

Aust J Crop Sci. 18(12):786-793 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p17
Compatibility and antimicrobial activity of Trichoderma spp. combined with diazotrophs and growth-promoting bacteria
Priscilla Costa Gobbi, Maria Laura Turino Mattos*
Embrapa Temperate Climate, 96010-971, Pelotas, Rio Grande do Sul, Brazil
*Corresponding author: Maria Laura Turino Mattos

Abstract: The study evaluated the compatibility, in vitro, of diazotrophs Bradyrhizobium spp., and plant growth-promoting bacteria (PGPB), Azospirillum brasilense and Bacillus spp., widely used as biofertilizers and biostimulantion soybean culture combined with as biofungicides/bionematicides Trichoderma harzianum and Trichoderma asperellum. Compatibility was evaluated through antagonistic activity and the minimum inhibitory concentration (MIC), using the techniques of dual cultures and disc diffusion, respectively. The experiment was conducted at the soil microbiology laboratory out at the Embrapa Temperate Climate, Pelotas, RS, Brazil. We adopted a completely randomized design (CRD) with three replications and the data were submitted to ANOVA followed by Tukey's test (p≤0.05) for compare of the mycelial growth variables, that showed significant difference. B. elkanii strain SEMIA.587 was compatible with all accessions of Trichoderma spp. A. brasilense strains Ab-V5 and Ab-V6, were compatible with the T. asperellum accession UFRA.T09. B. elkanii strain SEMIA.5019, B. japonicum strain SEMIA.5079 and B. diazoefficiens strain SEMIA.5080, B. subtilis strain CNPMS.B2084 and B. megaterium strain CNPMS.B119 inhibited from 47% to 83% the growth of Trichoderma spp. The MIC demonstrated that diazotrophs and PGPB are not sensitive to Trichoderma spp. B. elkanii strain SEMIA.587 can be used in combination with all Trichoderma spp. accessions, as well as the Ab-V5 and Ab-V6 strains of A. brasilense with the T. asperellum accession UFRA.T09 in the inoculation via a seeding furrow.
Keywords: Biofungicide; Dual Cultures; MIC; PGPB; Strains.
Introduction
The use of biological agents in soybean Glycine max (L.) has shown relevant results in the uptake of nitrogen and phosphorus, accumulation of plant biomass, synthesis of phytohormones, and increase in nodulation (Chagas et al., 2017; Moretti et al., 2020; Duré et al., 2022; Braccini et al., 2023), in addition to mitigating the environmental impacts caused by the use of chemical inputs (Hungria et al., 2015). The diazotrophs Bradyrhizobium spp. are widely used in biological nitrogen fixation (BNF) (Kaschuk et al., 2016; Mirriam et al., 2022) and its effectiveness supplies the totality of the nitrogen demand necessary for the crop (Hungria et al., 2020; Mahmud et al., 2020).
Brazilian legislation contains records of four strains of Bradyrhizobium spp. (B. japonicum SEMIA.5079, B. diazoefficiens SEMIA.5080, B. elkanii SEMIA.5019 and SEMIA.587) available for soybean crops (MAPA, 2011). However, some studies have shown that the association of these microorganisms with PGPB, through the so-called co-inoculation technique, has shown benefits for plant development (Iliäœić et al., 2017; Moretti et al., 2019; Garcia et al., 2021).
The main combinations involve the co-inoculation of Bradyrhizobium spp. and A. brasilense strains Ab-V5 and Ab-V6. Currently, this technique is applied in 25% of the soybean cultivation area (Santos et al., 2021) and results in phytohormone production and increases in root growth, benefiting the performance of Bradyrhizobium spp. in nodulation and, consequently, in BNF (Hungria et al., 2015; Ferri et al., 2017; Barbosa et al., 2021).
The genus Bacillus has been successfully employed with Bradyrhizobium spp. for inoculation, showing improvement in nodulation, growth, and shoot and root length (Atieno et al., 2012; Sibponkrung et al., 2020; Miljaković et al., 2022). Simultaneously, there has been an increase in the use of disease biocontrol agents based on Trichoderma spp., which in addition to controlling root pathogens and being a more sustainable alternative (Iturralde et al., 2020; Barbosa et al., 2022; Conte et al., 2022), show promising results in promoting growth (Conte et al., 2022), synthesizing indole acetic acid and auxins (Chagas et al., 2017), and increasing tolerance to abiotic stress (Tyśkiewicz et al., 2022).
Inoculation of soybean seeds with Trichoderma spp. via a seeding furrow is an increasingly used practice (Meyer et al., 2022) because, in addition to being efficient biocontrol agents (Conte et al., 2022), they have potential as plant growth promoters (Chagas et al., 2017; Junior et al., 2022). Using co-inoculation technology via seeding furrows, Trichoderma spp., when used in conjunction with PGPB and diazotrophs, enables increased efficiency in the inoculation process (Ferri et al., 2017; Brignoli et al., 2023). When using the co-inoculation technique, it is important that no organism is inhibitory to one another or interferes too much with the growth of the other (Whipps, 2001; Thomloudi et al., 2019).
