

Aust J Crop Sci. 18(12):905-915 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p217
Influence of auxin on growth, physiology, and yield of potato exposed to high temperature episode
Charles, O. Obiero*1, 2, Stephen, P. Milroy1, Richard, W. Bell2
1Potato Research Western Australia, College of Science, Health, Engineering and Education, Murdoch University, 90 South Street Murdoch, Western Australia 6150
2Agriculture Discipline, College of Science, Health, Engineering and Education, Murdoch University, 90 South Street Murdoch, Western Australia 6150
*Corresponding author: Charles, O. Obiero 
ORCID https://orcid.org/0000-0002-0926-7495
Charles Obiero current address: Washinton State University, Irrigated Agricultural Research and Extension Centre, 24106 N Bunn Road, Prosser, WA, 99350
Abstract: Literature suggests that gibberellic acid (GA) controls growth in potatoes grown at temperatures above 25 °C. However, potato responses to high-temperature episodes are more consistent with known effects of auxins. It was hypothesized that auxin inhibition is responsible for potato response to a high-temperature episode. This study investigated the influence of an auxin, Indole-3-acetid acid (IAA) and an auxin inhibitor, 2,3,5-triiodobenzoic acid (TIBA) (Expt. 1) and four concentrations of IAA (0, 5, 15, 45 µM) (Expt. 2) on potato plants exposed to nine days of 30 °C in greenhouse experiments. Plants were grown at the control (22 °C) before and after the end of a high-temperature episode. Plant growth regulators (PGRs) (i.e., IAA and TIBA) and the high temperature were applied simultaneously, shortly after tuber initiation. TIBA had a similar impact on plant and tuber growth at 22 °C as did the 30 °C treatment on plants without plant growth regulators (No-PGRs). IAA did not change the growth of plants and tubers under the 30 °C treatment. However, both PGRs increased photosynthesis (leaf 7 emerged before high-temperature application) and chlorophyll concentration index (CCI) (same leaf 7 and leaf 3 that emerged at 30 °C). In conclusion, TIBA impaired plant and tuber growth similarly to the 30 °C episode but IAA did not overcome the impact of the 30 °C episode. These findings suggest that auxin related processes play a role in how potato plants respond to high temperature episodes.
Keywords: gibberellins; heat stress; plant growth regulators; photosynthesis; tuber growth.
Abbreviations: µM_micromolar; ANOVA_Analysis of variance; CCI_Chlorophyl concentration index; D/N_Day/Night; DAP_Days after planting; DDI_Double distilled water; Expt._Experiment; GA_Gibberellic acid; GLM_General linear model; IAA_Indole-3-acetid acid; LSD_Least significance difference; No-PGRs_No plant growth regulators; PGRs_Plant growth regulators; Proc REG_Procedure Regression; SAS_Statistical Analysis Software; TIBA_2,3,5-triiodobenzoic acid.
Introduction
Literature indicates that gibberellins control plant and tuber growth in potato plants grown at temperatures above 25 °C. Specifically, it is showed that gibberellic acid (GA) inhibits tuber initiation thus reduce the number of tubers produced per plant (Lovell and Booth, 1967; Menzel, 1983) and stimulates shoot growth and stem elongation hence increase shoot dry matter and plant height (Lovell and Booth, 1967; Fernie and Willmitzer, 2001; Çalışkan et al., 2021). It also reduces starch synthase activity at the tuber level leading to repartitioning of the carbon that is otherwise meant for the tubers, into the shoots and stolons (Lovell and Booth, 1967; Booth and Lovell, 1972; Mares et al., 1981; Çalışkan et al., 2021).
The evidence linking GA to growth responses of potato plants at high temperatures is, however, inconsistent. Firstly, there are two contrasting findings on the influence of GA on the production of tubers in potato plants. While some studies show more but small-sized tubers in potato plants treated with GA (Struik et al., 1989; Šimko, 1994; Herman et al., 2016), others show less number of tubers with GA (Lovell and Booth, 1967; Menzel, 1980; Hartmann et al., 2011). At high temperatures, however, potato plants are usually showed to have less number of tubers per plant than the control (Menzel, 1980; Midmore and Prange, 1992).
The second question relates to shoot elongation in potato plants exposed to high temperatures. Potato plants exposed to a high-temperature episode show shorter shoots only at the end of a high-temperature period. When grown back at cooler conditions after the end of a high-temperature episode, all treatments have same height or high-temperature treatments are taller than the control plants (Obiero et al., 2019). In the literature, there is strong evidence that links GA to elongated shoots in potato plants (Kumar and Wareing, 1972, 1974; Hartmann et al., 2011) and the same shoot elongation is also observed in potato plants grown at high temperatures (Lovell and Booth, 1967; Menzel, 1980; Carrera et al., 2000; Rykaczewska, 2015).
Lastly, the stimulation of shoot growth in potato plants exposed to high temperatures also requires further clarification. Our previous study showed reduced growth on the main shoots in plants that had been exposed to a high-temperature episode; both at the end of the high-temperature period and at the final sampling. But the lateral shoots grew more after the end of the high-temperature period (Obiero et al., 2019). Such an influence of high temperature on branching in potato plants at high temperatures has been demonstrated before (Fleisher et al., 2006). Yet even Fleisher et al. (2006) did not observe this in cooler conditions after the end of the high-temperature period but only in potato plants that were under consistent influence of high temperatures. Further, such a shift in the shoot architecture of potato plants has never been demonstrated in any GA related studies (Lovell and Booth, 1967; Booth and Lovell, 1972; Sharma et al., 1998) nor in high temperatures studies where the reduction in tuber growth is linked to stimulated shoot growth (Menzel, 1980, 1983, 1985; Timlin et al., 2006).
Reduced growth and shorter main shoots at the end of high-temperature period combined with resumption of shoot elongation and rapid lateral shoot growth at cooler conditions following the end of the high-temperature (Obiero et al., 2019, 2022) are more like a response to an auxin effect. Auxins are known to control apical dominance (Cline, 1994; Kieber and Schaller, 2014) and disruption of their production and/or stimulation of cytokinin normally decrease the apical dominance and promote lateral bud development (Thimann, 1937, 1939; Herman et al., 2016). An auxin influence has been implicated in plant and tuber growth of potato plants (Harmey et al., 1966; Ponnampalam and Mondy, 1986; Farhan et al., 2010). However, little is known on the possible involvement of an auxin mechanism in potato plants exposed to high temperatures. There is also little understanding of how auxins might influence whole plant performance of potato in terms of leaf production, growth, and carbon partitioning especially to the tuber. Further, studies involving GA always attribute the response of potato plants directly to gibberellins. However, there are cases where the responses could be auxin-linked. For instance, Booth and Lovell (1972) attributed both the decrease in starch and the increase in sugars in the tubers of the potato cv. Majestic plants to GA but the shoot apices of the plants were removed which means an auxin metabolism was altered.
It was hypothesized that auxin inhibition is responsible for potato response to a high-temperature episode. This study investigated the influence of two plant growth regulators (PGRs) (an auxin; Indole-3-acetic acid (IAA) and an auxin inhibitor 2,3,5-triiodobenzoic acid (TIBA)) on plant and tuber growth of potato exposed to a 9-day episode of 30 °C. Then, evaluated the influence of four concentrations of IAA on potato plants exposed to the same 9-day episode of 30 °C. TIBA is known to inhibit auxin mechanism in plants including potato (Tsai and Arteca, 1984; Roumeliotis et al., 2012; Balla et al., 2016).
Results
In Expt. 1, the high temperature significantly reduced the whole plant (p = 0.002), the below-ground (p = 0.0001) and the tuber (p = 0.0001) dry matter but not the whole plant leaf and the shoot dry matter at the end of the experiment (Fig 1). The PGRs treatments also significantly influenced the whole plant (p = 0.0001), the below-ground (p = 0.002) and the tuber (p = 0.006) dry matter but also the whole plant leaf (p = 0.0001) and the shoot dry matter (p = 0.0001). However, the interactions of temperature and PGRs treatments had no significant influence on the whole plant, whole plant leaf, shoot, below-ground, and tuber dry matter per plant.
