

Aust J Crop Sci. 18(12):794-800 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p3402
In vitro and in vivo fungicidal and anti-ochratoxigenic activities of the essential oils of Backhousia citriodora and Lippia origanoides Kunth. in coffee fruits
Luana Isac Soares1, Sara Maria Chalfoun2, Rafaela Magalhães Brandão1, Vanuzia Rodrigues Fernandes Ferreira1, Wilder Douglas Santiago1, Richard Bispo Barbosa1, Caroline Lima Angélico2, Marcus Vinicius Prado Alves1, David Lee Nelson3, Maria das Graças Cardoso*1
1Departamento de Química, Universidade Federal de Lavras (UFLA), CEP 37200-900, Lavras, Minas Gerais, Brazil
2Empresa de Pesquisa Agropecuária de Minas Gerais, Campus Universitário, CEP 37200-900, Lavras, Minas Gerais, Brazil
3Programa de Pós-Graduação em Biocombustíveis, Universidade Federal do Vale do Jequitinhonha e Mucuri, CEP 39100-900, Diamantina, Minas Gerais, Brazil
*Corresponding author: Maria das Graças Cardoso 
Abstract: The fungicidal and anti-ochratoxigenic potentials of the essential oils (EO's) from Backhousia citriodora and Lippia origanoides were studied through in vitro and in vivo tests on coffee fruits. The EO's were extracted by hydrodistillation and characterized by gas chromatography using mass spectrometer (GC-MS) and flame ionization detectors (GC-FID) The in vitro antifungal activity of EOs against A. carbonarius and A. ochraceus was determined by measuring the percentage of inhibition of mycelial growth (MFC). The in vivo antifungal potential was evaluated by plating fractions of the pericarp and coffee beans (Coffea arabica L. and Catuaí) inoculated with spore suspensions and sprayed with essential oils (Blotter Test). The inhibition of OTA synthesis in vivo was quantified by High Performance Liquid Chromatography (HPLC). The geranial (57.55%) and neral (42.44%) isomers were the principal compounds from the B. citriodora EO, and carvacrol (65.53%), p-cymene (12.13%) and thymol (7.05%) were found in the EO from L. origanoides. The EO’s from L. origanoides and B. citriodora inhibited the growth of A. carbonarius at a minimum fungicidal concentration (MFC) of 250 μL L-1 and 500 μL L-1, respectively, and they inhibited the growth of A. ochraceus at a MFC of 500 μL L-1 and 250 μL L-1, respectively. The EO's presented an antitoxigenic effect on coffee fruits and can be promising for improving the quality and maintaining the safety of the coffee beans as well as the beverage.
Keywords: Coffea arabica; ochratoxin A; lemon myrtle; rosemary d'angola; antifungal; coffee beans.
Abbreviations: BOD_biochemical oxygen demand; EO(‘s)_essential oil(s); GC-FID_gas chromatography-flame ionization detector; GC-MS_gas chromatographer-mass spectrometer; HPLC_high-performance liquid chromatography; IARC_international cancer research agency; LD_detection limit; LQ_quantification limit; MA_malt agar; MAPA_Ministério da Agricultura e Pecuária; MFC_minimum fungicidal concentration; NRPS_nonribosomal peptide-synthetase; OTA_ochratoxin A; PBS_phosphate buffered saline; PKS_Polyketide synthases; RPM_revolution per minute.
Introduction
Coffee is one of the most widely consumed beverages in the world, having spread to different cultures and peoples. It is used in different ways and yields a wide variety of by-products that are produced and exported by more than 47 countries (ICO, 2019). The economic and commercial importance of coffee is immeasurable, especially in countries where the economy is directly linked to the coffee industry, such as Brazil, Vietnam and Colombia (Debastiani et al., 2019; dos Santos, Alvarenga and Boffo, 2020). Brazil stands out as the largest coffee exporter, with a yearly production of 40.6 million bags. This production represents great socioeconomic importance for the country's development, ensures the generation of jobs and taxes and contributes to the country's revenue (FAO, 2019). The world consumption of coffee is increasing every year, along with the concern of consumers with the quality of the beverage, and the coffee industry needs new technologies to meet a greater demand without decreasing the quality and sustainability of the production chain (dos Santos et al., 2020).
One of the factors that affect the quality and causes major economic impacts is the susceptibility of coffee fruits to the action of microorganisms throughout the entire production chain and especially in the post-harvest period. The filamentous fungi of the Aspergillus, Penicillium and Fusarium genus compromise the visual aspect of the grains and the quality of the taste and aroma of the beverage (Iamanaka et al., 2014). In addition, some species of the Aspergillus genus produce secondary metabolites, such as ochratoxin A (OTA), during their growth and development. This mycotoxin is toxic to animals and humans, and it is considered to be nephrotoxic, cytotoxic, carcinogenic, teratogenic and immunosuppressive (Chalfoun et al., 2018). The International Cancer Research Agency (IARC) classifies ochratoxin A in group 2B as being possibly carcinogenic in humans (IARC, 1993). According to Silva et al. (2020), regulatory authorities established that the maximum level of this substance permitted in coffee is 5 and 10 μg kg-1 of processed coffee in the European Union and Brazil, respectively. The contamination of coffee fruits by fungi that produce ochratoxin A occurs mainly during the process of drying on coffee terraces, a time that covers the grace period for synthetic antifungals used in the previous stages of the production chain (Vitoratos et al., 2013).