Information related to the compatibility of bacteria and fungi beneficial to the development of the soybean crop is still limited (Karuppiah et al., 2019; Mattos et al., 2020) and, for the most part, is limited to greenhouse evaluations (Ayoubi et al., 2012; Cadore et al., 2020; El-Nahrawy et al., 2020). Thus, the aim of this study was to evaluate the in vitro compatibility of diazotrophs (B. japonicum, B. diazoefficiens and B. elkanii) and PGPB (A. brasilense, B. subtilis, B. megaterium) with biocontrol fungi, T. asperellum and T. harzianum, by evaluating their antagonistic effects.
Results
The results obtained in the antagonism test, through the dual culture technique, demonstrated that all evaluated bacteria, diazotrophs and PGPB, had an inhibitory effect against the isolates of T. asperellum and T. harzianum (Table 2). As an exception, B. elkanii strain SEMIA.587 did not form an inhibition halo with the evaluated Trichoderma spp. isolates (Fig.1A), nor
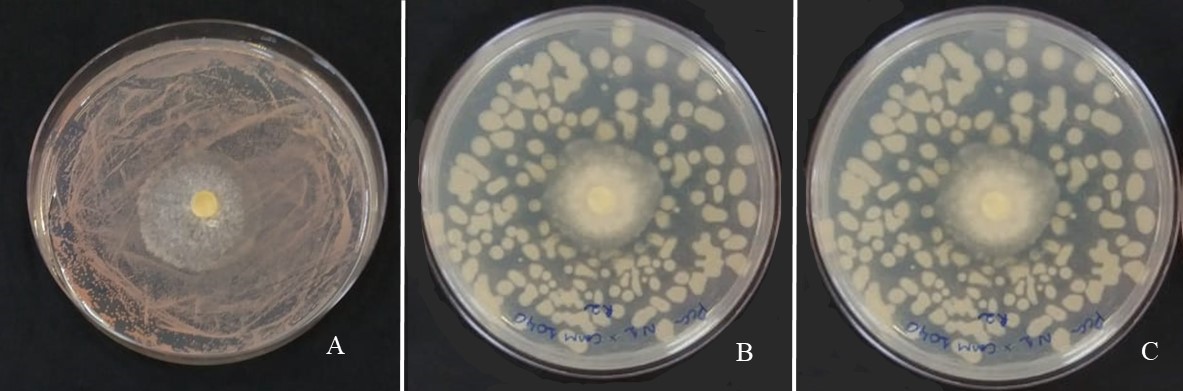
Fig 1. Antagonistic activity through the dual culture technique, without occurrence of formation of inhibition halo, after 120 hours of inoculation. Bradyrhizobium elkanii strain SEMIA.587 vs Trichoderma harzianum strain Esalq.1306 (A); Azospirillum brasilense strain Ab-V6 vs Trichoderma asperellum strain UFRA.T09 (B); and Azospirillum brasilense Ab-V5 vs Trichoderma aperellum strain UFRA.T09 (C). Photograph: Gobbi, PC.
Table 1. Selective medium and incubation conditions in a microbiological oven for the growth of different microorganisms used in the evaluation of antagonism and minimum inhibitory concentration. Embrapa Clima Temperado, Pelotas-RS, Brazil.
| Microorganism | Culture medium | Incubation conditions |
|---|---|---|
| Azospirillum spp. | Red Congo | 24 hours in a bacteriological oven at 28°C |
| Bacillus spp. | Casein-Peptone Dextrose Yeast Agar | 24 hours in a bacteriological oven at 28°C |
| Bradyrhizobium spp. | Yeast Mannitol Agar | 24 hours in a bacteriological oven at 28°C |
| Trichoderma spp. | Potato Dextrose Agar | 7 days in BOD at 27°C |
did A. brasilense strains Ab-V5 (Fig.1B) and Ab-V6 (Fig.1C) when faced with T. asperellum strain UFRA.T09.
When assessing PGPB compatibility of A. brasilense strains Ab-V5 and Ab-V6 with Trichoderma spp. isolates, the smallest halos of mycelial growth inhibitions occurred for T. asperellum strain UFRA.T09 (47.04% (F=6.976; df=4; p=0.006) and 62.85% (F=6.778; df=4, p=0.007), respectively; however, the latter did not differ significantly from T. harzianum strain Esalq.1306 (65.73%; Table 3).