The 30°C treatment had 17% less whole plant, 30% less below-ground and 32% less tuber dry matter than the control plants. TIBA-treated plants had 33% less whole plant, 37% less whole plant leaf, 40% less shoot, 28% less below-ground and 19% less tuber dry matter per plant than the No-PGRs plants. Those of the IAA-
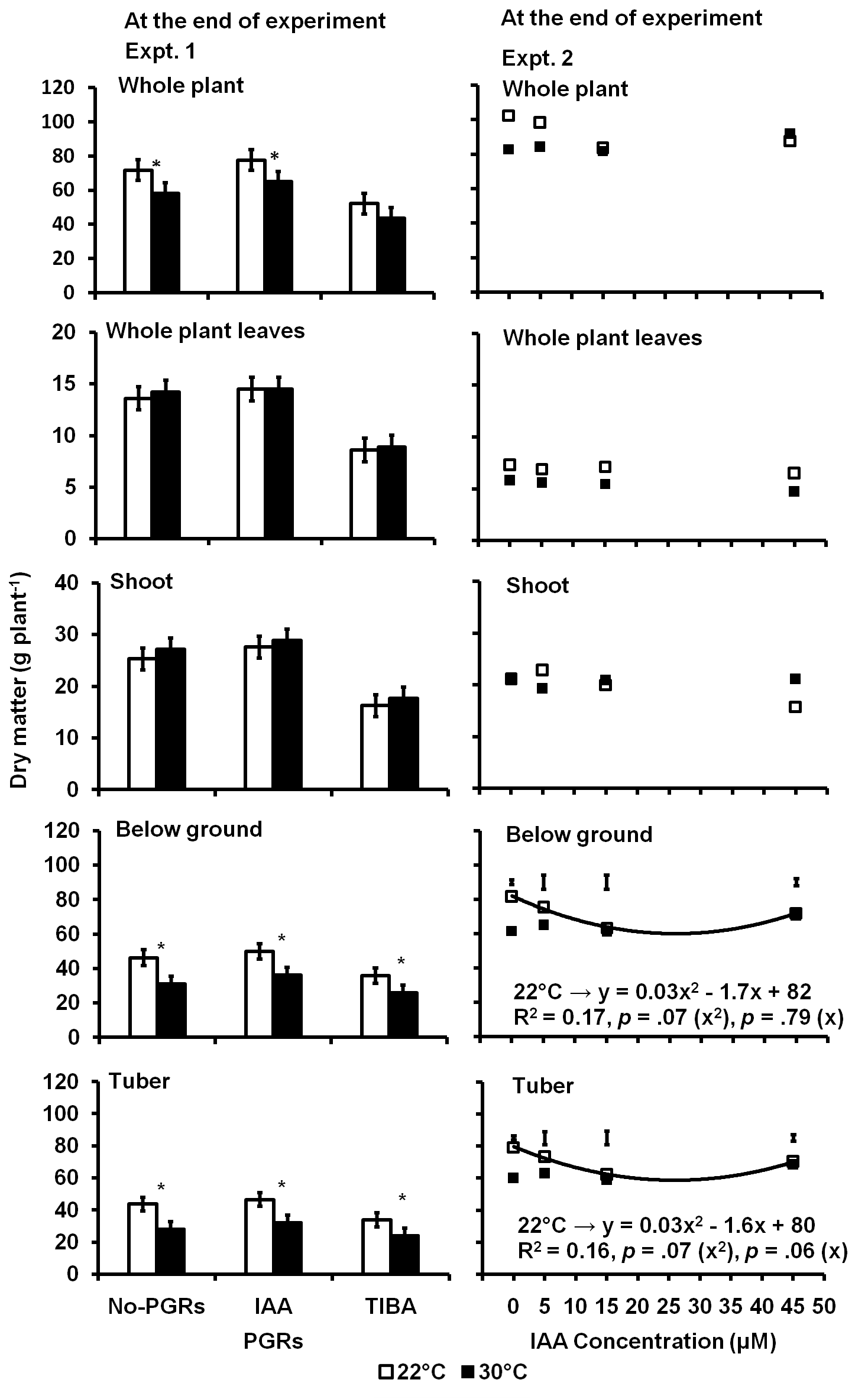
Fig 1. Whole plant, whole plant leaf, shoot, below ground and tuber dry matter per plant as affected by IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation. Data at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD 5% level of probability. * = significant means. Only significant regressions are shown.
treated plants were not significantly different from the No-PGRs treatments. The whole plant, whole plant leaf, shoot, below-ground, and tuber dry matter per plant in plants exposed to the 9-day episode of 30 °C was the same with or without IAA application. The whole plant and below ground dry matter in TIBA-treated plants in the control (22 °C) was also not different from those of the No-PGRs plants in the 30°C treatment. But the tuber dry matter in the TIBA-treated plants in the control (22 °C) was reduced in a similar manner as those of the No-PGRs in the 30 °C treatment.
In Expt. 2, the temperature marginally reduced the whole plant (p = 0.06) and the whole plant leaf (p = 0.09) dry matter per plant but significantly affected the below-ground (p = 0.03) and the tuber (p = 0.02) dry matter per plant at the end of the experiment. The IAA concentrations only caused a marginal (p = 0.09) increase on the shoot dry matter per plant but had no significant effects on the whole plant, the whole plant leaf, the below-ground and the tuber dry matter per plant. The interaction of temperature and IAA concentration had no significant impact on the whole plant, the
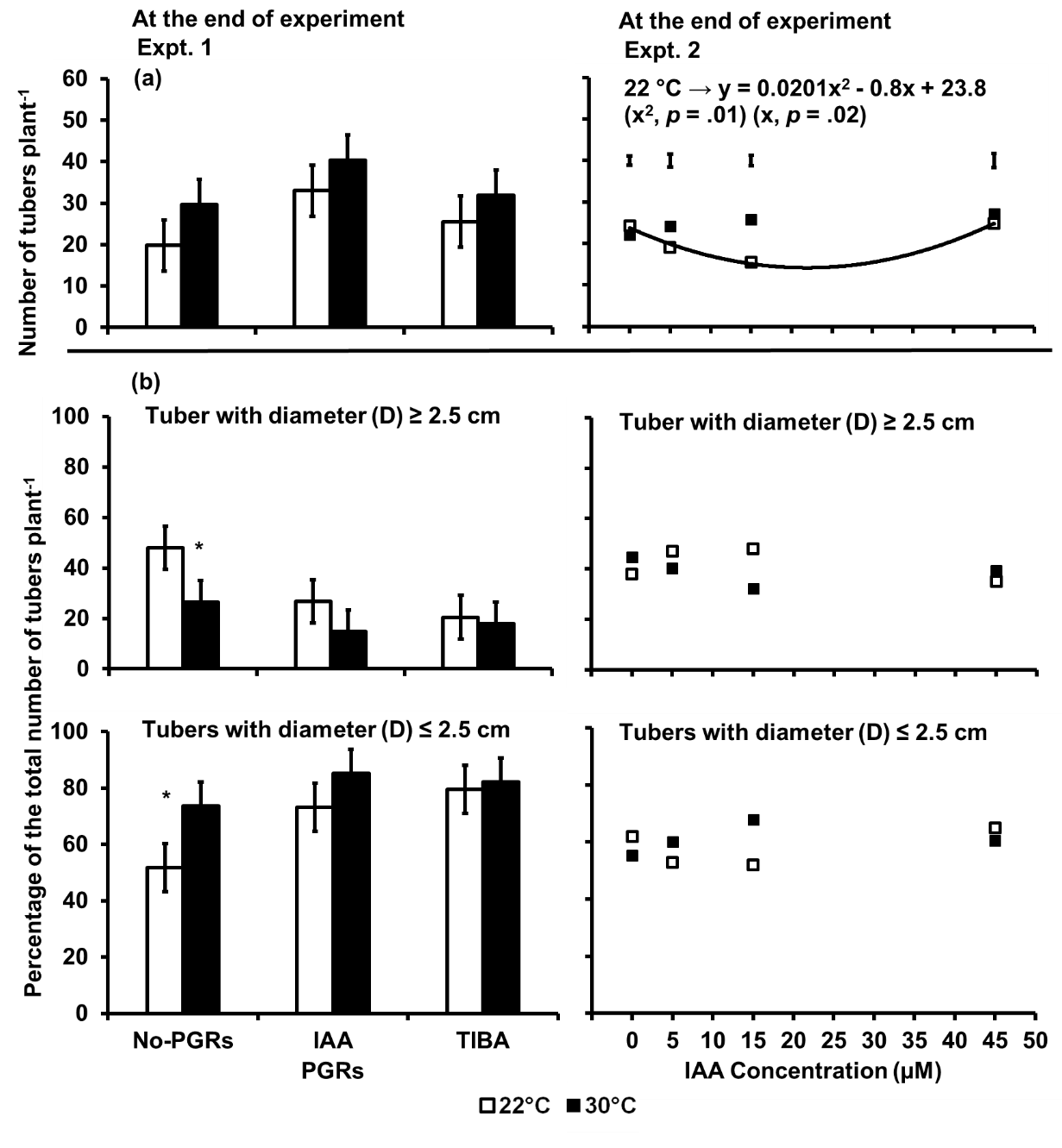
Fig 2. (a) The total number of tubers per plant and (b) Percent size distribution of tubers per plant as influenced by IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation. Tubers were categorized based on the diameter (D) ≤ 2.5 or ≥ 2.5 cm at the widest part. Data at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means. Only significant regressions are shown.
whole plant leaf, the below-ground, or the tuber dry matter but it significantly (p = 0.006) reduced the shoot dry matter per plant. The 30 °C treatment had 8% less whole plant, 12% less below-ground and 13% less tuber dry matter per plant than the control plants. When the untreated plants (treatments with 0 ppm IAA concentrations) were compared, the 30 °C treatment had 25% less tuber dry matter per plant than the control plants.
