blight-associated symptoms are more likely caused by toxins produced by the pathogen in the xylem of CB trees under certain conditions (Janse van Rensburg et al., 2001).
A number of abiotic causes have been listed as possibly contributing to root damage related ‘slow decline’, including nutritional deficiencies and/or imbalances, accumulation of toxic substances in the soil, soil pH, soil compaction, soil salinity and drought (Nel and Bennie, 1984). Studies by Milad et al. (1975) and Malewar et al. (1983) found that soil analysed from patches with declining trees in a young sweet orange orchard had higher levels of soluble salts and sodium (Na), as well as lower concentrations of soluble calcium (Ca) and magnesium (Mg). Soil salinity and poor irrigation water quality are also well-known contributors to severe yield losses, poor growth and an increase in the severity of several root rot diseases (Combrink et al., 1995).
The fact that the primary factor or factors causing root damage-related ‘slow decline’ described above, have not yet been clearly identified, indicates that the cause is likely a syndrome resulting from interactions among several parameters (biotic and abiotic). Due to this, one of the strategies utilised in South Africa against the development of slowly declining trees is to maintain the physicochemical characteristics associated with healthy trees within the soil environment. This may result in the maintenance of conditions that are unfavourable to the onset of the decline syndrome.
The identification of a range of interacting parameters positively or negatively associated with decline could possibly be utilised to prevent the gradual deterioration in healthy trees provided that the parameters can be managed effectively. The study investigated the relative importance of a total of 41 leaf, soil and trunk parameters to differentiate healthy trees from two categories of declining trees in two orchards. Trees already showing severe decline symptoms were not included in the study since many of the parameters associated with these trees were often outside of normal values.
The aim of the study was to use a multivariate analysis approach to identify the most important factors commonly associated with the decline syndrome in South Africa, and to arrange these factors according to their relative importance as related to the different levels of tree decline.
Results
Comparison of the two orchard trial sites
PCA analysis using the 41 parameters measured in the healthy trees (category 1) showed that the two orchards were distinctly different. This was evident from their distant locations on the negative (MP1) and positive (MP2) part of the F1 axis of the factorial plan which described 42.1 % of the variability (Fig. 2A). Because the orchards were separated along F1, factorial values of the measured parameters were projected along F1 (x-axis) to identify factors differing between the orchards (Fig. 2B). This showed that orchard MP1 located in the negative part of F1, was associated with higher levels of certain leaf elements (%Na, Mn (mg/kg), %S and Fe (mg/kg)), soil K (mg/kg) and Na (mg/kg), root starch (mg/kg) and citrus nematodes in soil and roots (Table 1; Fig. 2B). On the other hand, orchard MP2, located in the positive part of F1, was associated with higher levels of several leaf associated parameters (%P, %Ca, B (mg/kg) and leaf size (mm)), soil associated parameters ([Ca+Mg]/K, Mg:K, EC (mS/m) and pH) and a trunk associated parameter (citrus blight) (Table 1; Fig. 2B). The parameters that were similar for both orchards, situated in the middle of the factorial map, included leaf chlorophyll, % silt in the soil, bark Zn (mg/kg) content and %K in leaves (Fig. 2B). It is noteworthy that both orchards were associated with lower levels of the opposite parameters found in each orchard. When the parameters were ordered according to the factorial values from high to low for both orchards separately, univariant analysis revealed significant differences (P<0.05) between the values of most of the parameters for MP1 and MP2 (Table 1).
Change in soil-, leaf-, root- and trunk associated parameters according to tree decline appearance
Since the healthy trees of the two orchards differed significantly (Fig. 2), the analysis of the decline process was performed separately for each orchard. On the factorial plan (F1 x F2), the measured parameters for orchard MP1 described 13.6 % and 10.8 % of the variability respectively (Fig. 3A), whereas for orchard MP2, 14.9 % and 12.0 % of the variability was described by the two axes (Fig. 3B).
For both orchards, the position of the three categories was not random but occurred chronologically from categories 1 to 3 (Fig. 3). This was evident from the factorial plan where the difference in decline for the three categories occurred along both the first (F1) and second factor (F2) for MP1 (Fig. 3A), whereas it occurred mainly along F2 for MP2 (Fig. 3B).
Based on these results, a second PCA was conducted employing only the healthy (category 1) and the declining (category 3) trees within each of the orchards (Fig. 4). Category 2 trees were not included because there was a strong overlap with both the healthy and declining trees in this category. The PCA plot for the healthy and decline group showed that for orchard MP1 the two categories were separated on F1, whereas in orchard MP2 the two categories were separated on F2 (Fig. 4). The variability described by the two first factors (F1 and F2) in each of the orchards was only slightly higher (17 to 18% for F1 and 13 to 14% for F2 respectively) than when all three categories were used.
Ordering of the 41 parameters according to their F1 and F2 values for MP1 and MP2, respectively showed that very few of the most important parameters (highest factorial values) were similar between the two orchards for each of the tree categories (Fig. 4). For the healthy group, the shared parameters included Ca and Mg in the soil (Ca and Mg (mg/kg) and Ca:Mg), while parameters that were shared between the two orchards for the ‘decline’ group included leaf associated parameters (%Mg, %P) (Table 2 and Fig. 4).
When the values of Ca (mg/kg) were projected on Mg (mg/kg) for both categories and both orchards, a difference was observed in the distribution between both orchards (Fig. 5). In MP1 both eclipses were overlapping completely while in MP2 the two eclipses were differentiated with category 1 trees having a higher ratio compared to category 3 trees.
Univariate analysis (ANOVA) was conducted on the most important parameters based on the PCA factorial values ordered for the category 1 as well as 3 trees for each orchard. This showed that the parameters (ordering according to healthy and diseased trees) that were significantly different (P < 0.05) between the healthy and ‘decline’ group were mostly dissimilar between the two orchards (Table 2). Electrical conductivity and leaf %N were the only parameter differing significantly between the category 1 and 3 trees in both orchards, but the effects were opposite. Parameters that were significantly different (P < 0.05) between both categories that were specific to the MP1 orchard included soil Na and CEC and leaf concentrations of B, Mo, Fe and Zn. The parameters that were specific to the MP2 orchard included soil C, soil citrus nematodes and leaf %Na, %Mg and leaf size (Table 2).