In contrast, for PGPB, B. megaterium strain CNPMS.B119 and B. subtilis strain CNPMS.B2084, the highest percentage of inhibition occurred when confronted with T. asperellum strain UFRA.T09 (77.96% (F=71.28; df=4; p<0.05) and 68.44% (F=30.00; df=4; P<0.05), respectively) and did not differ for other evaluated isolates (Table 3).
Bradyrhizobium diazoefficiens strain SEMIA.5080 (F=4.486; df=4; p=0.025) and B. japonicum strain SEMIA.5079 (F=7.177, df=4; p=0.003) exhibited the lowest percentages of inhibition against the test agents T. asperellum strain UFRA.T12 (65.55% and 68.61%, respectively) and T. harzianum strain Esalq.1306 (74.63% and 66.67%, respectively). For B. elkanii strain SEMIA.5019, there were no significant differences between the evaluated Trichoderma spp. isolates (F=3.00; df=4; p=0.072; Table 3).
In the evaluation of the response of test agents to different antagonists, B. elkanii strain SEMIA.587 exhibited the lowest mean percentages of mycelial growth inhibition for T. asperellum strain UFRA.T06 (54.38%; F=20.426; df=7; p< 0.05), T. asperellum strain UFRA.T12 (47.02%; F=7.727; df=7; p=0.0003) and T. asperellum strain UFRA.T52 (49.12%; F=21.208; df=7; p<0.05) compared to other antagonists (Table 3). For T. asperellum strain UFRA.T09, the lowest suppression occurred in the interaction with A. brasilense strain Ab-V5 (F=34.05; df=7; p<0.05; Table 3).
The lowest percentage of mycelial inhibition for T. harzianum strain Esalq.1306 was observed with the antagonist B. subtilis strain CNPMS.B2084 (53.76%); however, it did not differ from B. elkanii strain SEMIA.587 (59.54%) or B. megaterium strain CNPMS.B119 (60.56%). The highest inhibition percentages for T. harzianum strain Esalq.1306 were verified in interactions with B. diazoefficiens strain SEMIA.5080 (74.63%), B. elkanii strain SEMIA.5019 (74.63%) and B. japonicum strain SEMIA.5079 (66.67%; F=16.432; df=7; p<0.05; Table 3).
Antagonists B. diazoefficiens strain SEMIA.5080, B. japonicum SEMIA.5079 and B. elkanii strain SEMIA.5019 also exhibited the highest inhibition values for T. asperellum strain UFRA.T09
(F=34.05; df=7; p<0.05) and T. asperellum strain UFRA.T12 (F=7.73; df=7; p=0.0004; Table 3). For T. asperellum strain UFRA.T06 and T. asperellum strain UFRA.T52, the highest percentage of inhibition occurred when confronted with A. brasilense strain Ab-V6 (80.28%; F=20.43; df=7; p<0.05 and 80.09%; F=21.21; df=7; p<0.05, respectively; Table 3).
The evaluation of microbial activity through the MIC demonstrated that diazotrophs and PGPB were not sensitive to the evaluated Trichoderma spp. isolates since there was no inhibition of bacterial growth at the different concentrations tested. Additionally, B. subtilis strain CNPMS.B2084 (Fig.2A), B. megaterium strain CNPMS.B119 (Fig.2B), B. elkanii strain SEMIA.5019 (Fig.2C), B. japonicum strain SEMIA.5079 (Fig.2D) and B. diazoefficiens strain SEMIA.5080 (Fig.2E) colonized the entire area of the plate, preventing the growth of Trichoderma spp. isolates at all evaluated concentrations.
The mycelial growth of T. harzianum strain Esalq.1306 and T. asperellum strain UFRA.T12 was observed up to a concentration of 1×105 conidia mL-1, while that for T. asperellum strain UFRA.T06, T. asperellum strain UFRA.T09 and T. asperellum strain UFRA.T52 occurred up to a concentration of 1×106 conidia mL-1 (Table 4). The evaluated Trichoderma spp. isolates showed reduced growth compared to the control, as shown in the antagonism test; however, for the most part, they did not differ from the control (Table 4).
Discussion
The formation of the halo of inhibition of diazotrophs and PGPB against the isolates of T. asperellum and T. harzianum did not nullify the development of the fungi after 120 hours of cultivation. However, all interactions, with and without halo formation, showed a reduction in mycelial growth in Trichoderma spp. isolates. Among the evaluated bacterial isolates, the diazotrophs B. diazoefficiens strain SEMIA.5080, B. japonicum strain SEMIA.5079 and B. elkanii strain SEMIA.5019 showed the greatest inhibitory effects against Trichoderma spp., and the interaction neutrality was verified for B. elkanii strain SEMIA.587 with Trichoderma spp. evaluation. Bécquer et al. (2013) found a neutral interaction between Sinorhizobium (Ensifer) meliloti and T. harzianum in an in vitro antagonism evaluation after 96 hours of cultivation, and although they did not show formation of an inhibitory halo, there was a reduction in the diameter of the T. harzianum colony.