In Expt. 1, both the temperature and the PGRs significantly influenced the total number of tubers per plant and their size distribution but not the interaction between them at the end of the experiment (Fig 2). Plants in the 30 °C treatment had 23% more tubers per plant and 38% fewer tubers with a diameter greater than 2.5 than in the control. The IAA-treated plants had 33% more tubers than the No-PGRs plants but the number of tubers per plant in the TIBA treatment was not significantly different from the No-PGRs plants. Plants treated with either IAA or TIBA had the same total number of tubers per plant as those in the 30 °C treatment. There were 45% fewer tubers with a diameter of greater than 2.5 cm in the IAA and TIBA-treated plants than in the No-PGRs plants.
However, the number of tubers with a diameter greater or less than 2.5 cm in either IAA or TIBA-treated plants in the control were not different from those in the 30 °C treatment.
In Expt. 2, there was no significant effect of the temperature, the IAA concentration or the interaction between temperature and the IAA concentration on the total number of tubers per plant or on the distribution of tubers per plant.
In Expt. 1, the leaf emergence (Fig 3(a)) and the shoot elongation (Fig 3(b)) above the seventh leaf tagged before the high temperature treatment was applied was significantly influenced by temperature only at the end of the high temperature period. However, temperature influenced the elongation of the whole shoot (Fig 3(c)) at both the end of the high temperature period and at the final sampling. The influence of PGRs on the leaf emergence, the elongation of the shoot above the seventh leaf tagged before the high temperature treatment was applied and the elongation of the whole shoot was only significant at the final sampling. The interaction between temperature and PGRs only influenced the
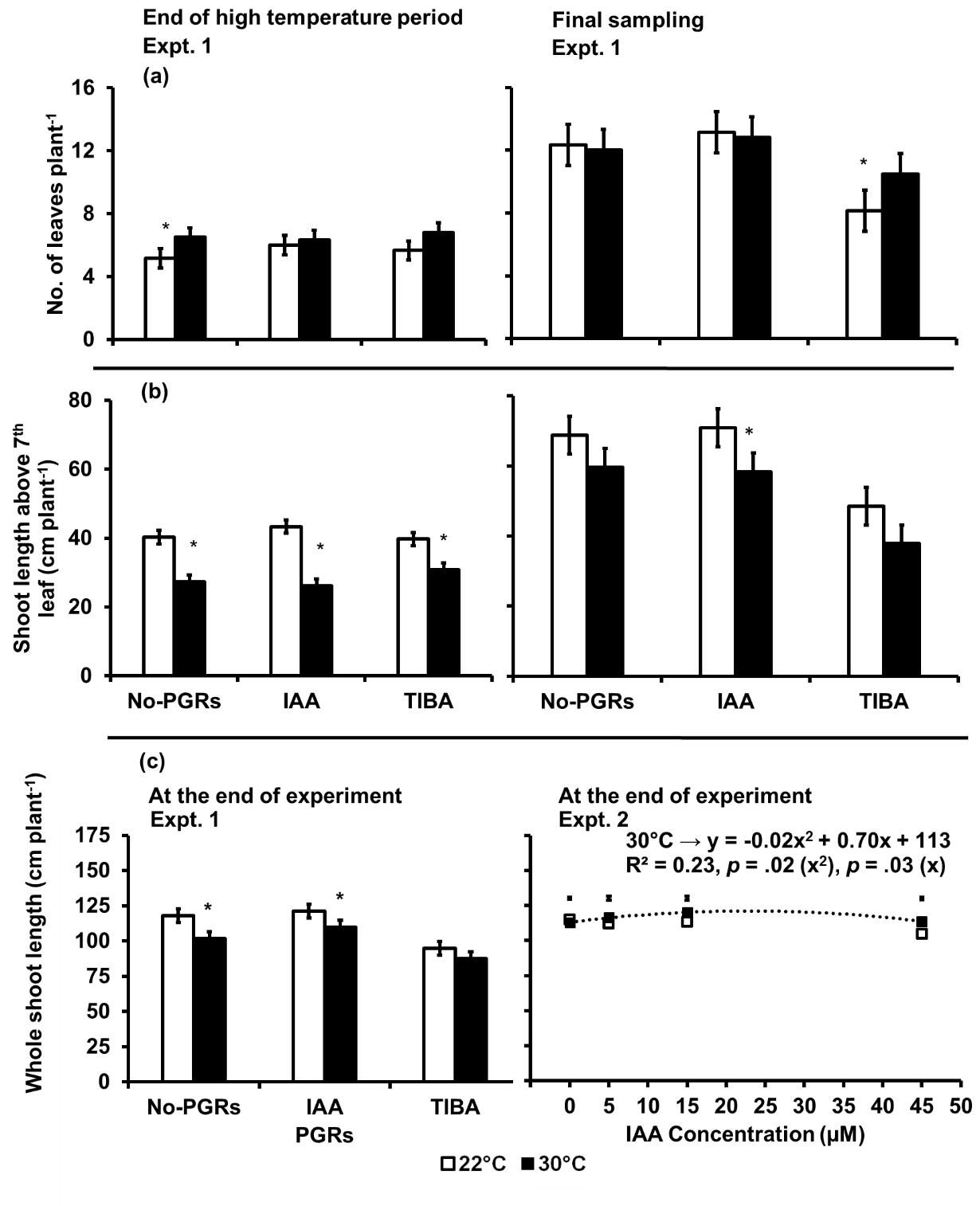
Fig 3. (a) Emergence of leaves above the seventh leaf, (b) Shoot length above the seventh leaf and (c)Whole shoot length per plant as influenced by the IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation. Data on emergence of leaves and shoot length above the seventh leaf are at end of the high temperature period and at the final sampling of Expt. 1. Those on the whole shoot length are at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means. Only significant regressions are shown.
elongation of shoot above the seventh leaf tagged before the high temperature was applied.
At the end of the high-temperature period, plants in the 30 °C treatment had 7% more leaves and 10% shorter shoot length above the seventh leaf than the control plants. At the final sampling, plants in the 30 °C treatment had 17% shorter shoot length above the seventh leaf than the control plants. The number of emerged leaves in the IAA and No-PGRs plants were not significantly different but TIBA-treated plants had 15% fewer emerged leaves than either the IAA-treated or the No-PGRs plants. The TIBA-treated plants had 17% shorter whole shoot length than the No-PGRs plants. The whole shoot length was the same with or without the IAA application in plants in the 30 °C treatment. However, the whole shoot length of the TIBA-treated plants at 22 °C was not different from the No-PGRs treatment at 30 °C. Plants treated with either IAA or TIBA in the 30 °C treatment had shorter shoot lengths above the seventh leaf than plants in the control at the end of the
high-temperature period. Further, the IAA-treated plants had shorter lengths than the TIBA-treated plants in 30 °C treatment.
In Expt. 2, neither temperature (p = 0.1), IAA concentration (p = 0.2) nor the interaction between temperature and IAA concentration (p = 0.53) had any significant effect on elongation of the whole shoot length in the potato plants.
In Expt. 1, the main shoot dry matter per plant at the end of the experiment was influenced only by the PGRs (p = 0.0001) while the dry matter partitioned to the lateral shoot compared to the main shoot only by the temperature (p = 0.0001) (Fig 4). Plants treated with TIBA had 38% less main shoot dry matter than the No-PGRs or the IAA-treated plants. The lateral shoots had 76% more dry matter compared to the main shoots of plants in the 30 °C treatment than in the control.