The effects caused by the presence of fungi and the synthesis of
Table 1. Chemical composition of the essential oils of Backousia citriodora and Lippia oiganoides Kunth.
| Percentage of constituent in the essential oil | ||
|---|---|---|
| Constituent | B. citriodora | L. origanoides |
| Carvacrol | - | 65.5307 |
| Geranial | 57.55 | - |
| Myrcene | - | 1.3542 |
| Neral | 42.44 | - |
| Caryophylene oxide | - | 1.5621 |
| p-Cymene | - | 12.1347 |
| Terpine-4-ol | - | 0.4647 |
| Thymol | - | 7.0471 |
| Trans-β-Caryophylene | - | 4.0466 |
| α-Phelandrene | - | 0.3046 |
| α-Pinene | - | 0.1753 |
| α-Terpinene | - | 0.8335 |
| α-Tujene | - | 0.6346 |
| Total percent | 99.99 | 99.6677 |
| Compound groups | ||
| Monoterpene hydrocarbons | - | 21.0165 |
| Oxygenated monoterpenes | 99.99 | 73.0425 |
| Sesquiterpene hydrocarbons | - | 4.0466 |
| Oxygenated sesquiterpenos | - | 1.5621 |
*The quantity of each chemical constituent in the essential oils is expressed as a percentage. (-) unidentified chemical constituent.
OTA in coffee fruits can be minimized or controlled with the use of products of natural origin or their derivatives. Secondary metabolites produced by plants act as potent antimicrobials, and they are capable of inhibiting the growth of various fungi, including toxigenic fungi (Stierle and Stierle, 2015). Essential oils have been explored in the control of the growth of fungi as well as in the production of OTA in fruits during the post-harvest period because they are composed of a complex mixture of terpenes and phenylpropanoids (Vitoratos et al., 2013; Kapetanakou et al., 2019). The aromatic species Backhousia citriodora and Lippia origanoides Kunth., respectively known as lemon myrtle and rosemary d'angola, contain essential oils that are rich in non-aromatic and aromatic monoterpenes such as citral and carvacrol, which exhibit a high toxicity to microorganisms and can act as fungicides during the post-harvest period and processing of coffee fruits (Sarrazin et al., 2015; Teixeira, 2019). The objectives of this work were to chemically characterize the essential oils from the leaves of Backhousia citriodora and Lippia origanoides Kunth., to evaluate the fungicidal potential against A. carbonarius and A. ochraceus, and to determine the anti-ochratoxigenic activity through in vivo tests on coffee fruits.
Results and Discussion
Chemical characterization of essential oils
The chemical constituents present in the essential oils extracted from the leaves of B. citriodora and L. origanoides are shown in Table 1. Citral, consisting of the mixture of the geranial (57.55%) and neral (42.44%) isomers, was the principal constituent of the essential oil from B. citriodora. Southwell et al. (2003) studied the chemical composition of the oil of lemon myrtle leaves obtained from commercial plantations in Australia and found the oxygenated monoterpenes, neral and geranial, to be the principal components. The compounds myrcene, linalool and citronellal were also identified, although in low concentrations. The results found by Southwell et al. (2003) partially corroborate those found in this study.
The principal compounds in the essential oil from L. origanoides were carvacrol (65.53%), p-cymene (12.13%) and thymol (7.05%). The results partially corroborate those found by Sarrazin et al. (2015), who analyzed the composition of the essential oil from L. origanoides Kunth. collected in the state of Pará, Brazil. They determined that carvacrol (47.2%), thymol (12.8%), p-cymene (9.7%) and p-methoxythymol (7.4%) were the principal components. Because essential oils are composed of volatile compounds and act in the defense system of the plants,
qualitative and quantitative differences in their components as a result of intrinsic, extrinsic and abiotic factors can occur (Dhouioui et al., 2016).
In vitro antifungal activity
A remarkable exponential antifungal activity against the microorganisms under study (Figure 1) was observed for the essential oils. The essential oil from L. origanoides inhibited the proliferation of the A. carbonarius and A. ochraceus fungi with minimum fungicidal concentrations (MFC) of 250 µL L-1 and 500 µL L-1, respectively. The mycelial growth of A. carbonarius and A. ochraceus was completely inhibited by the essential oil from B. citriodora, with MFCs of 500 µL L-1 and 250 µL L-1, respectively.
Essential oils act on fungi by several mechanisms. One of the mechanisms reported in the literature involves the ability of essential oils to alter the permeability and integrity of fungal cell membranes to affect their growth (Guo et al., 2017; Adelakun et al., 2016). These mechanisms are related to the lipophilic characteristic and the low molecular weight of the constituents present in essential oils. These substances interact easily with the fungal cell membrane and alter its functions (Pawar and Thaker, 2006).
For An et al. (2019), highly lipophilic oxygenated monoterpenes can exert antifungal activities through the rupture of the cell wall, membrane and cytoplasm. Consequently, they block the synthesis of components of cell walls, cytomembranes, cytoplasm and organelles and cause modifications in the growth and morphology of mycelia and spores. In addition, monoterpenes can negatively influence metabolic and energetic pathways, thereby interfering in the development of fungi.