Most of the individual parameters were not significantly different between both categories indicating that the differences between the categories were not strong. However, when a mathematical artificial index was calculated, significant differences were
Table 1. Differences, based on univariate analysis, between the healthy tree categories of orchards MP1 and MP2 for the most important leaf, soil, root and trunk associated parameters identified for each orchard in a principal component analysis.#
| Orchard MP1 | Orchard MP2 | F1 – value ^ | P-value ^ | T-ratio | |
|---|---|---|---|---|---|
| Ordered according to factorial values (F1) for MP1 | |||||
| % Na leaf | 0.033 | 0.018 | -0.9416 | <0.001 | 17.11 |
| Mn (mg/kg) leaf | 304.41 | 134.00 | -0.9347 | <0.001 | 15.20 |
| K (mg/kg) soil | 180.2 | 55.5 | -0.9176 | <0.001 | 10.15 |
| % S leaf | 0.39 | 0.28 | -0.9082 | <0.001 | 16.60 |
| Starch (mg/kg) roots | 7.7 | 2.2 | -0.8933 | <0.001 | 12.76 |
| Fe (mg/kg) leaf | 231.08 | 158.67 | -0.8599 | <0.001 | 8.11 |
| Na (mg/kg) soil | 10.47 | 5.91 | -0.8299 | <0.001 | 6.97 |
| Nem roots | 4.03 | 3.38 | -0.8265 | <0.001 | 7.09 |
| Nem soil | 4.38 | 3.84 | -0.8140 | <0.001 | 6.54 |
| Cu (mg/kg) leaf | 33.39 | 11.38 | -0.7988 | <0.001 | 7.21 |
| Ordered according to factorial values (F1) for MP2 | |||||
| B (mg/kg) leaf | 22.5 | 90.8 | 0.9187 | <0.001 | -13.27 |
| % P leaf | 0.10 | 0.13 | 0.9135 | <0.001 | -10.53 |
| (Ca+Mg)/K soil | 4.01 | 12.83 | 0.8174 | <0.001 | -6.83 |
| Leaf size (mm2) | 30.0 | 46.6 | 0.8119 | <0.001 | -8.62 |
| Mg:K soil | 1.32 | 4.95 | 0.7533 | <0.001 | -4.82 |
| % Ca leaf | 2.76 | 3.15 | 0.6995 | <0.001 | -4.90 |
| EC* (mS/m) soil | 3.33 | 3.56 | 0.6803 | <0.001 | -4.40 |
| Citrus blight trunk | 2.20 | 2.93 | 0.6573 | <0.001 | -5.11 |
| pH soil | 5.73 | 6.43 | 0.6386 | <0.001 | -4.09 |
# The parameters were selected based on the highest and lowest positioned parameters in MP1 and MP2 according to the first factorial axis of the principal component analysis (Fig. 2). *EC = electrical conductivity, ^F- and P-value for Student’s t-test.
observed. The mathematical artificial indices were calculated for each orchard using the parameters with the three highest factorial values for MP1 (ordered according to category 1 trees or category 3 trees) and in orchard MP2 the two highest factorial values for each of the ordered tree categories (Table 2). In orchard MP1, the calculated (Na soil + Ca soil + Fe leaf) index, ordered according to the healthy trees was significantly higher in category 1 than in category 3 trees, whereas the (B leaf + Mo leaf + % Sand) index for trees ordered according to the diseased trees was significantly lower in category 1 than category 3 trees (Table 2). For MP2 a significant difference was only observed for the index calculated in the diseased trees (%C soil + %Na leaf), with the index being higher in the category 3 than category 1 trees (Table 2).
Discriminant analysis
Selected leaf and soil parameters of the healthy and category 3 trees were evaluated separately using DA to investigate the importance of leaf versus soil parameters, and whether leaf and/or soil analysis could be used on its own to discriminate between healthy and declining trees. Using 13 of the 15 investigated leaf parameters explained 93.28% of the variability between the healthy and category 3 trees, and the two categories were evident as two different groups in both orchards. The two orchards themselves, furthermore, were also differentiated by being positioned on either side of the y-axis (Fig. 6). Parameters in MP1 that were correlated with healthy trees were identified as higher concentrations of leaf Na, Fe and Zn, while parameters correlated with diseased trees included higher concentrations of N and K. For MP2, higher concentrations of foliar N, K and P were correlated with healthy trees while higher concentrations of Mg and Ca were correlated with declining trees.
The results clearly indicate that several leaf and soil parameters did indicate associations with deteriorating citrus trees.
Discussion
In the current study, two citrus orchards with declining trees were selected in the Lowveld region in South Africa for their apparent similarity to each other concerning tree age, cultivar, soil type, management practices and climatic conditions. However, it
became clear that both orchards were not as similar as originally anticipated. Since the orchards were similar concerning soil physical parameters, the distinct differences that the multivariate analyses revealed between the category 1 trees of the two orchards were therefore due to a range of parameters and their interactions. The latter likely also contributed towards the observation that parameters differentiating the healthy and declining categories within each of the orchards differed. Interactions between parameters had a much stronger effect than the individual soil and leaf parameters since most of the measured soil and leaf nutrient elements were within the accepted levels for citrus production. This is not surprising as leaf and soil analyses are conducted annually and are subsequently used to adjust the fertilization program for the coming year to ensure optimal nutrition for the citrus trees.
The two orchards not only exhibited different soil and leaf nutrient characteristics, but the intensity of tree decline also differed. Disease incidence was markedly lower in MP2 compared to MP1, which was evident from more category 3 trees being present in MP1 as well as an additional category 4 tree that represented trees that were almost dead (data not shown). It is anticipated that the soil and root rhizosphere in MP2 was probably in a better condition than in MP1 and it might be beneficial to strive towards having these better conditions (as in MP2) in the healthy MP1 trees.