In Expt. 2, the main shoot dry matter at the end of the experiment was influenced only by the interaction between temperature and
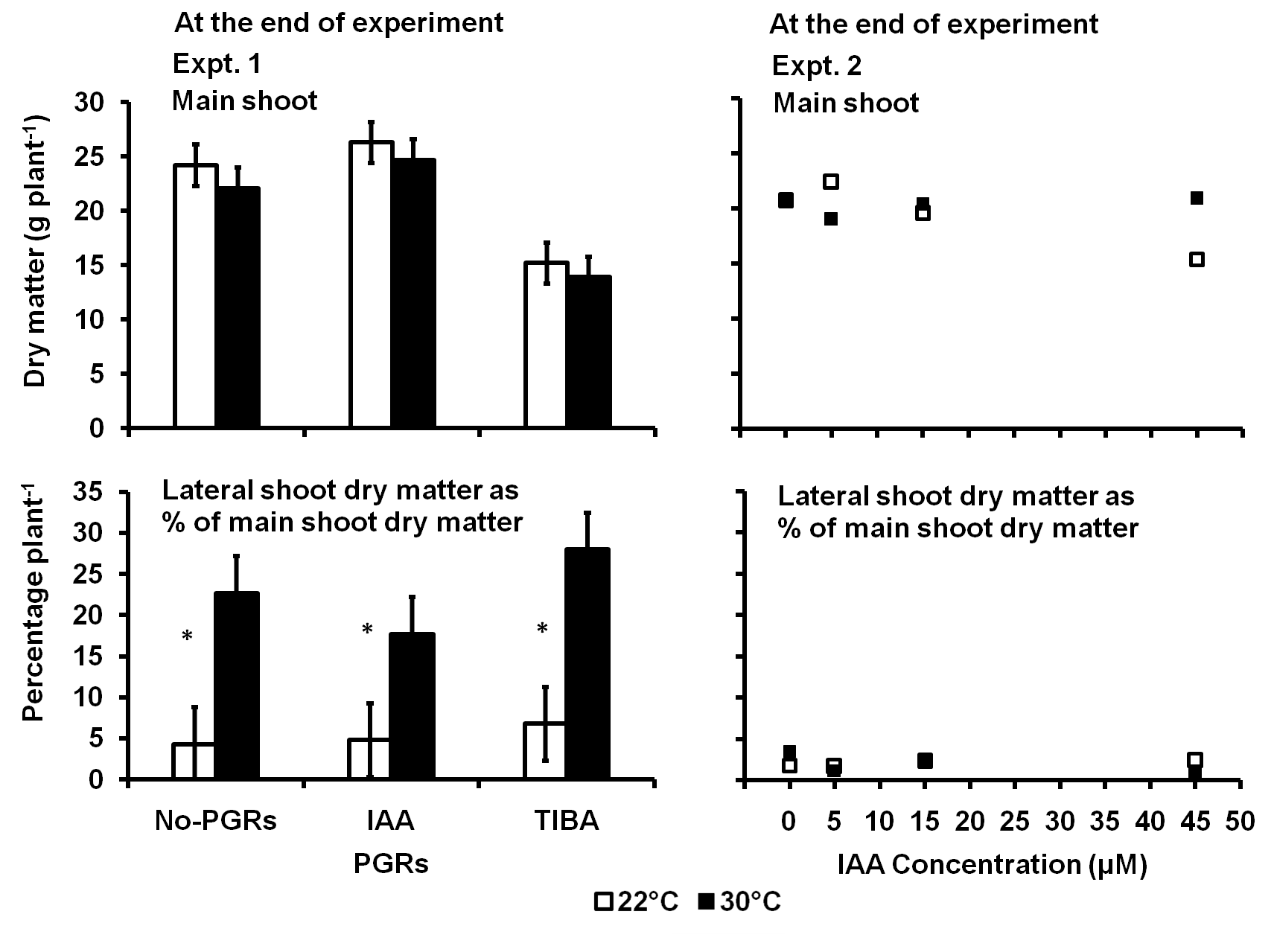
Fig 4. The main shoot dry matter and the dry matter on the lateral shoot as a percent of the main shoot dry matter per plant as influenced by IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day of 30°C applied shortly after tuber initiation. Data at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means.
the IAA concentration (p = 0.004). The main shoot dry matter per plant did not change with an increased concentration of IAA in the 30 °C treatments. However, the main shoot dry matter per plant in the control was less with increased concentration of IAA. Plants treated with 45 µM of IAA in 30 °C had 26% more dry matter on main the shoots than in the control.
In Expt. 1, the high temperature significantly increased the proportion of the dry matter partitioned to the shoot by 23% but decreased the proportion partitioned to the tubers by 17% at the end of the experiment (Fig 5). TIBA-treated plants partitioned 12% more dry matter to the shoot but 8% less dry matter to the tubers compared to the No-PGRs plants. The IAA-treated plants partitioned the same amount of dry matter to the shoot and tuber as the No-PGRs plants. However, there was no significant interaction effect between the temperature and the PGRs on dry matter partitioned either to the shoot or the tubers.
In Expt. 2, there was no significant influence of the temperature, the IAA concentration or the interaction between the temperature and the IAA concentration on dry matter partitioning in potato plants at the end of the experiment.
In Expt. 1, there was 29% less whole plant leaf area, 44% less main shoot leaf area and 83% more leaf area on the lateral shoots compared to the main shoot in plants in the 30 °C treatment than in the control at the end of the experiment (Fig 6). Plants treated with IAA had no statistically different whole plant and main shoot leaf area compared to the No-PGRs plants. However, the TIBA-treated plants had 48% less whole plant and 39% less main shoot
leaf area than the No-PGRs plants. Plants treated with either the IAA or TIBA were not significantly different from the No-PGRs plants in terms of the leaf area on the lateral shoots compared to the main shoot, but the TIBA-treated plants had 52% more leaf area on the lateral shoots compared to the main shoot than the IAA-treated plants. The interaction of the temperature and the PGRs was not significant for the whole plant or the main shoot leaf areas. However, the IAA-treated plants in 30 °C had less leaf area on the lateral shoots compared to the main shoot than either the No-PGRs or TIBA-treated plants. In 22 °C, none of the PGRs treatments was significantly different from the No-PGRs plants.
In Expt. 2, plants in 30 °C treatment had 26% less whole plant leaf area, 27% less main shoot leaf area and 42% more leaf area on the lateral shoots compared to the main shoots than in control. However, the concentration of IAA or the interaction of temperature and the IAA concentration was not significant.
In Expt. 1, at the end of the high-temperature period, the terminal leaflet of the seventh leaf in the PGRs-treated plants in 30 °C treatment was greener than the No-PGRs plants in the same 30 °C treatment (Fig 7). At the final sampling, plants in the 30 °C treatment had 15% less green terminal leaflet of the seventh leaf than in the control.
At the end of the high-temperature period in Expt. 1, the chlorophyll concentration index (CCI) in the terminal leaflet of the third leaf was the same with or without IAA application in 30 °C treatment. The CCI in the terminal leaflet of the third leaf in the TIBA-treated plants in the control was also not different from the No-PGRs plants in 30 °C treatment. At the final sampling, the
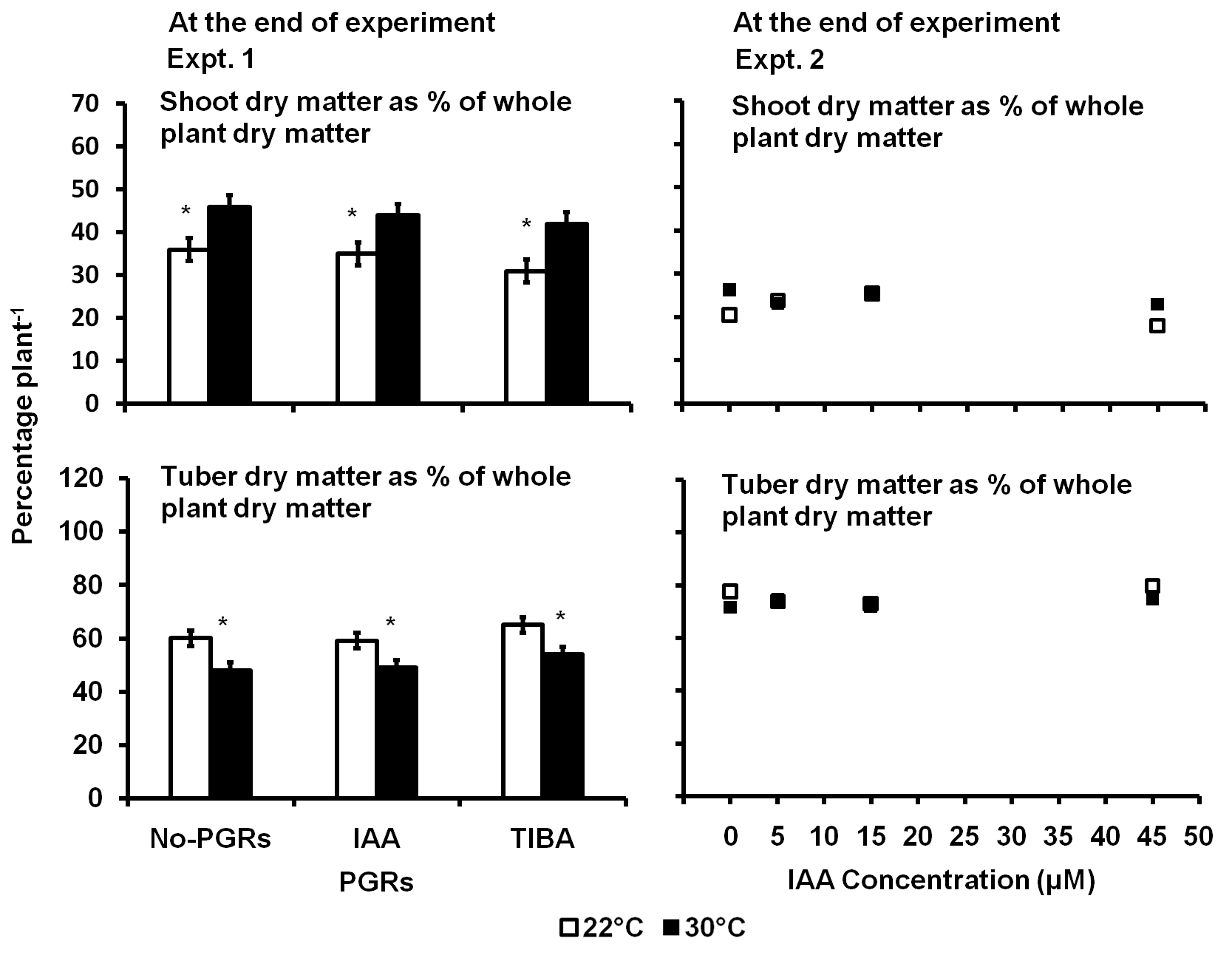
Fig 5. The proportion of dry matter partitioned to the shoot and tubers as influenced IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation. Data at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means.
terminal leaflet of the third leaf in the TIBA-treated plants was 36% less green in the 30 °C treatment than in the control.