Antifungal activity in Coffea arabica fruits
The values referring to the plating of coffee pericarps after treatments against A. carbonarius and A. ochraceus with the essential oils from B. citriodora and L. origanoides are shown in Figure 2, where the antifungal activity was evaluated by spore counts for A. carbonarius (Figure 2A) and with respect to the occurrence index for A. ochraceus (Figure 2B). During the plating of the grains, the presence of the inoculated fungi in the fruits was not observed because there was no rest period, a step that favors the penetration of microorganisms present in the pericarp of the green grain. Thus, the rest period was observed to contribute to the contamination of grains by pathogens present in the fruits.
The application of the essential oils resulted in an exponential decrease in pathogens in the pericarps, reducing the number of A. carbonarius spores by 71.43% and 69.57% for treatments with
Table 2. Effects of essential oils from B. citriodora and L. origanoides Kunth. on the synthesis of ochratoxin A by A. carbonarius in coffee pericarp.
| Treatment | Concentration | Production of OTA (µg Kg-1) |
Value – p2 | |||
|---|---|---|---|---|---|---|
| CF | - | 1.64 | CF | CN | 500 | 1000 |
| CN | 10% | 1.60 | 0.876 | - | - | - |
| B. citriodora | 500 µL L-1 | 1.48 | 0.014 | 0.088 | - | - |
| 1000 µL L-1 | 1.42 | 0.001 | 0.007 | 0.653 | - | |
| 2000 µL L-1 | 1.42 | 0.001 | 0.006 | 0.638 | 1.000 | |
| CF | - | 1.64 | CF | CN | 250 | 500 |
| CN | 10% | 1.60 | 0.907 | - | - | - |
| L. origanoides | 250 µL L-1 | 1.65 | 1.000 | 0.834 | - | - |
| 500 µL L-1 | 1.61 | 0.982 | 0.999 | 0.951 | - | |
| 1000 µL L-1 | 1.47 | 0.015 | 0.079 | 0.011 | 0.045 | |
Values of p² <0.05 differ by the Tukey test at the level of 5% probability; CF = Fungal control: only containing inoculum; CN = Negative control: 10% powdered milk solution.
Table 3. Effects of the essential oils from B. citriodora and L. origanoides Kunth. on the synthesis of ochratoxin A by A. ochraceus in coffee pericarp.
| Treatment | Concentration | Production of OTA (µg Kg-1) |
Value – p2 | |||
|---|---|---|---|---|---|---|
| CF | - | 1.16 | CF | CN | 500 | 1000 |
| CN | 10% | 1.17 | 1.000 | - | - | - |
| B. citriodora | 250 µL L-1 | 1.12 | 0.962 | 0.950 | - | - |
| 500 µL L-1 | 1.12 | 0.935 | 0.919 | 1.000 | - | |
| 1000 µL L-1 | 1.04 | 0.197 | 0.182 | 0.554 | 0.620 | |
| CF | - | 1.16 | CF | CN | 250 | 500 |
| CN | 10% | 1.17 | 1.000 | - | - | |
| L. origanoides | 500 µL L-1 | 1.11 | 0.160 | 0.131 | - | - |
| 1000 µL L-1 | 1.06 | 0.005 | 0.004 | 0.344 | - | |
| 2000 µL L-1 | 0.99 | 0.000 | 0.000 | 0.002 | 0.059 | |
Values of p² <0.05 differ by the Tukey test at the level of 5% probability; CF = Fungal control: only inoculum; CN = Negative control: 10% powdered milk solution.
the highest concentrations of the essential oils from B. citriodora and L. origanoides, respectively. The incidence of A. ochraceus also decreased by 74.86% and 51.33% in treatments with the highest concentrations of these essential oils.
The antifungal activity was observed at higher concentrations in vivo than was observed in vitro, probably because of the volatility of the essential oils. The coffee fruits were exposed to solar radiation and a variation in temperature during drying. The fact that the complexity of the investigated system was greater in in vivo tests was also a factor.
There are no reports in the literature of studies in which the biological potential of essential oils from B. citriodora and L. origanoides was evaluated against A. carbonarius and A. ochraceus in fruits of Coffea arabica. However, authors such as Sonker, Pandey and Singh (2015) evaluated the antifungal effect of the essential oil from Artemisia nilagirica on deteriorating fungi such as A. ochraceus in grapes. They observed that the essential oil, rich in monoterpenes, inhibited fungal growth, and the useful life of the grapes increased without modification of sensory properties of the fruits or phytotoxic effects. The results found by the authors and in the present study are in agreement, confirming that essential oils rich in monoterpenes, aromatic or not, are effective in microbiological control, both in vitro and in vivo.
Ochratoxin A in pericarps and Coffea arabica grains
The production of ochratoxin A in coffee pericarps infected with A. carbonarius and A. ochraceus (fungal controls) and the influence of the treatment with essential oils on the production of the toxin are shown in Tables 2 and 3, respectively. The OTA production in the coffee pericarp by the controls was 1.64 and 1.16 µg Kg-1 for A. carbonarius and A. ochraceus, respectively. In general, the values obtained are below the limits established by European and Brazilian legislation of 5 µg Kg-1 and 10 µg Kg- 1, respectively (EC, 2006; ANVISA, 2011). However, the production
of ochratoxin A was slightly lower in treatments with both essential oils, which showed a dose-dependent effect.