Our study showed that a combination of leaf, soil and trunk parameters may be associated with the gradual decline process in the two orchards studied in South Africa. This was evident from the chronological ordering of the three tree categories (healthy and two disease categories) on the factorial plan for each of the orchards when investigated independently. However, the selection of parameters for characterising declining trees requires improvement, since the 41 investigated parameters only had a marginal contribution towards the variability in the data set when using all three tree categories (24 and 27% for orchards MP1 and MP2, respectively) or when using only category 1 and category 3 trees (30 and 32% for orchards MP1 and MP2, respectively). This is also likely why an overlap was evident between the different disease categories in both orchards, making clear distinction between the disease categories difficult. This was especially evident for category 2 trees that had considerable overlap with category 1 and 3 trees in both orchards (Fig. 3). Overlapping could also be
Table 2. Differences, based on univariate analysis, between the healthy tree category 1 and decline category 3 in orchards MP1 and MP2 for the most important leaf, soil, root and trunk associated parameters identified for each orchard according to tree category in a principal component analysis#
| Cat 1 | Cat 3 | Factorial value | P-value | T-ratio | |
|---|---|---|---|---|---|
| Ordered according to factorial values (F1) for category 1 trees in MP1 | |||||
| Na (mg/kg) soil | 10.47 | 7.60 | 0.6976 | <0.001* | 4.41 |
| Ca (mg/kg) soil | 252.98 | 211.39 | 0.6910 | 0.092 | 1.75 |
| Fe (mg/kg) leaf | 231.08 | 188.90 | 0.6851 | <0.001* | 4.23 |
| CEC soil | 2.26 | 1.94 | 0.6745 | 0.047* | 2.07 |
| Mg (mg/kg) soil | 59.81 | 53.68 | 0.6217 | 0.289 | 1.08 |
| Zn (mg/kg) leaf | 107.94 | 51.52 | 0.6020 | <0.001* | 5.79 |
| pH soil | 5.74 | 5.55 | 0.5646 | 0.329 | 0.99 |
| Cu (mg/kg) leaf | 33.39 | 26.69 | 0.5232 | 0.093 | 1.74 |
| Index for Na soil + Ca soil + Fe leaf^ | 494.50 | 407.90 | NA | 0.004* | 3.14 |
| Ordered according to factorial values (F1) for category 3 trees in MP1 | |||||
| B (mg/kg) leaf | 22.53 | 38.55 | -0.5891 | <0.001* | -4.04 |
| Mo (mg/kg) leaf | 1.76 | 2.23 | -0.565 | <0.001* | -4.02 |
| % Sand soil | 90.13 | 90.53 | -0.4967 | 0.364 | -0.92 |
| %P leaf | 0.102 | 0.116 | -0.4904 | 0.066 | -1.97 |
| %N leaf | 2.58 | 2.79 | -0.4771 | <0.001* | -4.34 |
| EC (mS/m) soil | 3.33 | 3.43 | -0.3629 | 0.014* | -2.76 |
| %K leaf | 1.41 | 1.47 | -0.3008 | 0.558 | -0.60 |
| %Mg leaf | 0.29 | 0.32 | -0.2126 | 0.152 | -1.47 |
| Index for B leaf + Mo leaf + % Sand^ | 114.4 | 131.3 | NA | <0.001* | -4.25 |
| Ordered according to factorial values (F2) for category 1 trees in MP2 | |||||
| K (mg/kg) soil | 55.45 | 58.73 | -0.5773 | 0.718 | -0.36 |
| %N leaf | 2.66 | 2.40 | -0.5085 | <0.001* | 5.85 |
| Leaf size | 46.6 | 32.5 | -0.3785 | <0.001* | 2.87 |
| Nem soil^ | 3.80 | 3.52 | -0.3527 | 0.008* | 2.87 |
| Phythopthora soil | 40.5 | 33.1 | -0.3442 | 0.460 | 0.75 |
| Ca:Mg soil | 1.90 | 1.91 | -0.3383 | 0.911 | -0.11 |
| EC (mS/m) soil | 3.56 | 3.41 | -0.2577 | 0.003* | 3.53 |
| Index for K soil + %N leaf^ | 58.11 | 61.13 | NA | 0.118 | 1.35 |
| Ordered according to factorial values (F2) for category 3 trees in MP2 | |||||
| %C soil | 0.23 | 0.49 | 0.7382 | <0.001* | -8.25 |
| %Na leaf | 0.018 | 0.024 | 0.7286 | 0.010* | -2.92 |
| Mg:K soil | 4.61 | 4.98 | 0.7037 | 0.593 | -0.54 |
| (Ca+Mg)/K soil | 12.83 | 13.79 | 0.6619 | 0.649 | -0.46 |
| %P leaf | 0.128 | 0.129 | 0.6276 | 0.837 | -0.21 |
| Bark Zn trunk | 69.07 | 49.19 | 0.5701 | 0.231 | 1.25 |
| %Mg leaf | 0.31 | 0.40 | 0.4894 | <0.001* | -4.10 |
| Index for %C soil + %Na leaf^ | 0.248 | 0.514 | NA | < 0.001 | -8.28 |
#Parameters selected were the parameters with the highest factorial values in MP1 or MP2 according to the first (MP1) or second (MP2) factorial axis (Fig. 4).; ^ The calculated index was created with the three highest factorial values for MP1 (ordered according to category 1 or 3 trees) and two for MP2 for each ordered category; *indicates a significant difference (P < 0.05) according to the Student’s T-Test of least significant difference; Nem Soil = citrus nematodes in soil log transformed; EC = Electrical conductivity; CEC = Cation exchange capacity.
ascribed to trees being in different stages of decline at any specific time and, while some of the parameters lean towards severe decline, the symptoms might not be visible in the tree yet.
The ability of the 41 parameters to differentiate the tree categories on the factorial plan differed in the two orchards. This was apparent from the disease categories being chronologically separated along F1 and F2 for orchard MP1 on the factorial plan, but only along F2 for orchard MP2 when using either all three tree categories or only two (Figs. 3 and 4). This could be due to the overall better health status of trees in orchard MP2. The distinctiveness of the two orchards, although both had similar soil types, rootstocks, cultivars, age and orchard practices, was also supported by multivariate analysis of the healthy trees (Fig. 2). In both orchards, it might have been more meaningful to monitor the
tree condition over a longer period, starting at an early stage to ensure that changes in the parameters are detected and can be dealt with in time.
The fact that only soil and leaf associated factors were identified as being indicators of gradually declining trees in citrus, rather than biological factors such as citrus blight, root associated citrus nematodes and Phytophthora in the soil, was expected. This is due to these biological agents being known for their ability to kill a tree within a few months to a year (Donovan, 2007; Schoeman et al., 2017). In contrast, the gradual decline process in the two orchards under investigation developed slowly and was only evident approximately 10 years after planting. Furthermore, it takes many years for a tree to move from category 1 to category 3.

Fig 1. Photographs of citrus trees representing the three disease categories that were investigated in the current study.
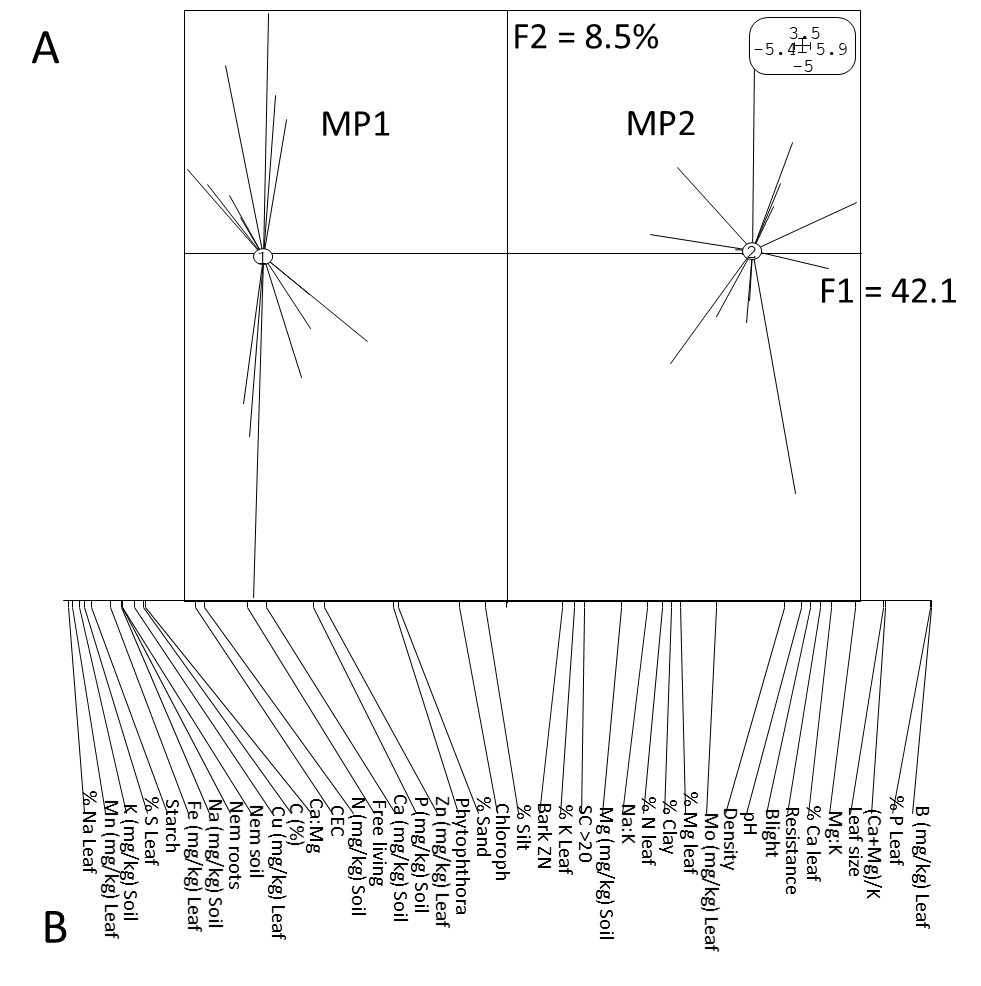
Fig 2. (A) Principle component analysis (PCA) factorial plan of healthy (category 1) citrus trees from two citrus orchards (MP1 an MP2) for the first two factorial axes (F1 and F2) and (B) ordering of 41 parameters used in the PCA according to their factorial values with parameters on the left indicating the highest positively correlated parameters associated with orchard MP1 and the parameters on the right indicating those having the highest positive correlation with orchard MP2.