In the 30 °C treatment, the IAA and TIBA-treated plants had higher photosynthesis per unit area of the terminal leaflet of the seventh leaf both during the high-temperature period and after plants were grown back at cooler conditions; except at the beginning of the high temperature treatment (IAA and TIBA-treated plants) and 64 DAP (in TIBA-treated plants) (Fig 8). In the control, the IAA-treated plants had slightly higher photosynthesis than the No-PGRs plants during the high-temperature period. However, six days before the final sampling, the rates in the IAA-treated plants were slightly lower than in the No-PGRs plants. The TIBA-treated plants initially had slightly higher photosynthesis at the beginning of the high temperatures than the No-PGRs plants. But the rates in the TIBA-treated plants were lower than in the No-PGRs plants both during the high-temperature period and after the end of the high-temperature period. Overall, IAA improved photosynthetic performance of the terminal leaflet of the seventh leaf during the high-temperature period and after plants were exposed to cooler base temperatures following an episode of the high temperatures. TIBA was only beneficial during the high-temperature period.
Discussion
Potato responses under high-temperature episodes are more consistent with known effects of an auxin mechanism (Obiero et al., 2019). This study explored the role of an auxin mechanism in potato exposed to high-temperature episode. There were four main
findings: (a) The auxin inhibitor (TIBA) reduced plant and tuber growth in the control (22 °C) to a similar extent as the 9-day episode of 30 °C. (b) Auxin (IAA) application did not enhance dry matter partitioning to the tubers nor the growth of the potato plants exposed to the 9-day episode of 30 °C. (c) Both the auxin and auxin inhibitor increased the chlorophyll concentration index in the seventh leaf (emerged before the high temperature was applied) and in the third leaf (emerged during the high temperature period) and (d) The auxin and the auxin inhibitor enhanced photosynthesis in the terminal leaflet of the seventh leaf of the potato plants exposed to the 9-day episode of 30 °C.
The similarity of the extent to which plant and tuber growth was reduced in plants at 22 °C when treated with TIBA to the plants that were exposed to the 9-day episode of 30 °C is consistent with the hypothesis that the negative impacts of an episode of high temperatures on plant and tuber growth in potato could be associated with an auxin inhibition. The dry matter in the tubers, whole plant, below-ground plant parts and on the main shoot, the leaf area on the whole plant and on main shoot, the main shoot length, the total number of tubers per plant and the size distribution of tubers (small and large tubers) all responded quantitatively or qualitatively in the TIBA treated plants at the control (22 °C) the same was as was in the No-PGRs plants exposed to the 9-day episode of 30 °C (Fig 1 to 6).
The reduced tuber growth in the TIBA-treated plants at 22 °C and in the No-PGRs plants exposed to the 30°C episode was most likely caused by reduced leaf growth. The whole plant and main shoot leaf area in the TIBA-treated plants at 22 °C was reduced to a
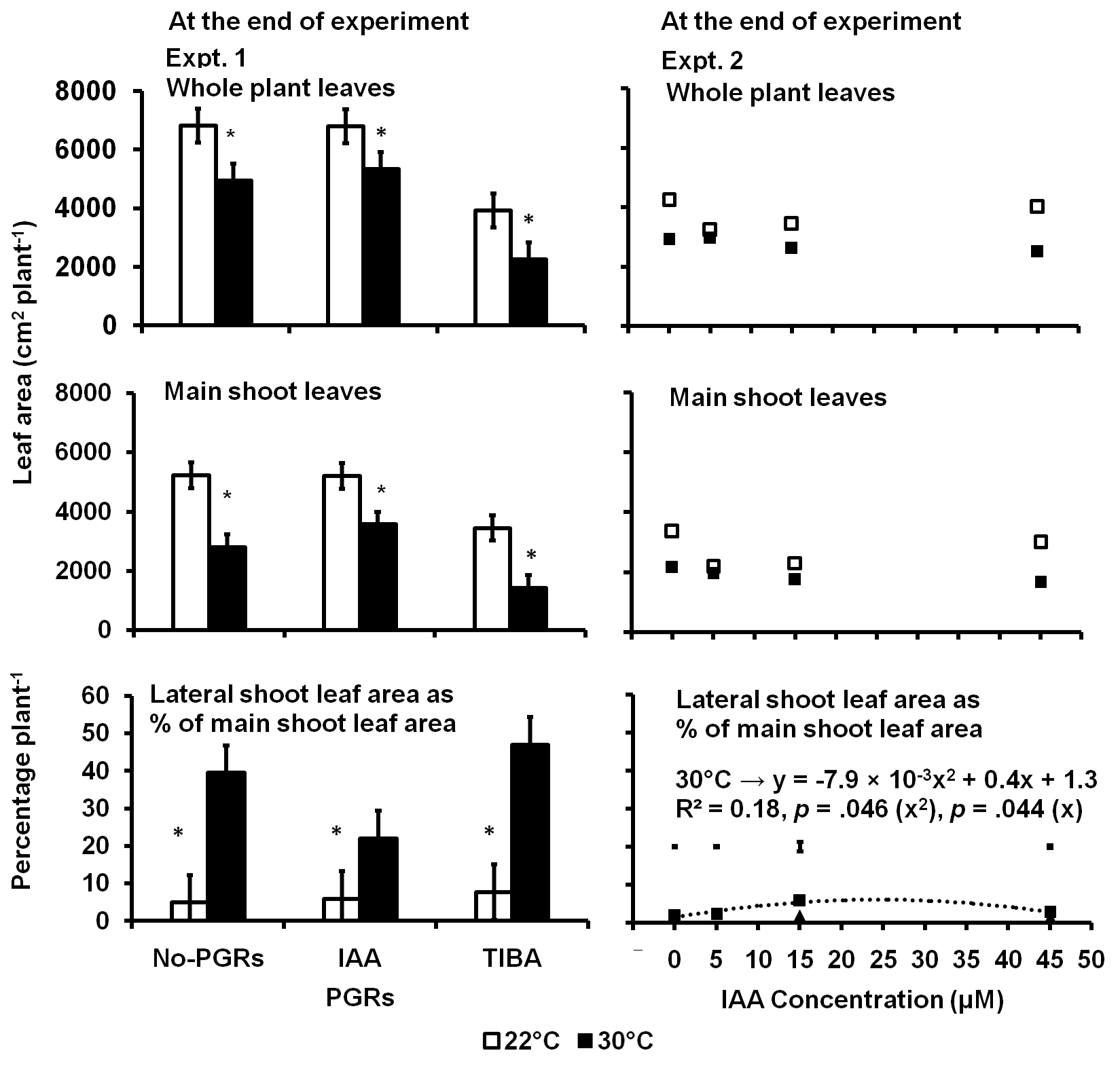
Fig 6. Whole plant and main shoot leaf area per plant and leaf area on lateral shoots as a percent of the main shoot leaf area as influenced by IAA and TIBA (Expt. 1) and IAA concentration (Expt. 2) in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation. Data at the end of Expt. 1 and 2. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means.
similar extent as those of the No-PGRs plants exposed to the 9-day 30 °C episode (Fig 6). Hence, less carbon was available for whole plant growth and partitioning to the tubers. There was also a further reduction in leaf area in the TIBA-treated plants exposed to the 9-day episode of 30 °C. This only means that the high-temperature episode aggravated the negative influence of the auxin inhibitor on leaf growth. This is consistent with the findings of Sakata et al. (2010) in which low levels of IAA was found in the anthers of barley plants and in Arabidopsis grown at 29 °C than at 20 °C D/N. However, further clarity is necessary because other studies showed increased levels of IAA in the anthers of cotton and in Arabidopsis exposed to high temperatures (Gray et al., 1998; Franklin et al., 2011; Min et al., 2014). In the present study, however, the reduced leaf area implies that less photosynthate was available for both plant and tuber growth in TIBA-treated plants at 22 °C as well as those of the high-temperature treatment. This is consistent with the results of our previous study in which we found that the reduced plant and tuber growth under the high-temperature episode was more closely related to the reduced whole plant carbon production than to the inhibition of starch synthase or the production gibberellins (Obiero et al., 2019; Obiero et al., 2020).