| Antagonists | Trichoderma harzianum Esalq.1306 |
Trichoderma asperellum UFRA.T06 |
Trichoderma asperellum UFRA.T09 |
Trichoderma asperellum UFRA.T12 |
Trichoderma asperellum UFRA.T52 |
|---|---|---|---|---|---|
| Azospirillum brasilense Ab-V6 | 5.00 | 1.67 | 0.00 | 0.87 | 1.33 |
| Azospirillum brasilense Ab-V5 | 5.33 | 4.00 | 0.00 | 2.17 | 3.67 |
| Bacillus subtilis CNPMS.B2084 | 5.33 | 2.66 | 1.67 | 2.00 | 2.33 |
| Bacillus megaterium CNPMS.B119 | 3.00 | 5.67 | 3.00 | 1.67 | 1.33 |
| Bradyrhizobium elkanii SEMIA.587 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Bradyrhizobium elkanii SEMIA.5019 | 3.00 | 3.33 | 4.00 | 4.00 | 3.67 |
| Bradyrhizobium japonicum SEMIA.5079 | 2.67 | 1.67 | 1.33 | 2.67 | 1.17 |
| Bradyrhizobium diazoefficiens SEMIA.5080 | 3.00 | 1.00 | 2.00 | 1.67 | 1.33 |
| Coefficient of variation (CV%) | 52.57 | 72.33 | 99.39 | 63.08 | 69.30 |
Table 2. Values of inhibition halos (mm) of the antagonists, diazotrophs and plant growth-promoting bacteria, against the test agents, Trichoderma spp. Embrapa Clima Temperado, Pelotas-RS, Brazil.
The in vitro compatibility of B. elkanii strains SEMIA.587 and SEMIA.5019 evaluated by Mattos et al. (2020) was also shown to be the most compatible with the different isolates of T. asperellum. The strains belonging to B. elkanii produce rhizobiotoxins and present intrinsic resistance to antibiotics, unlike strains of B. japonicum (Minamisawa et al., 1996). The production of rhizobiotoxin limits endogenous ethylene production in soybean plants in low land soils, with poor natural drainage, favoring plant nodulation (Mattos et al., 2019). Thus, the synthesis of rhizobiotoxins can be a factor in the neutral interaction of B. elkanii SEMIA.587 and Trichoderma spp. verified in the present study.
The use of B. elkanii together with T. harzianum favored soybean production (Silva et al., 2018), and co-inoculation of B. elkanii and T. asperellum resulted in increased plant height and root dry mass production, indicating its application in cowpea, Vigna unguiculata (L.) Walp. (Fabaceae) (Costa et al., 2020).
Biological agents are considered compatible when they do not have a suppressive effect on each other in vitro co-cultivation or in rhizosphere colonization assays (Thomloudi et al., 2019). Antagonistic effects in vitro partially reflect the response of an interaction in culture systems (Prasadd and Babu, 2017) since microorganisms can colonize different ecological niches in the rhizosphere without interfering with each other’s growth (Niu et al., 2020), making the antagonistic activity notorious when space and nutrients are limited, and perhaps this is the main effect of the antagonistic action of fungi (Carvalho et al., 2014).
The use of the consortium B. japonicum and T. harzianum in the soybean crop resulted in an increase in the shoot and root length and in the N content compared to the use of B. japonicum alone (El-Nahrawy et al., 2020). The co-inoculation of B. japonicum and T. harzianum increased the fresh root mass without interfering with nodulation by B. japonicum, with soybean plants presenting nodules with the same ultrastructure as those inoculated only with B. japonicum. In addition, no fungal structures were observed below the root epidermis, indicating that T. harzianum colonized the soybean rhizosphere and remained on the surface of the roots (Iturralde et al., 2020).
The in vitro antagonism of Bradyrhizobium spp. against T. asperellum was evaluated by Mattos et al. (2020), and unlike the results obtained in this study, the authors observed the antagonistic action of B. elkanii strain SEMIA.587, B. elkanii strain SEMIA.5019, B. japonicum strain SEMIA.5079 and B. diazoefficiens strain SEMIA.5080 against T. asperellum strain UFRA.T09 and the formation of halos ranging from 1 to 5 mm diameter, demonstrating low potential for suppression of Bradyrhizobium spp. This occurred similarly in the present study, with halos ranging from 1.00 to 4.00 mm (Table 2) in the
evaluations of Bradyrhizobium spp. against T. asperellum and T. harzianum isolates.