Concerning individual parameters, in orchard MP1 (orchard with more severe decline), a few leaf parameters (Mo, Zn and B) were identified that were significantly different between declining and healthy trees, which were unique to this orchard. Molybdenum was significantly higher in declining trees than in healthy trees. This element is known to be linked to stress related responses such as pathogens (Kaiser et al., 2005), which might be the reason why levels were higher in the declining trees (Table 2). Since it is anticipated that the higher Mo concentration is stress related, this would indicate that the declining trees are under continued stress.
Lower Zn levels in declining trees (due to less effective uptake by the roots) could result in these trees being more susceptible to diseases. Singh and Tripathi (1985) observed an antagonistic effect of Fe and Zn availability due to Zn deficiency. In orchard MP1, boron levels were too low irrespective of the tree category (normal range of B for citrus is 40-200 mg/kg) which could also render the trees more susceptible to diseases and it might be the reason for this orchard being in poorer health than MP2.
Another element that is noteworthy to mention is Na levels in leaves. Na levels were significantly higher in healthy trees of MP1
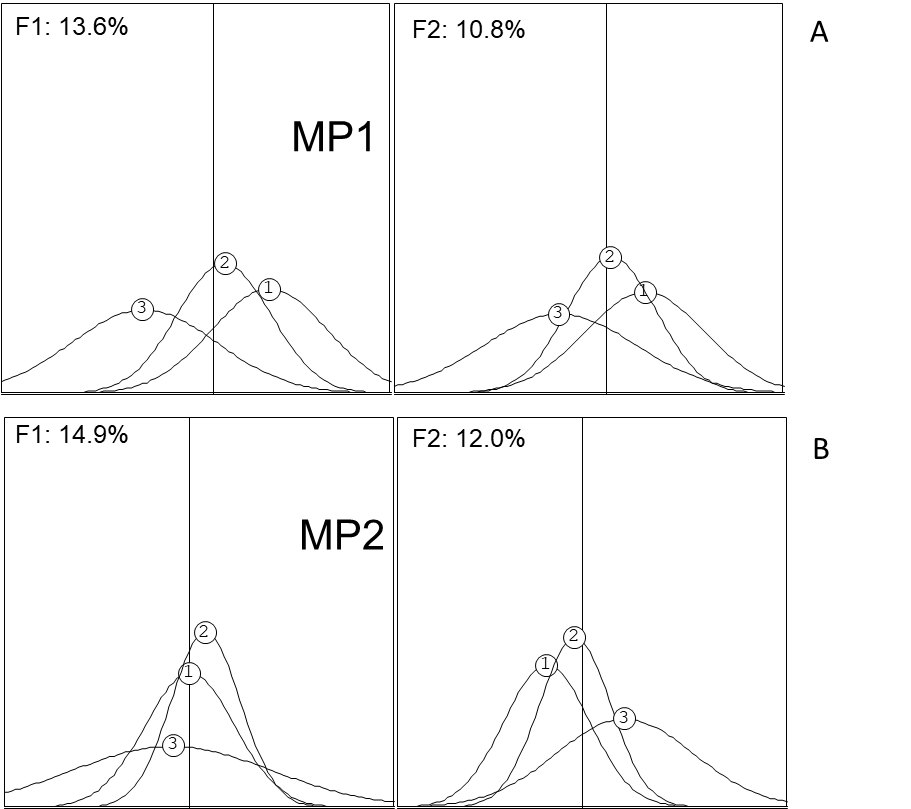
Fig 3. Principal component analysis (PCA) factorial plans of two citrus orchards MP1 (A) and MP2 (B) containing three disease categories respectively, of which the positions on the factorial plans are shown as Gauss curves along the first (F1) and second (F2) axis. Each Gauss curve represents the same area, but the basis is proportional to the variability within each category.
compared to healthy trees in MP2. Furthermore, in MP2, the leaf Na levels were significantly higher in category 3 than in category 1 trees. Altogether this supports the importance of high Na levels in leaves as being a parameter associated with declining trees.
Results further indicated that soil or leaf Mg, Ca, K, B, P and pH, albeit in either one of the orchards, were determining parameters in soil and leaves as was evident from their high factorial values in either category 1 or 3 trees. In a study conducted by Srivastava and Singh (2009), soil properties of diseased and healthy trees were compared and it was found that Ca values (mg/kg) were significantly lower in the soil of diseased trees. In MP2, the Ca/Mg ratio was also higher in the healthy trees compared to the diseased trees, while the Ca/Mg ratio of the healthy trees in MP2 was also higher than the ratio of the healthy trees of MP1 (Fig. 5), possibly providing another substantiating reason why this orchard was not as healthy as MP2. Conversely, ratios can be too high (>7) also resulting in nutritional deficiencies in trees (Aso and Bustos, 1981).
One of the first recommendations that emerged from our study is to reinforce the contribution of four-leaf elements (Mo, B, Zn and Na) in the orchards most affected by the decline of trees. Additionally, leaf Mg, Ca, K, and P may also be important. As for the other elements, none were necessary directly linked to disease incidence but these soil and leaf nutrients are known to play an important role in citrus health, vigour and yield as individual elements as well as in balance with other elements.
To further assess the interaction of some of these elements, a mathematical artificial index was calculated for each of the tree categories to simulate interactions between the elements and highlight the importance of these interactions. In MP1, the calculated indices were significantly different between both tree categories for both the healthy tree index (Na soil + Ca soil + Fe leaf), as well as the diseased tree index (B leaf + Mo leaf + % sand). This strengthens the idea that interactions play an important role in tree health and that health or disease susceptibility is not linked to a specific element but rather a combination of parameters. In MP2, only the calculated index for the diseased trees differed significantly, while the calculated index for the healthy trees was not significantly different between the two tree categories. This can probably be explained by the fact that the parameters differentiating the three tree categories in MP2 were positioned along the F2 axis only.