The presence of ochratoxin A could not be determined in coffee beans whose fruits were infected with A. ochraceus. Although the proliferation of fungi in coffee beans whose fruits were infected with A. carbonarius was not observed, contamination by OTA (1.16 µg Kg-1) was observed. The level was 29.27% lower than that found in the pericarps. The application of both essential oils resulted in a decrease in OTA production by 18.68% relative to the fungal control (Table 4).
The data obtained in this work corroborate the results obtained by Aldred et al. (2008), who investigated the effectiveness of essential oils from cinnamon, cloves, and laurel and that of the antioxidant resveratrol against the production of OTA by Penicillium verrucosum and A. westerdijkiae in wheat grains. Wang et al. (2018) stated that the reduction in OTA production caused by the principal compound in the essential oil from cinnamon, cinnamaldehyde, is a consequence of the disregulation in the expression of genes (PKS, NRPS, VeA, LaeA and VelB) responsible for OTA biosynthesis. The authors studied the antifungal and antitoxigenic effects of cinnamaldehyde at the morphological and structural levels, together with the inhibition of OTA synthesis at the transcriptional level, and determined that cinnamaldehyde can act as a safe and effective natural agent against OTA contamination.
Research by Hua et al. (2014) showed that citral, the only compound present in the essential oil under study (B. citriodora), is capable of suppressing the transcription of the PKS genes responsible for OTA biosynthesis. Previously, Tian et al. (2012) suggested that compounds rich in terpenes and terpenoids can easily cross cell membranes to induce biological responses. This principle can help us to understand the effect of aromatic monoterpenes, such as carvacrol, the principal component in the essential oil from L. origanoides, on toxigenic fungi. The phenolic hydroxyl of the molecule can form hydrogen bonds with enzymes to modify their activities and, as a consequence, it can
Table 4. Effects of essential oils from B. citriodora and L. origanoides Kunth. on the synthesis of ochratoxin A by A. carbonarius in coffee beans.
| Treatment | Concentration | Production of OTA (µg Kg-1) |
Value – p2 | |||
|---|---|---|---|---|---|---|
| CF | - | 0.91 | CF | CN | 500 | 1000 |
| CN | 10% | 0.90 | 0.989 | - | - | - |
| B. citriodora | 500 µL L-1 | 0.86 | 0.025 | 0.068 | - | - |
| 1000 µL L-1 | 0.76 | 0.000 | 0.000 | 0.000 | - | |
| 2000 µL L-1 | 0.74 | 0.000 | 0.000 | 0.000 | 0.510 | |
| CF | - | 0.91 | CF | CN | 250 | 500 |
| CN | 10% | 0.90 | 0.996 | - | - | - |
| L. origanoides | 250 µL L-1 | 0.81 | 0.000 | 0.001 | - | - |
| 500 µL L-1 | 0.76 | 0.000 | 0.000 | 0.071 | - | |
| 1000 µL L-1 | 0.74 | 0.000 | 0.000 | 0.013 | 0.898 | |
Values of p² <0.05 differ by the Tukey test at the level of 5% probability; CF = Fungal control: only inoculum; CN = Negative control: 10% powdered milk solution.
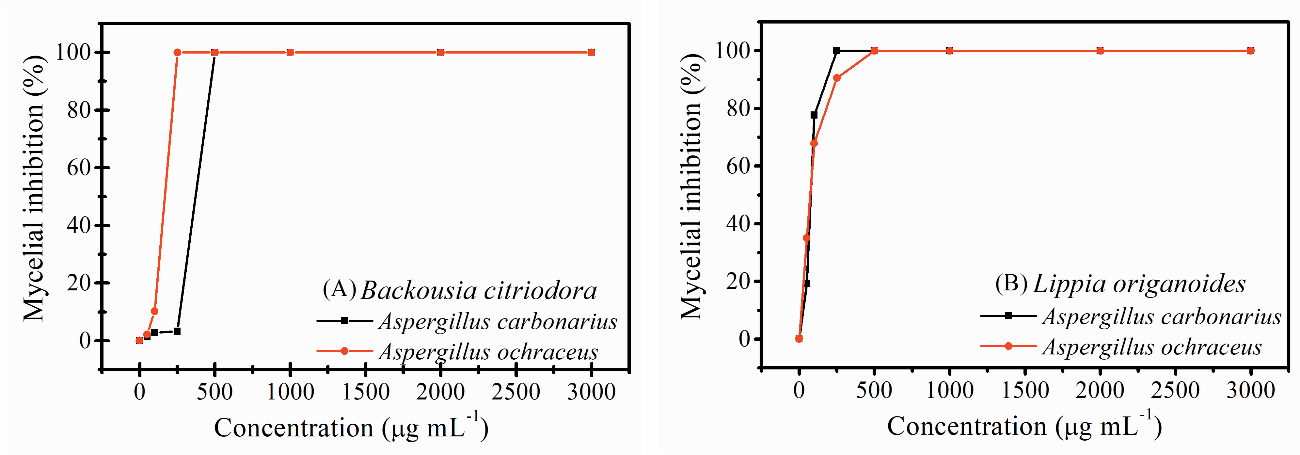
Fig 1. Inhibition of mycelial growth of Aspergillus carbonarius and Aspergillus ochraceus by the essential oils from Backousia citriodora and Lippia origanoides Kunth.
cause changes in the behavior of the microorganism and in the production of secondary metabolites, such as toxins (Goncalves and Romano, 2017).