Bacillus subtilis strain CNPMS.B2084 and B. megaterium strain CNPMS.B119 showed the lowest results of mycelial growth inhibition in T. harzianum strain Esalq.1306, when compared to other bacterial isolates, although there was formation of an inhibition halo. The antagonism of Bacillus spp. against phytopathogenic fungi is often related to the production of secondary metabolites with antibiotic properties and competition for nutrients and space (Harba et al., 2020; Miljaković et al., 2022); however, evaluations of the effect of these microorganisms against beneficial fungi in vitro are still limited.
The investigation of the in vitro compatibility of several isolates of Bacillus spp. and Trichoderma spp. showed strong antagonistic activity, making prior analysis a necessary step for application in mixtures (Fuga et al., 2016). The combined application of B. subtilis and T. harzianum proves to be compatible, providing protection against Rhizoctonia solani Kühn; however, the best results were obtained when applied separately (Abeysinghe, 2009). According to Li et al. (2005), B. subtilis exerts antifungal activity against the growth of T. harzianum, inducing the formation of chlamydospores.
The compatibility of A. brasilense strains Ab-V5 and Ab-V6 varied among the evaluated Trichoderma spp. isolates, being more inhibitory against T. asperillum strains UFRA.T6 and UFRA.T12 and more compatible with T. asperillum UFRA.T09 without the occurrence of the formation of an inhibition halo. Co-inoculation with T. harzianum and A. brasilense in beans, Phaseolus vulgaris L. (Fabaceae), decreased the number of nodules compared to inoculation with T. harzianum; however, there was an increase in the weight of nodules (Öğüt et al., 2005) and increased concentrations of micronutrients in grains (Öğüt et al., 2006).
The assessment of MIC demonstrated that diazotrophs and PGPB are not sensitive to the tested Trichoderma spp. isolates since they did not show reduced growth compromised in the presence of the fungus. The inhibition potential of a microorganism may be related to competition for resources available in the substrate, speed of multiplication and colonization capacity, which defines efficiency as an antagonist of Trichoderma spp. isolates (Benítez et al., 2004) or even to an evaluation method.
Scorzoni et al. (2007) when comparing assessment methods of MIC of an antimicrobial, observed that the agar diffusion technique was less sensitive than the microdilution technique, concluding that the effectiveness of a method may not always be adequate for an antimicrobial due to differences in physical, volatile and diffusion properties. According to Muniz et al. (2018),
Table 3. Mean percentage of inhibition of mycelial growth (±SE) of the antagonists, diazotrophs and plant growth-promoting bacteria, against the test agents, Trichoderma spp. Embrapa Clima Temperado, Pelotas-RS, Brazil.
| `1 | Trichoderma harzianum Esalq.1306 |
Trichoderma asperellum UFRA.T06 |
Trichoderma asperellum UFRA.T09 |
Trichoderma asperellum UFRA.T12 |
Trichoderma asperellum UFRA.T52 |
|---|---|---|---|---|---|
| Azospirillum brasilense Ab-V6 | 65.73±5.12 bB | 80.28±2.05 aA | 62.85± 3.41 bC | 80.09±5.12 aA | 72.15± 1.65 abABC |
| Azospirillum brasilense Ab-V5 | 63.47±1.63 aB | 67.61±0.81 aBC | 47.04 ±2.30 bD | 64.61±3.07 aAB | 64.38±5.48 aBCD |
| Bacillus subtilis CNPMS.B2084 | 53.76±1.54 cC | 56.81±1.24 bcCD | 68.44±0.48 aBC | 61.87±1.39 bBC | 53.42±0.79 cDE |
| Bacillus megaterium CNPMS.B119 | 60.56±0.81 bBC | 60.09±0.81 bCD | 77.96±1.07 aA | 59.36±1.21 bBC | 62.10±0.91 bCD |
| Bradyrhizobium elkanii SEMIA.587 | 59.54±0.74 aBC | 54.38±0.23 bD | 61.67±1.50 aC | 47.02±0.54 cC | 49.12±1.09 cE |
| Bradyrhizobium elkanii SEMIA.5019ns | 74.63±1.49 A | 62.74±4.97 CD | 73.66±2.34 AB | 68.46±4.63 AB | 78.79±3.15 A |
| Bradyrhizobium japonicum SEMIA.5079 | 66.67±2.77 cAB | 78.92±2.73 abAB | 82.26±0.93 aA | 68.61±3.42 bcAB | 76.51±1.91 abcAB |
| Bradyrhizobium diazoefficiens SEMIA.5080 | 74.63±2.28 abA | 79.71±2.79 aA | 76.88±1.94 abAB | 65.55±4.31 bAB | 79.29±1.34 aA |
Significant effect by ANOVA (p<0.05; ns, p>0.05). Means within a row followed by the same lower-case letter, or within a column followed by the same capital letter, do not differ significantly (Tukey test: p≤0.05).