It may be possible to manipulate some of the above-mentioned parameters to induce a change in the soil environment in the root zone of trees in orchard MP1 so that it can become more similar to the root zone of trees in orchard MP2 since these trees showed superior root and tree health. Of course, experimental studies will have to be conducted to investigate the impact of the manipulation of soil nutrient balances such as Mg and Ca levels. No examples could be found of studies in citrus where the manipulation of the nutrients or potential impact of interactions between elements in the soil and or leaves were studied to reduce disease pressure.
The DA showed that the nutrient elements in the leaves were strong indicators of changes in the tree categories since a clear separation between healthy and declining categories was observed for both orchards for leaf parameters (Fig. 6). It, however, also confirmed that the leaf elements that are indicative of declining trees were site-specific. It is therefore important to have a history of leaf analyses to identify changes in leaf nutrient concentrations over time. Since there is no fixed trend in either a decrease or increase of elements in declining trees, as well as a fixed threshold level for trees deteriorating over time, it seems that comparison of annual leaf analyses to detect changes in nutrient concentrations has value. Causes for such changes should be investigated to avoid progressive decline of the trees. Additionally, leaf samples of trees with different levels of disease should be compared before a conclusion is drawn and recommendations are made. It is worth mentioning that interactions between the nutrient elements, more than the actual values, probably play a major role in development or inhibition of declining trees. Further studies will be required to confirm the value of correcting nutrient ratios in trees to slow down
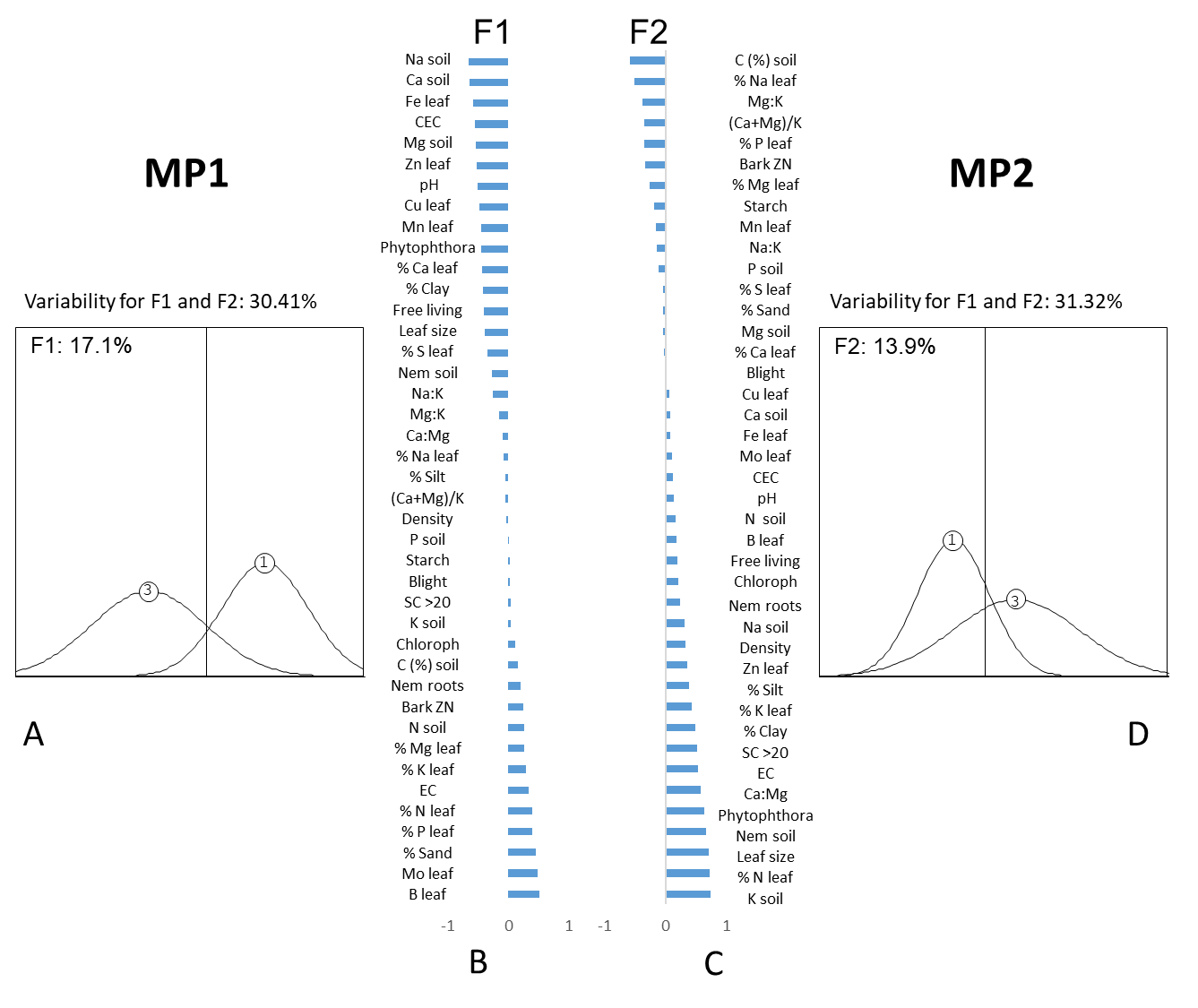
Fig 4. Principal component analysis (PCA) factorial plans of two citrus orchards MP1 (A) and MP2 (D) containing two disease categories respectively, of which the positions on the factorial plans are shown as Gauss curves along the horizontal (F1) and vertical (F2) axis. Each Gauss curve represents the same area, but the basis is proportional to the variability within each category. The 41 parameters that were evaluated in PCA analysis for orchard MP1 (B) and orchard MP 2 (C) are sorted according to their F1 (for MP1) and F2 (for MP2) values respectively. The height of the histogram bars indicates the size of the factorial values, and whether the parameter increased (positive value) or decreased (negative value) for a disease category relative to the other disease categories.
the progress of tree decline, as soon as the tree decline syndrome has been identified.
A similar tendency was observed with Ca and Mg ratios for both orchards and both categories (Fig. 5). The results indicate that a higher Ca:Mg ratio was linked to improved health as seen in MP2. Srivastava & Singh (2009) studied soil fertility and plant nutrition linked to citrus decline and when calculating Ca against Mg ratios, they were also higher in the healthy trees compared to the declining trees, even if this was not significant. It confirms that differences can be minimal, yet still result in considerable differences especially since the elements cannot be isolated and should be evaluated in relation to the other elements and through their interactions.
Materials and methods
Orchard trial sites
Two experimental sites (MP1 and MP2) with decline symptoms were selected in the Mpumalanga province, South Africa. Orchard MP1 was located near Karino (25°27'0.37"S; 31°4'39.14"E) and contained 20-year-old Delta Valencia (Citrus sinensis Osbeck) trees on Rough Lemon (Citrus jambhiri) rootstock. Orchard MP2 was located near Nelspruit (25°26'4.23"S; 30°59'36.95"E) and contained
19-year-old Midknight Valencia (C. sinensis) trees on Rough Lemon rootstock.