In conclusion, neral (42.44%) and geranial (57.55%) were the principal constituents in the essential oil from B. citriodora, and carvacrol (65.63%), p- cymene (12.13%) and thymol (7.04%) were found to be the principal constituents in the essential oil from L. origanoides. The essential oils were effective in inhibiting the mycelial growth of A. carbonarius and A. ochraceus in vitro and exerted antifungal and anti-ochratoxigenic effects on fungi in pericarps and coffee beans. Essential oils reduced the formation of A. carbonarius spores and the rate of occurrence of A. ochraceus in coffee pericarps. For the first time, the influence of essential oils on the proliferation of toxigenic fungi and on the inhibition of ochratoxin A biosynthesis in coffee fruits was demonstrated. The essential oils from B. citriodora and L. origanoides could be favorable for the environment. They generate a minimum amount of residues in the protection of coffee and other foods contaminated by toxigenic fungi because the oils are volatile. Thus, they are promising for the development of new natural antifungals to guarantee food quality and safety.
Material and Methods
Collection of plant material and extraction of essential oils
The B. citriodora and L. origanoides species were collected at the Horto de Plantas Medicinais, Universidade Federal de Lavras (UFLA) (21° 13' south latitude and 44° 58' west longitude) in March 2018. The exsiccata of B. citriodora and L. origanoides were deposited in the ESAL Herbarium under registration numbers 30300 and 23660, respectively. The essential oils were extracted by hydrodistillation using the Clevenger apparatus modified in accordance with the procedure described by Anvisa (2010).
Chemical characterization of essential oils
The compounds comprising the essential oils were identified by gas chromatography coupled to mass spectrometry (GC/MS, Shimadzu Corporation, model 110 QP 2010 Plus, Kyoto, Japan), and the quantification of each constituent was obtained by normalizing areas (%) of peaks obtained by gas chromatography with a flame ionization detector (GC/FID, Shimadzu GC-17A) as described by Brandão et al. (2020).
The Van den Dool and Kratz (1963) equation was used to calculate the retention index, and the standards used were the homologous series of n-alkanes (nC8-nC18). The compounds identified yielded mass spectra and retention indices with up to 95% similarity with those of the equipment libraries (FFNSC 1.2, NIST 107 and NIST 21) and with the literature retention indices of Adams (2017).
Isolation of fungi and preparation of the spore suspension
The fungal isolates of the Aspergillus genus were obtained by direct plating on coffee beans by the Blotter Test method (Tempe, 1963). The identification of the species of the selected isolates proceeded according to the isolation of the microorganisms in Czapeck Yeast Agar (CYA, HiMedia Laboratories Pvt. Ltd., Munbai, India) after incubation at 25 °C and 37 °C for a period of 7 days according to the identification keys proposed by Samson et al. (2014).
The isolates that presented the set of favorable characteristics were selected, that is, greater speed of mycelial growth and high potential for the production of ochratoxin A. The selected isolates were identified as Aspergillus carbonarius and Aspergillus ochraceus. The fungi were incubated in a manner similar to a procedure described elsewhere (Brandão et al., 2020).
In vitro antifungal activity of essential oils
The antifungal activities of the essential oils were evaluated in vitro according to a modification of the method described by
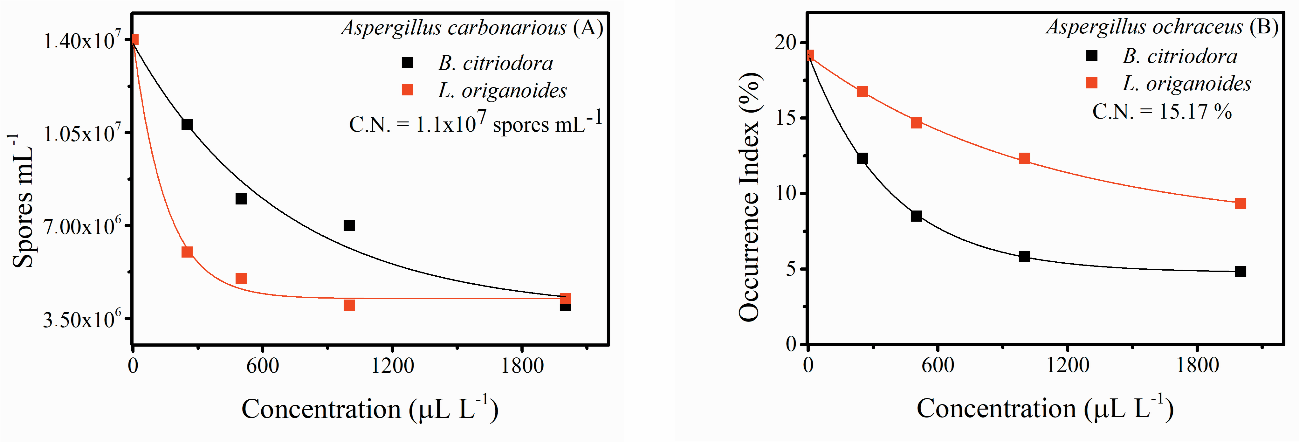
Fig 2. Antifungal activity of essential oils from B. citriodora and L. origanoides against contamination of Coffea arabica L. beans. Influence of the action of essential oils on: (A) spore count of A. carbonarius and (B) index of occurrence of A. ochraceus. C.N.- Negative control: 10% powdered milk solution.