Table 4. Mean mycelial growth (±SE) and minimum inhibitory concentration (MIC) of five accessions of Trichoderma spp. against eight bacterial accessions using the disk diffusion technique. Embrapa Clima Temperado, Pelotas-RS, Brazil.
| Fungi | Concetration | Control | Azospirillum brasilense Ab-V6 |
MIC | Azospirillum brasilense Ab-V5 |
MIC | Bradyrhizobium elkanii SEMIA.587 |
MIC | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Trichoderma harzianum Esalq.1306 |
1x108 | 13.72 ± 0.61 | a | 9.59 ± 1.57 | b | NA(1) | 14.89 ± 0.87 | a | NA | 12.97 ± 0.50 | a | NA | 0.000 | |
| 1x107 | 13.49 ± 0.50 | a | 9.26 ± 1.10 | b | NA | 11.05 ± 1.04 | b | NA | 10.25 ± 0.38 | b | NA | 0.000 | ||
| 1x106 | 11.59 ± 0.51 | a | 9.10 ± 0.33 | b | NA | 7.65 ± 0.33 | b | NA | 8.93 ± 0.50 | b | NA | 0.000 | ||
| 1x105ns | 8.93 ± 0.27 | 8.44 ± 0.83 | NA | 8.85 ± 0.96 | NA | 7.38 ± 0.65 | NA | 0.368 | ||||||
Trichoderma asperellum UFRA.T06 |
1x108 | 14.94 ± 0.59 | a | 11.08 ± 0.87 | b | NA | 15.80 ± 0.69 | a | NA | 13.52 ± 1.05 | ab | NA | 0.008 | |
| 1x107 | 13.07 ± 0.40 | a | 9.70 ± 1.24 | b | NA | 14.99 ± 0.76 | a | NA | 13.68 ± 0.50 | a | NA | 0.004 | ||
| 1x106 | 11.51 ± 0.74 | ab | 12.89 ± 0.68 | a | NA | 9.67 ± 0.97 | b | NA | 12.50 ± 0.30 | ab | NA | 0.033 | ||
Trichoderma asperellum UFRA.T09 |
1x108ns | 8.49 ± 0.44 | 8.92 ± 0.40 | NA | 10.49 ± 1.38 | NA | 9.54 ± 0.36 | NA | 0.333 | |||||
| 1x107 | 8.80 ± 0.47 | a | 8.89 ± 0.39 | a | NA | 9.81 ± 0.39 | a | NA | 10.29 ± 0.19 | a | NA | 0.043 | ||
| 1x106 | 9.87 ± 0.57 | a | 6,53 ± 0.22 | a | NA | 8.84 ± 0.55 | a | NA | 6.53 ± 0.22 | b | NA | 0.001 | ||
Trichoderma asperellum UFRA.T12 |
1x108 | 11.47 ± 0.10 | a | 8.34 ± 0.44 | b | NA | 8.26 ± 0.69 | b | NA | 9.11 ± 0.28 | b | NA | 0.000 | |
| 1x107ns | 9.67 ± 0.23 | 8.75 ± 0.26 | NA | 9.77 ± 0.99 | NA | 8.94 ± 0.80 | NA | 0.626 | ||||||
| 1x106ns | 6.87 ± 2.31 | 1.83 ± 1.83 | NA | 8.46 ± 0.79 | NA | 4.42 ± 2.73 | NA | 0.168 | ||||||
| 1x105ns | 7.40 ± 0.18 | 6.93 ± 0.14 | NA | 6.62 ± 0.45 | NA | 7.06 ± 0.21 | NA | 0.298 | ||||||
| Trichoderma asperellum UFRA.T52 | 1x108 | 16.50 ± 0.29 | a | 15.25 ± 0.25 | ab | NA | 13.19 ± 0.94 | b | NA | 13.75 ± 0.63 | b | NA | 0.008 | |
| 1x107ns | 12.85 ± 0.89 | 13.25 ± 0.63 | NA | 13.00 ± 0.41 | NA | 10.25 ± 1.03 | NA | 0.060 | ||||||
| 1x106ns | 10.61 ± 1.06 | 6.89 ± 2.35 | NA | 6.22 ± 2.10 | NA | 4.57 ± 2.75 | NA | 0.290 | ||||||
(1)No antifungal activity; Significant effect by ANOVA (p<0.05; ns, p>0.05); Means within a row followed by the same lower-case letter, do not differ significantly (Tukey test: p<0.05).