The orchards contained healthy trees and a range of trees that differed in their stages of decline. The declining trees were distributed randomly throughout the orchard and not in a pattern normally followed by a developing disease where adjacent trees are diseased.
Three ‘decline’ categories were designated to the selected trees with: category 1 containing visually healthy trees (control); category 2 containing trees with smaller, yellowish leaves and a less dense tree canopy appearance, and category 3 containing trees with a combination of typical root damage or root disease related problems (twig die-back in upper parts of the trees, small, discoloured leaves and a considerably less dense tree canopy) (Fig. 1). Fifteen trees belonging to each category were randomly chosen in both orchards, resulting in a total of 45 data trees per orchard. Although the three categories were present in both orchards, orchard MP2 contained fewer declining trees than MP1.
The experimental orchards followed similar management and production practices to ensure that fruit from the orchards can be exported, which included specific fertilization and pest and disease control (nematodes and Phytophthora respectively) programs. Standard fertilization programmes were applied according to
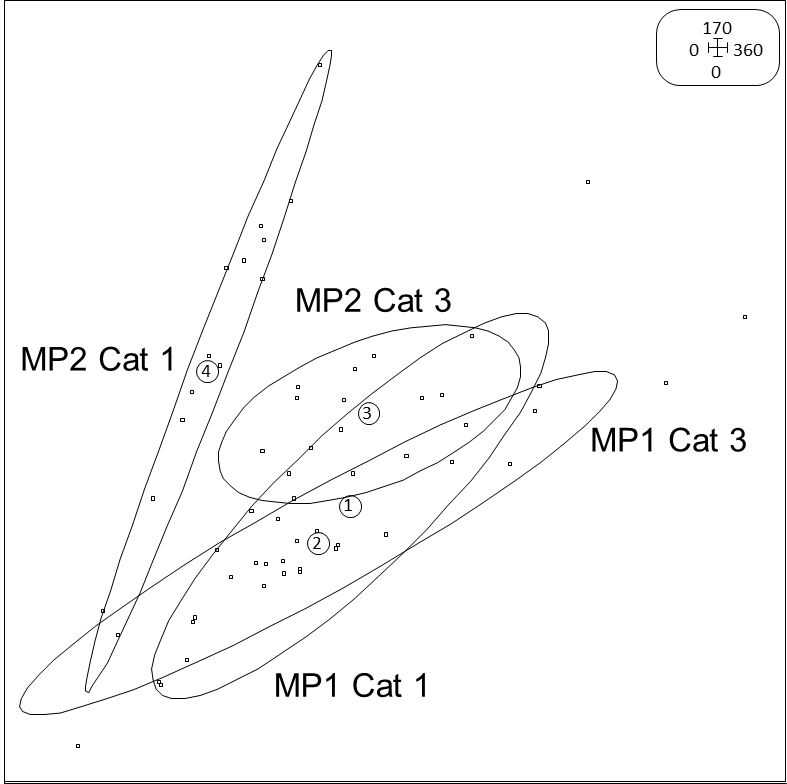
Fig 5. Scatter graph of the soil Ca and Mg values measured in two orchards (MP1 and MP2) for healthy (Cat1) and declining (Cat 3) tree categories. Eclipses indicate the variance per category and orchard.
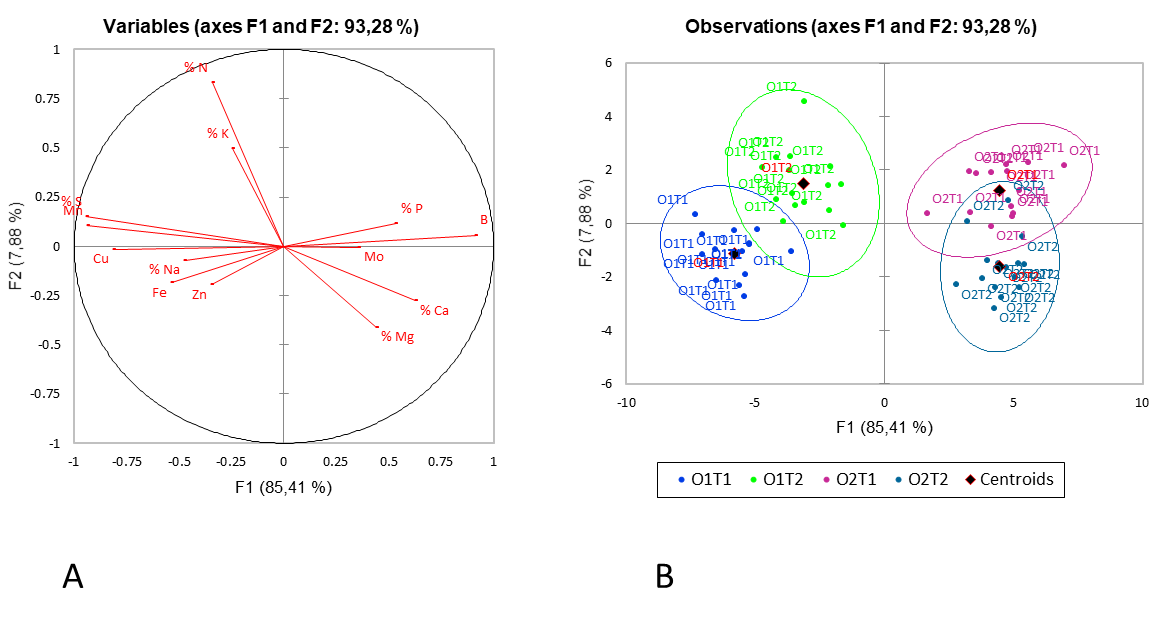
Fig 6. Discriminant analysis for 13 leaf parameters between healthy (T1) and declining (T2) trees in orchard MP1 (O1) and orchard MP2 (O2). The correlation chart on the left (A) and the observation chart on the right (B), indicate a separation between the two disease categories (T1 and T2), as well as the two orchards MP1 & MP2 (O1 and O2).
recommendations based on the soil and leaf analyses collected during autumn. All trees within the orchard were treated similarly when management practices were applied. Both orchards utilised a micro-jet irrigation system, while the soil type was loamy sand (85% sand, 10% silt and 5% clay in MP1 and 86% sand, 11% silt and 3% clay in MP2).
Sampling time of soil, leaf, root and trunk parameters
A combination of abiotic and biotic factors, totalling 41 parameters, were measured at each of the experimental trees in both orchards. The measured parameters included 22 soil associated parameters, 15 leaf associated parameters, two root associated parameters and
two trunk associated parameters. Soil, root, leaf and trunk sampling and analyses were performed in May 2012 and 2013 (2-year period) during the scheduled routine leaf and soil analysis for fertilisation, i.e., before harvest, on each of the experimental trees. None of the selected trees changed their status from one category to another over the 2-year period.