Brandão et al. (2020). The isolates (A. carbonarius and A. ochraceus) were reactivated in Petri dishes containing Malt Agar culture medium (MA, HiMedia Laboratories Pvt. Ltd., Munbai, India) at 25 °C for 10 days. After this period, the plating was performed by replicating the fungus in the center of the plate containing 50, 100 250, 500, 1000, 2000 and 3000 μL L-1 concentrations the essential oils (B. citriodora and L. origanoides) in 20 mL of MA medium. Petri dishes containing just the culture medium and the microorganism were used as the fungal control. All the treatments were incubated in BOD in the dark for 15 days at 25 °C, and orthogonal measurements of the diameter of the mycelial growth were performed on the last day of incubation. These analyses were performed in triplicate. The percentage of inhibition of mycelial growth by the essential oils was calculated according to Brandão et al. (2020).
Preparation of coffee beans for the in vivo test
The in vivo experiments were performed on coffee beans of the species Coffea arabica L. cv Catuaí Amarelo, 2017/2018 crop, harvested at the berry maturation stage. The fruits were disinfected with a 1% hypochlorite solution and washed in running water. At the semi-dry state, in which the berries contained 18-20% moisture, the spore suspensions (106 spores mL-1) of the fungi (A. carbonarius and A. ochraceus) were added to the coffee beans and incubated. The treatments were accomplished by spraying the essential oils from B. citriodora and L. origanoides, diluted in a 10% powdered milk solution in the concentrations of 250, 500, 1000 and 2000 µL L-1, over the A. carbonarius and A. ochraceus cultures. Fungal control (fungal spore suspension only) and negative control (fungal spore suspension and 10% powdered milk solution) were used. The fruits, when reaching 12% moisture, were submitted to mechanical processing to remove the pericarp. Mechanical processing was performed by collecting pericarps and grains separately.
Antifungal activity of essential oils on Coffea arabica fruits
The antifungal potentials of the essential oils were evaluated by plating fractions of pericarp and coffee beans using the Blotter Test method (Tempe, 1963; Angélico and Lima, 2012). The pericarps were plated without disinfection, whereas disinfestation of the grains was performed to observe the microbiota present inside. The disinfestation of the grains was achieved by treatment with 70% alcohol for one minute, followed by treating with 1% sodium hypochlorite for 30 seconds and washing three times with sterile distilled water.
Petri dishes (15 cm in diameter) containing two sheets of sterile filter paper moistened with 10 mL of sterile distilled water were used. For each repetition of in vivo treatments (triplicate), 25 fractions of pericarp or grain were plated per plate and incubated in a BOD at 25 °C with a photoperiod of 12 hours for 10 days.
The results for A. carbonarius (pericarps and grains) were expressed by counting spores mL-1 in a Newbauer chamber and
adding 10 mL of distilled water containing 1% Tween 80 to the plate. The results for A. ochraceus (pericarps and grains) were expressed by identifying the main fungal genera from the samples with the aid of a Phoenix CP 608 stereoscopic microscope. The incidence of A. ochraceus was determined by the Mckinney equation (Mckinney and Davis, 1925), usually used in the calculation of the occurrence index:
\[OI\ (\%) = \frac{\left( f \times n_{0} \right) + \ \left( f \times n_{1} \right) + \ \left( f \times n_{2} \right) + \ \left( f \times n_{3} \right) + \ \left( f \times n_{4} \right)}{FN100}\]
where f is the number of individuals for each grade; F is 25 (total number of individuals); N is 4 (maximum attributable score). Notes (0, 1, 2, 3, 4) were estimated according to the area occupied in the pericarp or grain by the fungus and represented by the weighted average of the notes attributed to this occurrence, where n0 = 0 (note for the absence of fungus in the region); n1 = 1 (note for 1% to 25% of the region occupied by the fungus); n2 = 2 (note for 25% to 50% of the region occupied by the fungus); n3 = 3 (note for 50% to 75% of the region occupied by the fungus); n4 = 4 (score for 75% to 100% of the region occupied by the fungus).
Anti-ochratoxigenic activity in pericarps and Coffea arabica beans
The anti-ochratoxigenic activity of essential oils on samples of pericarps and grains contaminated by A. carbonarius and A. ochraceus was analyzed according to the official method of the MAPA (Ministério da Agricultura e Pecuária), published in the Diário Oficial da União, through the Normative Instruction SDA No. 09, of March 24, 2000 (DOU, 2000). The concentrations evaluated were the MFC and two concentrations greater than the MFC that was determined in the in vitro test.