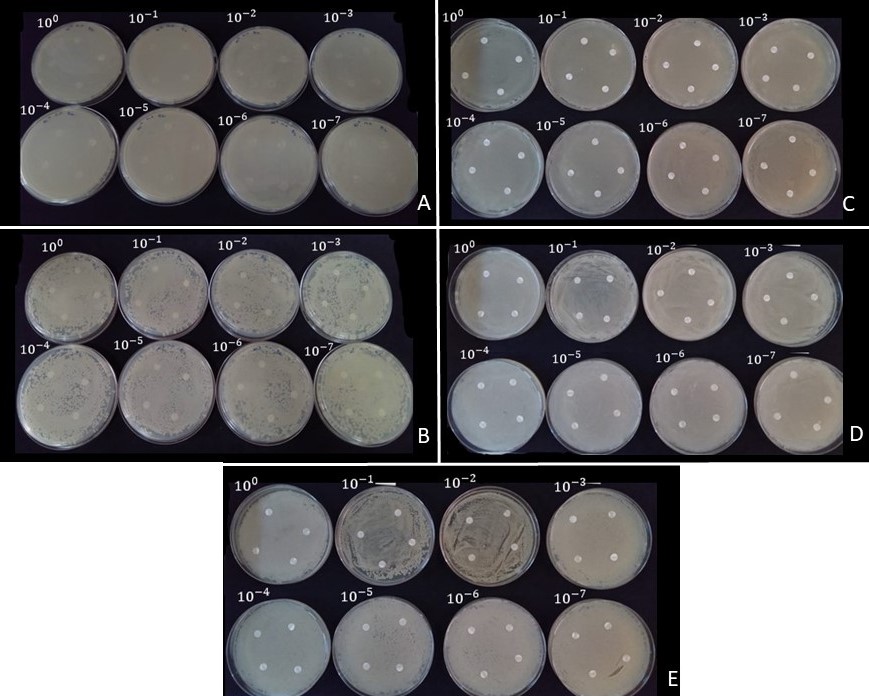
Fig 2. Antimicrobial activity of Trichoderma spp. assessed by determining the minimum inhibitory concentration using the disk diffusion sensitivity test, without occurrence of inhibition of bacterial growth at the different concentrations tested. Exemplarys of Trichoderma asperellum strain UFRA.T06 evaluated with, Bacillus subtilis strain CNPMS.B2084 (A); Bacillus megaterium strain CNPMS.B119 (B); Bradyrhizobium elkanii strain SEMIA.5019 (C); Bradyrhizobium japonicum strain SEMIA.5079 (D); Bradyrhizobium diazoefficiens strain SEMIA.5080 (E). Photograph: Gobbi PC.
T. harzianum has the capacity for superficial colonization in a culture medium rich in sucrose, reaching 50% of growth after 48 hours. However, the standard means of Clinical and Laboratory Standards Institute (NCCLS, 2003), for disc diffusion sensitivity analyses, Müeller–Hinton medium, does not contain a source of sugar, which could have limited the multiplication and colonization of fungi in this study.
Materials and methods
The experiment was conducted at the soil microbiology laboratory at Embrapa Clima Temperado, Pelotas, RS, Brazil (31°42’ South and 52°24’ West). Eight bacterial isolates were used: A. brasilense strains Ab-V5 and Ab-V6; B. subtilis strain CNPMS.B2084; B. megaterium strain CNPMS.B119; B. elkanii strains SEMIA.587 and SEMIA.5019; B. japonicum strain SEMIA.5079; and B. diazoefficiens strain SEMIA.5080. Five isolates of Trichoderma spp. were evaluated, with four isolates of T. asperellum (strains UFRA.T06, UFRA.T09, UFRA.T12 and UFRA.T52) and an isolate of T. harzianum, strain Esalq.1306, preserved in Coleção de Microrganismos Multifuncionais de Clima Temperado (CMMCT).
The compatibility of the microorganisms was evaluated through the antagonistic activity and the antimicrobial activity in the MIC using dual culture (Brito et al., 2018) and disc diffusion techniques (NCCLS, 2003), respectively.
Recovery and cultivation of biological isolates
Bacterial and fungal isolates were recovered and cultivated in Petri dishes (90 mm diameter) containing 25 mL of selective medium according to the characteristics of each species (Table 1). In a laminar flow chamber, smear loops of bacterial colonies, previously incubated in a microbiological oven, were removed
(Table 1), obtaining a concentration of 1–2×108 CFU mL-1 according to tube n°0.5 of the MacFarland scale, through spectrophotometer reading (Logen Scientific SF325NM) at a wavelength of 625 nm (absorbance: 0.8-0.13).
Antagonism of diazotrophs and PGPB against Trichoderma spp.
For the in vitro evaluation of antagonism, 100 μL aliquots of the bacterial suspension were seeded using the Drigalski loop spreading method in Petri dishes (90 mm diameter) containing 25 mL of medium Casein-Peptone Dextrose Yeast Agar (PCA). In this evaluation, PCA was used for all bacterial isolates due to better visualization of mycelial growth and the halo of inhibition.