Soil associated parameter analyses (22 abiotic and biotic parameters)
Soil chemical and physical parameters (abiotic). Soil samples were collected as described by Coetzee (2018), with each soil sample consisting of 100 g of soil per tree, collected at a maximum depth of 20 cm in the root-zone under the tree canopy. The samples, collected during the same week were placed in cooler boxes and sent to Nvirotek Laboratories (Pty) Ltd (Hartebeespoort dam, South Africa) for analysis. The analysed soil elements included calcium (Ca) (mg/kg), magnesium (Mg) (mg/kg), phosphate (P) (mg/kg), nitrogen (N) (mg/kg), carbon (C) (%), sodium (Na) (mg/kg), and potassium (K) (mg/kg). Additionally, salt-value (cmol (+)/kg), pH, electrical conductivity (EC) (mS/m), density (g/cm3), and percentage sand, silt and clay were determined. The analyses were conducted by Nvirotek Laboratory (Pty) Ltd according to the methods described in The Non-Affiliated Soil Analysis Work Committee (1990). The following ratios were also included in the evaluated parameters: Na:K, Mg:K, Ca:Mg and (Ca + Mg)/K.
Soil compaction (abiotic). Soil compaction was measured using a penetrometer. Readings (three per tree) were collected for each tree in the root-zone under the tree canopy at a depth of 0-5 cm, 5-10 cm and deeper than 20 cm.
Soil citrus nematode and free-living nematodes (biotic). Soil (250 g) samples were collected at a maximum depth of 20 cm as one composite sample in the root-zone under the tree canopy. The composite sample consisted of three subsamples taken from the root-zone around the trunk. The number of second stage citrus nematode larvae (J2) and free-living nematodes in the soil were determined according to the method of Whitehead and Hemming (1965).
Soil Phytophthora inoculum (biotic). Soil samples (200 g) were collected from each tree at a maximum depth of 20 cm in the root-zone under the tree canopy. Phytophthora nicotianae population densities in the soil were determined semi-quantitatively with the leaf baiting technique according to a standard procedure (Grimm and Alexander, 1973) followed at the Citrus Research International (CRI) Diagnostic laboratory (Nelspruit, South Africa).
Leaf associated parameter analyses (15 abiotic parameters)
Leaf chemical characteristics. Twenty randomly collected leaves were sampled per tree as described by Coetzee (2018) and were sent to Nvirotek Laboratories (Pty) Ltd for analysis. Leaf nutrient concentration (B (mg/kg), Cu (mg/kg), Fe (mg/kg), Mn (mg/kg), Mo (mg/kg), Zn (mg/kg), Ca (%), K (%), Mg (%), N (%), Na (%), P(%) and S (%)) was analysed by Nvirotek Laboratories (Pty) Ltd (Hartebeespoort dam, South Africa) using routine analysis protocols according to methods described in Kalra (1998).
Leaf size. Leaf size was determined by randomly collecting 30 leaves per tree and measuring the leaf surface area (mm2) using a Li-3100 area meter (Li-Cor Inc. Lincoln Nebraska USA).
Chlorophyll content. Thirty leaves per tree were sampled and chlorophyll content was determined by means of a mobile SPAD meter (SPAD 502 Plus - Chlorophyll Meter, Spectrum Technologies, Inc., Aurora, Illinois, USA).
Root associated parameter analyses (two abiotic and biotic parameters)
Starch content (abiotic). Starch content (mg/kg) in the roots was determined using 100 g fibrous roots of each sampled tree, collected up to a depth of 20 cm. Roots were washed, placed in brown paper bags, dried in an industrial oven (70°C for 24 hours), grounded to a fine powder and analysed by Nutrilab (Department of Animal and Wildlife Sciences, University of Pretoria, Pretoria, South Africa), according to the AOAC official method (996.11-Total Starch in Cereal Products) (AOAC, 1984).
Citrus nematode (biotic). Root samples (50 g) were collected up to a maximum depth of 20 cm in the root-zone under the tree canopy. Tylenchulus semipenetrans was extracted from the roots according to the method of Van der Vegte (1973), and the number of adult T. semipenetrans female nematodes in the roots was recorded per 10 g roots.
Trunk associated parameter analyses (two abiotic and biotic parameters)
Zinc content (abiotic). Samples of trunk wood and bark were collected by drilling a 1 cm deep hole in each tree trunk using a 1 cm wide wood drill bit. These samples were analysed by a commercial laboratory (Labserve (Pty) Ltd, Nelspruit, South Africa) for zinc content (mg/kg) using the digestion method of Bessinger (1985) and analysing the Zn concentration in the extract using a Varian ICP-OES optical emission spectrometer (Varian, Inc.).
Citrus blight (biotic). The Blight status of each tree was determined by using the Blight syringe water uptake test of Lee et al. (1984) which measures the quantity of water that can be injected into the tree trunk in a given period. A single hole was drilled into each tree trunk, with a battery-operated hand drill (3 mm drill bit), after which a 100 mℓ surgical disposable syringe without a needle filled with 10 mℓ of water was inserted into the hole. The time of water up-take was measured, and trees were allocated to either one of two categories namely < 30 s indicating a healthy tree and > 30 s indicating a Blight positive tree (Lee et al., 1984).
Statistical analyses
The data collected from each tree over the two years were averaged for multi- and univariate analyses. This was done to reduce variability that might have been introduced due to climatic differences between seasons. Multivariate analyses were conducted using the software package ADE-4 (Thioulouse et al., 1997). A PCA was performed on the 15 control trees (category 1) of each orchard, which indicated that the orchards were distinctly different (see Results). PCAs were therefore subsequently conducted for each orchard separately. Either three ‘decline’ categories or only two categories (healthy = category 1 trees and declining = category 3 trees) were used for the analyses of each orchard.
Data, based on multivariate analysis, were analysed using the statistical program Genstat for Windows 18th Edition. The “Student” t-test (5% significance level) was used to test whether the means of the different leaf, soil, root and trunk associated parameters for the two disease categories in both orchards (MP1 and MP2) differed significantly. Subsequently, a mathematical index (sum of the parameters) was calculated for each disease category for MP1 and MP2. The selected parameters used were based on the highest factorial values. For MP1, three parameters were selected while in MP2, only two parameters. A significant probability (P ≤ 0.05) indicates that the two groups’ means tested differ significantly at the 5% level.
Discriminant analysis as described by Rencher (2002) was used to assess if the leaf (13 parameters) and soil (14 parameters) parameters could be used to correctly predict/identify the health status of trees. For DA the size of the smallest group must be larger than the number of predictor variables.
Conclusion
The overall finding of our study was that several of the measured leaf and soil parameters were indicators of plant stress. Although the leaf nutrients reflected a difference between both tree categories, it is difficult to manipulate these directly while, the soil parameters on the other hand can be manipulated much easier. It is therefore important to determine the soil nutrients and their interactions that create a healthy soil rhizosphere. If the soil nutrient status can be manipulated in the diseased trees to simulate the soil conditions of the healthy trees, it is anticipated that the tree conditions will be rectified and trees will become healthier. Although each orchard will have a different ‘soil nutrient health status’ and therefore no easy warning, preventative or curative options are available, it is anticipated that serious losses due to gradual decline in tree appearances can be prevented. Leaf nutrient status can be additionally used to alert the grower of a potential decline.