A representative homogeneous sampling of pericarps and beans was obtained among the treatments and controls. The samples were ground separately in their entirety to obtain a particle size of 20 mesh. Then, 25.00 g of the powder was subjected to extraction of OTA. The samples were stirred for 10 minutes in 200 mL of 3% methanol:sodium bicarbonate solution (1:1, v/v), at a speed of 254 rpm. The extract was vacuum filtered, and 10-mL aliquots of the filtrate were diluted to 100 mL with PBS buffer. The diluted sample was passed through an immunoaffinity column (OchratestTM WB - Vicam, Watertown. USA) at a flow rate of 2 to 3 mL per minute using a 60 mL syringe. Four mL of methanol (Merck, Darmstadt, Germany, PA grade) was added to the immunoaffinity column, and the methanol was left in contact with the antibodies for three minutes. The OTA present in the sample was eluted from the immunoaffinity column using a flow rate of 2 to 3 mL min-1. The solvent was evaporated on a water bath, the residue was re-dissolved with 400 μL of the methanol: acetonitrile: water: acetic acid (35:35:29:1) mobile phase, and 20 μL of each filtrate was injected directly into the HPLC system (HPLC, Shimatzu, Kyoto, Japan). The HPLC was equipped with two high-pressure pumps (SPD-M20A), a degasser (DGU 20A3), an interface (CBM-20A), an automatic injector (SIL-10AF) and a fluorescence detector (RF-10 AXL). Separations were performed using an Agilent-Zorbax Eclipse XDB-C18 column (4.6 x 250 mm, 5 μm) connected to an Agilent-Zorbax Eclipse XDB-C18 4-Pack column (4.6 x 12.5 mm, 5 μm). Twenty microliters of the samples and standard were injected, and the solutes were eluted with an isocratic system of methanol:acetonitrile:water:acetic acid (35:35:29:1) having a flow rate of 0.8 mL min-1. External standardization was used to quantify OTA (Sigma-Aldrich®, São Paulo, Brazil) in the samples, performed using the analytical curve (y = 3799.6 x -3149, with a determination coefficient (r2) of 0.999. The limits of detection (LD) and quantification (LQ) were 0.24 and 0.8 μg Kg-1, respectively. All OTA analyses were performed in triplicate.
Statistical analysis
The ANOVA test was used to compare the concentrations in each essential oil under study (B. citriodora and L. origanoides) in the in vivo anti-ochratoxigenic test (Montgomery, Peck and Vining 2020), whereas the Tukey's test (Tukey, 1949) was employed for multiple comparisons. The software used in the analyses was R (version 3.5.1).
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento Pessoal de Nível Superior - Brasil (CAPES) - Financial Code 001. The authors thank the Central de Análise e Prospecção Química da Universidade Federal de Lavras for furnishing the equipment for the chromatographic analysis, EPAMIG (Empresa de Pesquisa Agropecuária de Minas Gerais) for permission to perform the experiments and the Horto de Plantas Medicinais da UFLA for furnishing the plant species.
References
Adams RP (2017) Identification of Essential Oils Components by Gas Chromatography/ Mass Spectroscopy, (Part B). 4a ed. Carol Stream: Allured, p. 469.
Adelakun OE, Oyelade OJ, Olanipekun BF (2016) Use of Essential Oils in Food Preservation. In: Essential Oils in Food Preservation, Flavor and Safety, ACAD Press, 71–84.
Aldred D, Cairns-Fuller V, Magan N (2008) Environmental factors affect efficacy of some essential oils and resveratrol to control growth and ochratoxin A production by Penicillium verrucosum and Aspergillus westerdijkiae on wheat grain. J. Stored Prod. Res. 44: 341–346.
An P, Yang X, Yu J, Qi J, Ren X, Kong Q (2019) α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control. 98: 42–53.
Angélico CL, Lima C (2012) Aplicação do agente biológico Cladosporium cladosporioides (Fresen) de Vries “Cladosporin” como bioprotetor da qualidade do café (Coffea arabica L.) In: http://www.sbicafe.ufv.br/handle/123456789/6623#.XjhHQ3GHqYQ. (May 2020, date last accessed).
ANVISA - Agência Nacional de Vigilância Sanitária (2011) Diário oficial da união. Resolução - RDC No - 7, de 18 de fevereiro de 2011. Dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos. In: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0007_18_02_2011_rep.html. (20 May 2020, date last accessed).
ANVISA - Agência Nacional de Vigilância Sanitária (2010) Métodos de Farmacognosia. In: Farmacopeia Brasileira, Fiocruz. 5, 198-199. In: http://portal.anvisa.gov.br/documents/33832/260079/5%C2%AA+edi%C3%A7%C3%A3o+-+Volume+1/4c530f86-fe83-4c4a-b907-6a96b5c2d2fc. (20 May 2020, date last acesse).
Brandão RM, Ferreira VRF, Batista LR, Alves E, Lira NA, Bellete BS, Scolforo JRS, Cardoso MG (2020) Antifungal and antimycotoxigenic effect of the essential oil of Eremanthus erythropappus on three different Aspergillus species. Flavour Fragr. J. 35: 524-33.