Sink capacity in terms of the number of tubers per plant was also consistent with results of our previous experiments (Obiero et al., 2019). There were slightly or significantly more tubers per plant in
all the high-temperature treatments and with the PGRs relative to the No-PGRs plants at 22 °C (Fig 2). This means that the reduced tuber dry matter in both the TIBA-treated plants at 22°C and the high-temperature treatment was not due to the lack of enough sinks for the deposition of starch.
While the application of TIBA had a similar effect on potato growth, morphology and yield as did the high-temperature episode, the IAA treatment did not overcome the negative impact the high-temperature episode. In the present study, the potato plants were treated with 10 µM (Expt. 1) or 0, 5, 15 and 45 µM (Expt. 2) concentration of IAA. The PGRs were applied on the foliage; it is also likely that some of the solution went into the growth mix because the foliage was sprayed to the point of runoff. Auxins are produced at the growing tip of the plant and move basipetally through the stems where they inhibit the growth of the lateral buds (Snow, 1929; Goldsmith, 1977; Aloni et al., 2006). This has been demonstrated in experiments in which suitable amounts of auxins applied to decapitated plants inhibited the growth of lateral buds (Skoog and Thimann, 1934; Fanu, 1936; Thimann, 1939). As such, there would have been some responses with the exogenously applied auxins in the present study with the right concentration (Kumar and Wareing, 1974), site (decapitated shoots), method and frequency of the application (Ponnampalam and Mondy, 1986) and or even with right interaction with other PGRs such as gibberellic
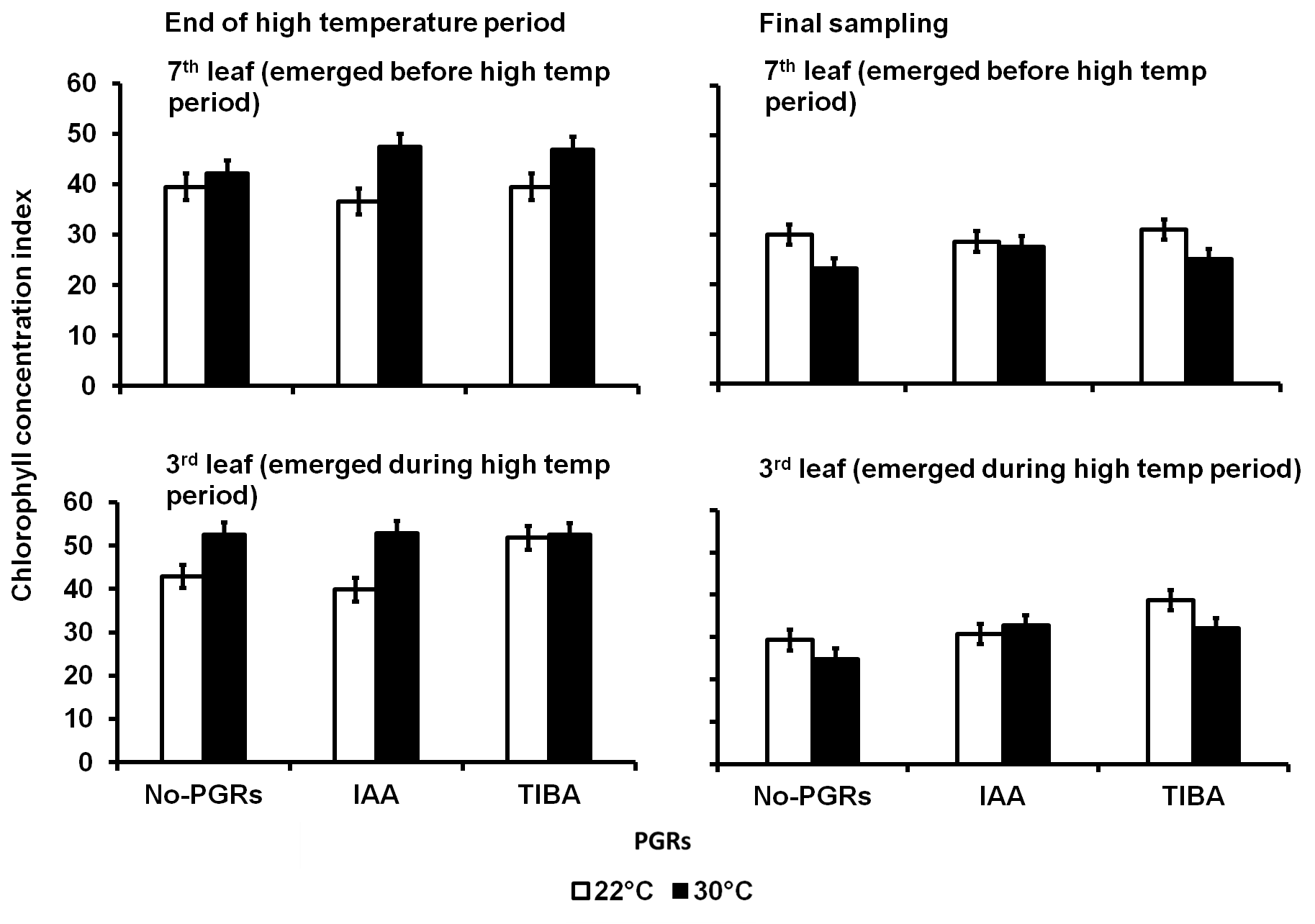
Fig 7. Leaf chlorophyll concentration index (CCI) in the terminal leaflet of the seventh leaf tagged before the high temperatures were applied and in the third leaf that emerged during the high-temperature period in potato plants exposed to a 9-day episode of 30°C applied shortly after tuber initiation in Expt. 1. Data at the end of the high temperature period and at the final sampling of Expt. 1. PGRS = Plant growth regulators. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. * = significant means.
acid and cytokinins (Kumar and Wareing, 1972; Davies et al., 1986; Kumlay, 2014). These present some of the possible areas for further study.
Materials and methods
Two greenhouse experiments (Expt. 1 and 2) were conducted at Murdoch University, Perth (32° 04′ S; 115° 50′ E), Western Australia. Expt. 1 investigated the influence of two plant growth regulators (PGRs) (IAA and TIBA) while Expt. 2 evaluated the effect of four concentrations of IAA on potato plants exposed to nine days of 30 °C shortly after tuber initiation. Expt. 1 was conducted from March to June 2017 and Expt. 2 from July to September 2017.
Expt. 1 was a 2 × 3 factorial with two levels of temperatures: the control (22 °C) and a treatment involving exposure of potato plants to a high temperature of 30 °C for nine days, and three PGRs (No-PGRs, IAA and TIBA). Expt. 2 was 2 × 4 factorial with the same two levels of temperatures as in Expt. 1 and four levels of IAA concentration (0, 5, 15 and 45 µM). Both experiments were in a split-plot design. Temperature treatments were the main plot factors while PGRs were subplots.
Potato cv. Royal Blue plants were grown in a greenhouse at 22 °C before and after the end of the high-temperature episode in both experiments (Expt. 1 and 2). In both experiments, the high-temperature treatments were applied for nine days of 30 °C after tuber presence was confirmed. This was immediately after plants had been treated with the PGRs on day 37 (Expt. 1) and 41 (Expt. 2) after planting (DAP). At the end of the nine days, plants in the 30 °C treatment were transferred back to 22 °C and grown together with the control plants for another 23 (Expt. 1) or 20 (Expt. 2) days before the final sampling.
Greenhouses were set and managed as described in Obiero et al. (2019). In Expt. 1, the solar duration ranged from 12 hours (hr) in March (at planting) to 10 hr in June (at the final sampling). In Expt. 2, the solar duration ranged from 10 hr and 20 min in July (at planting) to 12 hr in September (at the final sampling). The mean relative humidity during the high-temperature period in the 30 °C treatment was 52% (Expt. 1) and 61% (Expt. 2). In the control (22 °C), the mean relative humidity for the duration of the experiment was 70% (Expt. 1) and 61% (Expt. 2).
In Expt. 1, the air temperatures during the high-temperature period in the 30 °C treatment ranged from 29 to 31 °C with a mean of 30°C. In the control (22 °C) treatment, the day and night temperatures ranged from 21 to 22 °C with a mean of 21.5 °C. In Expt. 2, the day temperature differed from the night temperature due to the malfunction of an air conditioner in one greenhouse. The mean nighttime (7.00 pm to 7.00 am) and daytime (7.00 am to 7.00 pm) temperatures were 25 °C and 29 °C, respectively. The day and nighttime temperature fluctuation allowed for a better comparison to field situation but contrasted to Expt. 1. The day and nighttime air temperatures of the control (22 °C) treatment in Expt. 2 were similar during the high-temperature period. They ranged from 21 to 22 °C with a mean of 21.5 °C.