Acknowledgements
Authors would like to acknowledge Regina Cronje from Agricultural Research Council-Tropical and Subtropical Crops, Nelspruit, South Africa, Dr. Wilma du Plooy, Charl Kotze and Elaine Basson, Citrus Research International, Nelspruit, South Africa for their valuable inputs with this manuscript.
References
AOAC (1984) Official methods of analysis, 14th ed. Association of Official Analytical Chemists, Inc., Arlington, Virginia, USA.
Aso PJ, Bustos VN (1981) Conditions relating Mg deficiencies in citrus orchards of Tucman. Rev Ind Agric Tucumán. 57:9-13.
Benfradj N, Migliorini D, Luchi N, Santini A, Boughalleb-M’Hamdi N (2017) Occurrence of Pythium and Phytopythium species isolated from citrus trees infected with gummosis disease in Tunisia. Arch Phytopathol PFL. 50:286-302. doi: 10.1080/03235408.2017.1305479
Bessinger F (1985) Methods of plant analysis by autoanalyzer techniques. Information Bulletin No. D-3. Soil and Irrigation Research Institute, Private Bag X79, Pretoria, South Africa.
Burnett HC, Nemec S, Patterson M (1982) A review of Florida citrus blight and its association with soil edaphic factors, nutrition and Fusarium solani. Trop Pest Manage. 28:416-422.
Coetzee JGK (2018) Fertilization of citrus. CRI Integrated Production guidelines. Volume 2:1-142. https://www.citrusres.com/system/files/documents/production-guidelines.
Combrink NJJ, Labuschagne N, Barnard RO, Kotze JM (1995) The effect of chloride on four different citrus rootstocks. S Afr J Plant Soil. 12(3):95-98.
Derrick KS, Timmer LW (2000) Citrus Blight and other diseases of recalcitrant etiology. Annu Rev Phytopathol 38:181–205.
Donovan N (2007) Managing sudden death in citrus. Primefact 755. NSW Department of Primary Industries.
DuCharme EP (1971) Tree loss in relation to young tree decline and sand hill decline of citrus in Florida. Proc Fl State Hortic 84:48-52.
El-Borai FE, Duncan LW, Graham JH (2002) Infection of citrus roots by Tylenchulus semipenetrans reduces root infection by Phytophthora nicotianae. J Nematol. 34(4):384-389.
Gottwald TR, Garnsey SM, Borbon J (1998) Increase and patterns of spread of Tristeza Virus infections in Costa Rica and the Dominican Republic in the presence of the brown citrus aphid, Toxoptera citricida. Phytopathology. 88:621-636.
Graham JH (1995) Root generation and tolerance of citrus rootstocks to root rot caused by Phytophthora nicotiannae. Phytopathology. 85:111-117.
Grimm GR, Alexander AF (1973) Citrus leaf pieces as traps for Phytophthora parasitica from soil slurries. Phytopathology. 63:540-541.
Hall DG, George J, Lapointe SL (2015) Further investigations on colonization of Poncirus trifoliate by Asian citrus psyllid. Crop Prot. 72:112-118.
Janse van Rensburg JC, Labuschagne N, Nemec S (2001) Occurrence of Fusarium-produced naphthazarins in citrus trees and sensitivity of rootstocks to isomarticin in relation to citrus blight. Plant Pathol. 50:258-265.
Kaiser BN, Gridley KL, Brady JN, Phillips T, Tyerman SD (2005) The role of molybdenum in agricultural plant production. Ann Bot. 96(5):745-754. doi: 10.1093/aob/mci226
Kalra YP (1998) Handbook of reference methods for plant analysis. CRC Press, London, 287pp.
Lee RF, Marais LJ, Timmer LW, Graham JH (1984) Syringe injection of water into the trunk: a rapid diagnostic test for citrus blight. Plant Dis. 68:511–513.
Malewar GU, Varade SB, Jadhav NS (1983) Evaluation of nutritional status of citrus orchards of Marathwada region (Maharashtra state) by leaf and soil analysis. In: Proc. Int. Citrus Symp., pp. 14 – 21. Bangalore, India: Indian Institute of Horticultural Research.
Maseko BOZ, Couthino TA (2002) Pathogenicity of Phytophthora and Pythium species associated with citrus root rot in South Africa. S Afr J Bot. 68:327-332.
Milad S, Bakhati HK, El-Hakim M, Moutafa FB (1975) The effect of different soil conditions upon citrus growth. Agric Res Rev. 53(3):31-40.
Nel DJ, Bennie TP (1984) Soil factors affecting tree growth and root development in a citrus orchard. S Afr J Plant Soil. 1:39-47.
Nemec S, Zablotowicz RM, Chandler JL (1989) Distribution of Fusarium spp. and selected microflora in citrus soils and rhizospheres associated with healthy and blight-diseased citrus in Florida. Phytopathology. 21:141-146.
Pretorius MC, Le Roux HF (2017) Nematode pests of citrus. In: Fourie H, Spaull VW, Jones RK, Daneel MS, De Waele D (eds.) Nematology in South Africa: A view from the 21st Century (pp. 151-182). Cham, Switzerland: Springer International Publishing. DOI:10.1007/978-3-319-44210-5.
Rencher AC (2002) Methods of Multivariate Analysis. 2nd Ed. Brigham Young University, John Wiley & Sons, Inc. ISBN 0-471-41889-7 (cloth), USA.
Safdar A, Javed N, Khan SA, Safdar H, Ul Haq I, Abbas H, Ullah Z (2013) Synergistic effect of a Fungus, Fusarium semitectum, and a nematode, Tylenchulus semipenetrans, on citrus decline. Pak J Zool. 45(3):643-651.
Schoeman M, Labuschagne N, Calitz F (2017) Efficacy of fungicides, plant resistance activators and biologcal control agents against guava wilt disease caused by Nalanthamala psidii. S Afr J Plant Soil. 34(2):119-124.
Singh V, Tripathi BR (1985) Studies on chlorosis in Sweet Orange in Agra Region of Uttar Pradesh. J Ind Soc Soil Sci 33(2):333-338.
Srivastava AK, Singh S (2009) Citrus decline: Soil fertility and plant nutrition. J Plant Nutr. 32:197-245.
Suit RF, DuCharme EP (1947) Citrus decline. Citrus Ind. 28(7):8-13.
Suit RF, Knorr LC (1949) Progress Report on citrus decline. Proc Fl State Hortic. 62:45-49.
The Non-affiliated Soil Analyses Work Committee (1990) Handbook of standard soil testing methods for advisory purposes. Soil Sci SA, Pretoria, South Africa.
Thioulouse J, Chessel D, Dolédec S, Olivier JM (1997) ADE-4: a multivariate analysis and graphical display software. Stat Comput. 7:75-83.
Van der Vegte FA (1973) A new method of estimating the numbers of citrus nematodes (Tylenchulus semipenetrans) in root samples. Nematol Soc S Afr Newsl. 4:11-12.
VSN International (2015) Genstat for Windows 18th Edition. VSN International, Hemel Hempstead, UK. Web page: Genstat.co.uk
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol. 55:25-38.
Young RH, Wutscher HK, Albrigo LG (1980) Relationship between water translocation and zinc accumulation in citrus trees with and without blight. J Am Soc Hortic Sci. 105:444-447.