Chalfoun SM, Angelico CL, de Resende MLV (2018) Brazilian Coffee Quality: Cultural, Microbiological and Bioactivity Aspects. World J. Adv. Res. Rev. 6: 50-58.
da Silva SA, Pereira RG, de Azevedo NL da Glória EM., Chalfoun SM, Batista LR (2020) Fungi associated to beans infested with coffee berry borer and the risk of ochratoxin A. Food Control, 113: 107204.
Debastiani R, dos Santos CE, Ramos MM, Souza VS, Amaral L, Dias JF (2019). Elemental extraction factor from ground to drinking coffee as a function of the water temperature. Nucl Instrum Meth B, 477: 154-158.
Dhouioui M, Boulila A, Chaabane H, Zina MS, Casabianca H (2016) Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Ind Crops Prod. 83: 301–306.
DOU - Diário Oficial da União (2000) Métodos de Referência para Análise de Ocratoxina A em Café Verde, Diário Oficial da União, Instrução Normativa SDA, n°. 09, 24/03/2000, seção 1, 35-41.
dos Santos HD, Alvarenga YA, Boffo EF (2020) 1H NMR metabolic fingerprinting of Chapada Diamantina/Bahia (Brazil) coffees as a tool to assessing their qualities. Microchem. J. 152: 104293.
EC - European Commission (2006) Commission Regulation nº 1881/2006, of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, Brussels, 364, 5-2.
FAO - Food and Agriculture Organization of the United Nations (2019). Faostat: Food and Agriculture Organization Statistic: Crops. http://www.fao.org/statistics/en/. (20 May 2020, date last accessed).
Guo N, Zang YP, Cui Q, Gai QY, Jiao J, Wang W, Zu YG, Fu YJ (2017) The preservative potential of Amomum tsaoko essential oil against E. coil, its antibacterial property and mode of action. Food Control, 75: 236–245.
Hua H, Xing F, Selvaraj JN, Wang Y, Zhao Y, Zhou L, Liu X, Liu Y (2014) Effect of Essential Oils on Aspergillus ochraceus Growth and Ochratoxin A Production. PLoS ONE, 9: e108285.
Iamanaka BT, Teixeira AA, Teixeira ARR, Copetti MV, Bragagnolo N, Taniwaki MH (2014) The mycobiota of coffee beans and its influence on the coffee beverage. Food Res. Int. 62: 353–358.
ICO - International Coffee Organization (2019). Total production by all exporting countries, 2019. http://www.ico.org/prices/po-production.pdf. (3 May 2020, date last accessed).
IARC - International Agency for Research on Cancer (1993). Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the evalution carcinogenic risks to humans. 56: 489-421.
Kapetanakou AE, Nestora S, Evageliou V, Skandamis PN (2019) Sodium alginate–cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin A production. Food Res. Int. 119: 876-85.
Mckinney HH, Davis R (1925) Influence of soil temperature and moisture on infection of young wheat plants by Ophiobolus graminis. J. Agric. Res. 31: 827-840.
Montgomery DC, Peck EA, Vining GG (2012) Introduction to linear regression analysis. John Wiley & Sons. 672. ISBN: 978-0-470-54281-1.
Pawar VC, Thaker VS (2006) In vitro efficacy of 75 essential oil against Aspergillus niger. Mycoses. 49: 316-323.
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubé S, Szigeti G, Yaguchi T, Frisvad JC (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78: 141–173.
Sarrazin SLF, da Silva LA, Oliveira RB, Raposo JDA, da Silva JKR, Salimena FRG, Maia JGS, Mourão RHV (2015) Antibacterial action against food-borne microorganisms and antioxidant activity of carvacrol-rich oil from Lippia origanoides Kunth. Lipids Health Dis. 1:1-8.
Sonker N, Pandey AK, Singh P (2015) Efficiency of Artemisia nilagirica (Clarke) Pamp. essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 95: 1932-1939.
Southwell IA, Russell MF, Smith RL (2003) Chemical composition of some novel aromatic oils from the australian flora. Acta Hortic. 597: 79-89.
Stierle AA, Stierle DB (2015) Bioactive Secondary Metabolites Produced by the Fungal Endophytes of Conifers. Nat Prod Commun. 10: 1671–1682.
Teixeira ML, Marcussi S, Rezende DACS, Magalhães ML, Nelson DL, Cardoso MG (2019) Essential Oil from Lippia origanoides (Verbenaceae): Haemostasis and Enzymes Activity Alterations. Med. Chem. 15: 207-214.
Tempe J (1963) The blotter method for seed health testing. Proc. Int. Seed Test. Assoc. 28: 133-151.
Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y (2012) The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 130: 520–527.
Tukey J (1949) Comparing Individual Means i/n the Analysis of Variance. Biometrics. 5: 99–114.
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 11: 463-471.
Vitoratos A, Bilalis D, Karkanis A, Efthimiadou A (2013) Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. NOT BOT HORTI AGROBO. 41: 86-92.
Wang L, Jin J, Liu X, Wang Y, Liu Y, Zhao Y, Xing F (2018) Effect of Cinnamaldehyde on Morphological Alterations of Aspergillus ochraceus and Expression of Key Genes Involved in Ochratoxin A Biosynthesis. Toxins. 10: 340-352.