The growth medium, planting bags, planting and subsequent plant management in both experiments were also conducted as described in Obiero et al. (2019). In each experiment, six extra potato plants were used to check for the presence of tubers before the high temperature was applied.
PGRs in Expt. 1 were applied at the rate of 10 µM corresponding to 1.75 ppm for IAA and 80 µM corresponding to 39.98 ppm for TIBA. In Expt. 2, the following concentrations of IAA were used: 0, 5, 15 and 45 µM corresponding to 0.0, 0.875, 2.62 and 7.87 ppm of IAA
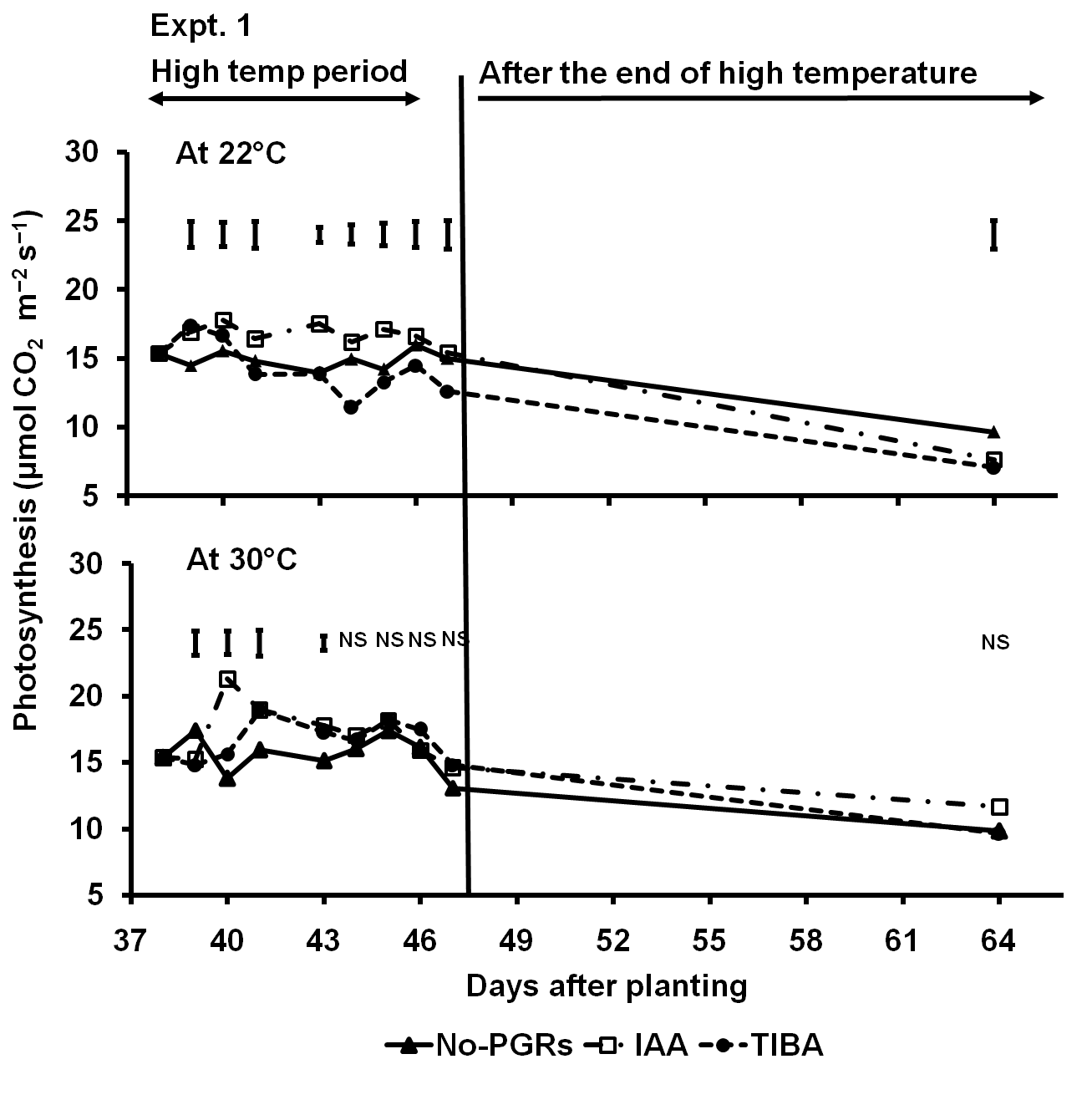
Fig 8. Net photosynthesis per unit area of the terminal leaflet of the seventh leaf as influenced by IAA and TIBA in potato plants in the control (22°C) or in the high temperature (30°C) treatment. Data are before the high temperature was applied, during the high temperature and after the end of the high temperature in Expt. 1. No-PGRs = No plant growth regulators. The vertical bars are LSD at 5% level of probability. NS = Not significant.
respectively. The IAA concentrations were adapted from Kumar & Wareing (1974) and those of TIBA from Roumeliotis et al. (2012).
The different PGRs were weighed and dissolved in 10 mL 1N NaOH. Then the solutions of the different PGRs were transferred to a 1-Litre measuring cylinder and diluted with DDI (double deionized water). Similar amount of DDI water containing only the 10 mL 1N NaOH was also prepared for the No-PGRs treatment (Expt. 1) and the "0" IAA concentration (Expt. 2). All solutions were prepared 5 hr before being applied and were kept in the dark at 2 to 4 °C.
To minimize any impact of direct solar radiation on the PGRs after application, application was conducted one hour after sunset. During application of the PGRs, all potato plants were first moved outside of the greenhouse and divided into two temperature-treatment groups: 22 °C and 30 °C (the high-temperature treatment group). In each group, there were three (Expt. 1) or four (Expt. 2) treatments. The foliage of six plants per treatment in each group was sprayed to the point of runoff with 400 mL of the PGRs (Expt. 1) or the different concentrations of IAA (Expt. 2) using a 500-mL hand-held sprayer. It is possible that some of the solutions also entered the growth media.
Immediately after application of the PGRs, the high-temperature treatment groups in both experiments were moved to another greenhouse and the high temperature started. The 22 °C treatment groups were moved back to the greenhouse already maintained at
22 °C. To minimize the chances of wet plants with different PGRs or IAA concentration coming into contact, plants with the same PGRs or IAA concentration were kept close together but not in contact until the following day. On the following day, when all plants were dry, plants in all treatments were completely randomized within and between benches of the greenhouse. Plants from different treatments on the same bench were placed as close as possible but not in contact. Further randomization was conducted every three days during the high-temperature period and weekly after plants had been returned to the control condition.
Only one destructive sampling was conducted in Expt. 1 and 2. This was the final sampling at the end of both experiments. The data on leaf area, plant height, and dry weights were also collected following the methods as described in Obiero et al. (2019). Plants were also sampled for the total number and the different size categories of tubers.
The length of the main shoot above the seventh leaf (the leaf that was tagged a day before the high temperature was applied) and the number of the newly emerged leaves above the seventh leaf were recorded at the end of the high-temperature period and at the final sampling in Expt. 1.
Net photosynthetic measurements were only carried out in Expt. 1. They were conducted on the terminal leaflet of the seventh leaf from the apex of the plant. The leaf was tagged a day before the high-temperature treatment commenced. The measurements commenced the day before the high temperatures were applied and continued daily through the high-temperature period with the final measurement made before the final sampling. The measurements were conducted between 12 noon and 2 pm. Light intensity was set to 1200 µmol m-2 s-1 using the light source on the LCpro+ Photosynthesis System (470 and 660 nm). The temperature and humidity of the measurement chamber were set to match those in the greenhouse.
Leaf chlorophyll concentration index (CCI) was measured on the terminal leaflets of two different leaves: the seventh leaf from the apex (as described above) and the third leaf that emerged after treatments were commenced. CCI measurements were conducted at the end of the high-temperature period and at the final sampling in Expt. 1. The measurements were conducted using the SPAD chlorophyll meter CCM-200 Plus (Apogee Instruments, Inc. USA) at the same time of the day as the photosynthetic measurements.
In Expt. 1, the effects due to temperature, PGRs treatment and their interaction were subjected to two-way analysis of variance (ANOVA). The analysis was conducted through the General Linear Model (GLM) procedure of the Statistical Analysis Software (SAS), University Edition (SAS Institute, North Carolina, USA). Significant means were compared using the Fisher’s Least Significant Difference (LSD) and reported at 5% level of probability unless otherwise specified.
In Expt. 2, linear or curvilinear regression models were used to test the effect of IAA concentration on the leaf area, the number of tubers and their size distribution and the dry matter variables (tuber, below ground, leaf, stem, shoot, and whole plant) using the SAS Procedure (Proc) REG. The significance of the regression coefficients was also tested. Improvement of significance was tested through the R-squared (R2). Only the regression models with improved terms are reported. No data required transformation even though the different size category of the tubers, the dry matter and leaf area on the lateral shoots and the senesced leaves on the main shoot were analyzed as percentages of the total number of tubers per plant, the dry matter or leaf area on the main shoot and the dry matter of the whole plant leaf, respectively.
Conclusion
The similarity in the tuber, whole plant, below ground and main shoot dry matter; the leaf area of the whole plant and on main shoot; the main shoot length, and the total number of tubers per plant and the size distribution of tubers (small and large tubers) per plant between the TIBA treatment in the control (22 °C) and the plants exposed to the 9-day episode of 30 °C is consistent with the hypothesis that an auxin inhibition has a role in the response of potato to a high-temperature episode. Addition of an auxin, however, did not overcome the negative effects of the high-temperature episode on plant and tuber growth. A positive response, perhaps, would have been possible with the right concentration; site, method, frequency, and timing of application in relation to the high-temperature treatment and or with interaction with the right plant growth regulator(s). Further research is recommended to determine the role of auxins in potato plants exposed to an episode of high temperatures both for understanding the mechanism of action and for potential agronomic intervention.
Acknowledgements
COO thanks the Australia Government for financial support through a Research Training Program Scholarship. Thanks to the Western Australia State Agricultural Biotechnology Centre, Murdoch University for use of facilities. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author’s contribution
This work was carried out in collaboration of all authors. Author COO designed the study, carried out the actual experiments, collected the data, conducted statistical analysis and wrote the first draft under the supervision of authors SM and RB. The final draft was read and approved by all authors.
Conflict of interest
All authors declare that no conflict of interest exist.
References
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 97(5):883–893.
Balla J, Medveďová Z, Kalousek P, Matiješčuková N, Friml J, Reinöhl V, Procházka S (2016) Auxin flow-mediated competition between axillary buds to restore apical dominance. Sci Rep. 6(1):35955.
Booth A, Lovell PH (1972) The effect of pre-treatment with gibberellic acid on the distribution of photosynthate in intact and disbudded plants of Solanum tuberosum L. New Phytol. 71(5):795–804.
Çalışkan S, Hashemı M S, Akkamış M, Aytekin Rİ, Bedir M (2021) Effect of gibberellic acid on growth, tuber yield and quality in potatoes (Solanum tuberosum L.). Turkish J. Field Crops. 26(2):136–146.
Carrera E, Bou J, García‐Martínez JL, Prat S (2000) Changes in GA 20‐oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 22(3):247–256.
Cline MG (1994) The role of hormones in apical dominance. New approaches to an old problem in plant development. Physiol Plant. 90(1):230–237.
Davies WJ, Metcalfe J, Lodge TA, da Costa AR (1986) Plant growth substances and the regulation of growth under drought. Funct. Plant Biol. 13(1):105–125.
Fanu B (1936) Auxin and correlative inhibition. New Phytol. 35(3):205–220.
Farhan HN, Sagar G, Oxley E (2010) Non-destructive and precise studies of the growth of individual potato tubers (Solanum tuberosum L.) under field conditions. ABJNA. 1(3):296–306.
Fernie AR, Willmitzer L (2001) Molecular and biochemical triggers of potato tuber development. Plant Physiol. 127(4), 1459–1465.
Fleisher DH, Timlin DJ, Reddy VR (2006) Temperature influence on potato leaf and branch distribution and on canopy photosynthetic rate. Agron J. 98(6):1442–1452.
Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, Wigge PA, Gray WM (2011) Phytochrome-Interacting Factor 4 (PIF4) regulates auxin biosynthesis at high temperature. PNAS. 108(50):20231.
Goldsmith M (1977) The polar transport of auxin. Annu. Rev. Plant Physiol. 28(1):439–478.
Gray WM, Östin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. PNAS. 95(12):7197.
Harmey MA, Crowley MP, Clinch PEM (1966) The effect of growth regulators on tuberisation of cultured stem pieces of Solanum tuberosum L. Eur. Potato J. 9(3):146–151.
Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S (2011) Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 155(2):776–796.
Herman DJ, Knowles LO, Knowles NR (2016) Differential sensitivity of genetically related potato cultivars to treatments designed to alter apical dominance, tuber set and size distribution. Am. J. Potato Res. 93(4):331–349.
Kieber JJ, Schaller GE (2014) Cytokinins. The Arabidopsis Book / ASPB, 12, e0168.
Kumar D, Wareing PF (1972) Factors controlling stolon development in the potato plant. New Phytol. 71(4):639–648.
Kumar D, Wareing PF (1974) Studies on tuberization of Solanum andigena. New Phytol. 73(5), 833–840.
Kumlay AM (2014) Combination of the Auxins NAA, IBA, and IAA with GA3 improves the commercial seed-tuber production of potato (Solanum tuberosum L.) under in vitro conditions. BioMed Res Int. 2014:1–7.
Lovell PH, Booth A (1967) Effects of gibberellic acid on growth, tuber formation and carbohydrate distribution in potato (Solanum tuberosum L.). New Phytol. 66(4):525–537.
Mares DJ, Marschner H, Krauss A (1981) Effect of gibberellic acid on growth and carbohydrate metabolism of developing tubers of potato (Solanum tuberosum L.). Physiol Plant. 52(2):267–274.
Menzel CM (1980) Tuberization in potato at high temperatures: Responses to gibberellin and growth inhibitors. Ann. Bot. 46(3):259–265.
Menzel CM (1983) Tuberization in potato at high temperatures: Gibberellin content and transport from buds. Ann. Bot. 52(5):697–702.
Menzel CM (1985) Tuberization in potato at high temperatures: Interaction between temperature and irradiance. Ann. Bot. 55(1):35–39.
Midmore DJ, Prange RK (1992) Growth responses of two Solanum species to contrasting temperatures and irradiance levels: Relations to photosynthesis, dark respiration and chlorophyll fluorescence. Ann. Bot. 69(1):13–20.
Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol. 164(3):1293–1308.
Obiero CO, Milroy SP, Bell RW (2019) Importance of whole plant dry matter dynamics for potato (Solanum tuberosum L.) tuber yield response to an episode of high temperature. Environ. Exp. Bot. 162:560–571.
Obiero CO, Milroy SP, Bell RW (2020) Photosynthetic and respiratory response of potato leaves of different ages during and after an episode of high temperature. J Agron Crop Sci. 2006(3):352–362.
Obiero CO, Milroy SP, Bell RW (2022) Increasing frequency of high-temperature episodes in potato growing regions of Western Australia and its impacts on plant and tuber growth. Arch. Agron. Soil Sci. 68(14):1988–2004.
Ponnampalam R, Mondy NI (1986) Effect of foliar application of indoleacetic acid on the total glycoalkaloids and nitrate nitrogen content of potatoes. J. Agric. Food Chem. 34(4):686–688.
Roumeliotis E, Kloosterman B, Oortwijn M, Kohlen W, Bouwmeester HJ, Visser RGF, Bachem CWB (2012) The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J. Exp. Bot. 63(12):4539–4547.
Rykaczewska K (2015) The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. Am. J. Potato Res. 92(3):339–349.
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyawaza Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. PNAS. 107(19):8569.
Sharma N, Kaur N, Gupta AK (1998) Effect of chlorocholine chloride sprays on the carbohydrate composition and activities of sucrose metabolising enzymes in potato (Solanum tuberosum L.). J. Plant Growth Regul. 26(2):97–103.
Šimko I (1994) Sucrose application causes hormonal changes associated with potato tuber induction. J. Plant Growth Regul. 13(2):73.
Skoog F, Thimann KV (1934) Further experiments on the inhibition of the development of lateral buds by growth hormone. PNAS. 20(8), 480–485.
Snow R (1929) The young leaf as the inhibiting organ. New Phytol. 28(5):345–358.
Struik PC, Geertsema J, Custers CH (1989) Effects of shoot, root and stolon temperature on the development of the potato (Solanum tuberosum L.) plant. III. Development of tubers. Potato Res. 32(2):151–158.
Thimann KV (1937) On the nature of inhibitions caused by auxin. Am. J. Bot. 24(7):407–412.
Thimann KV (1939) Auxins and the inhibition of plant growth. Biol. Rev. 14(3):314–337.
Timlin D, Rahman SML, Baker J, Reddy VR, Fleisher D, Quebedeaux B (2006) Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron J. 98(5):1195–1203.
Tsai D-S, Arteca RN (1984) Inhibition of IAA-induced ethylene production in etiolated mung bean hypocotyl segments by 2,3,5-triiodobenzoic acid and 2-(p-chlorophenoxy)-2-methyl propionic acid. Physiol Plant. 62:448–452.