A 6 mm diameter mycelium disc of an isolate previously grown in a Petri dish containing medium Potato-Dextrose Agar (PDA) (Table 1) was removed and deposited in the center of a Petri dish seeded with the antagonist (bacterial suspension), incubated inverted in a microbiological oven at 28°C, and evaluated after 120 hours of incubation.
The antagonistic activity was evaluated by measuring the mycelium growth diameter, including the inhibition halo, in the presence and absence of the antagonist, with the aid of a digital pachymeter. From these data, the mean percentage values of inhibition of the mycelial growth (ZI) of the test agent (Brito et al., 2018) were calculated using the following equation:
\[ZI = \frac{R1 - R2}{R1} \times 100\]
where:
R1- Diameter of the mycelium in the absence of the antagonist
R2- Diameter of the mycelium in the presence of the antagonist
The experiment was conducted in a CRD with three replications and a total of 40 interactions between fungi and bacteria.
Antimicrobial activity of Trichoderma spp. on bacterial isolates
The antimicrobial activity of Trichoderma spp. was evaluated by determining the MIC using a disc diffusion sensitivity test (NCCLS, 2003). The inoculum suspension of each accession was prepared from colonies incubated in selective medium at 28°C, 24 hours (Table 1). Each bacterium was suspended in 0.85% saline solution (autoclaved at 120°C, 20 min), and the cell concentration was adjusted to 1-2×108 CFU mL-1, according to tube n°0.5 of the MacFarland scale. Subsequently, 100 μL of each bacterial suspension was seeded in a Petri dish (90 mm diameter) containing 25 mL of Mueller-Hinton medium using the Drigalski loop spreading method. Then, glass microfiber filter paper discs 934-AHTM (Whatman®) of 6 mm diameter that were previously autoclaved (120°C, 20 min) were placed on the surface of the agar using sterile tweezers and then inoculated with 10 µL (Rabanal et al., 2002) of the test agent (Trichoderma spp.) at different concentrations. For this, the fungi were cultivated in Petri dishes, as shown in Table 1, and the initial concentration of 1×108 conidia mL-1 was determined by counting conidia in a Neubauer chamber. Serial dilutions were then performed (100 to 10-7) in a 0.85% saline solution. The plates were incubated inverted at 35°C, 48 hours. A positive control of the test agent (Trichoderma spp.) was performed at the different tested concentrations. The data were evaluated after the incubation period and the MIC was determined as the lowest concentration of the test agent responsible for inhibiting the total and partial growth of bacterial isolates. The experiment was conducted in a CRD with four repetitions.
Data analysis
The normality and homoscedasticity of the ZI and mycelial growth data in the different MICs were analyzed using Shapiro-Wilk and Bartlett tests, respectively. As the statistical assumptions were met, an analysis of variance (ANOVA) was performed, followed by the Tukey test (p<0.05), to compare the variables that showed significance using R v.3.4.1 software (R Development Core Team, 2022).
Conclusions
Compatibility and antimicrobial activity responses obtained in the present study confirm the importance of diazotrophs and PGPB that compose inoculants in the action of biocontrol of fungal agents. There was a neutral interaction of B. elkanii strain SEMIA.587 with all Trichoderma spp. Bacillus subtilis strain CNPMS.2084 and Bacillus megaterium strain CNPMS.B119 have the potential to inhibit the growth of Trichoderma spp. In contrast, A. brasilense strains Ab-V5 and Ab-V6 did not have suppressive mechanisms affecting T. asperellum UFRA.T09. Future research is needed to investigate the mechanisms of B. elkanii strain SEMIA.587, a producer of rhizobiotoxin, which limits endogenous ethylene production in low land soils with poor natural drainage, for greater compatibility with Trichoderma spp.
Acknowledgements
The authors acknowledge CNPq, CAPES and Fundação Araucária-STI, entities connected to the project NCT- Microrganismos Promotores do Crescimento de Plantas Visando à Sustentabilidade Agrícola e à Responsabilidade Ambiental” – MPCPAgro, for supporting this work.
References
Abeysinghe S (2009) Effect of combined use of Bacillus subtilis CA32 and Trichoderma harzianum RU01 on biological control of Rhizoctonia solani on Solanum melongena and Capsicum annuum. Plant Pathol. J. 8: 9-16.
Ayoubi N, Zafari, D, Mirabolfathy, M (2012) Combination of Trichoderma species and Bradyrhizobium japonicum in control of Phytophthora sojae and soybean growth. J. Crop Prot. 1: 67-79.
Atieno M, Herrmann L, Okalebo R, Lesueur D (2012) Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J. Microbiol. Biotechnol. 28(7): 2541-2550.
Barbosa JZ, Hungria M, Prior SA, Moura MC, Poggere G, Motta, ACV (2022). Improving yield and health of legume crops via co-inoculation with rhizobia and Trichoderma: A global meta-analysis. Appl. Soil Ecol. 176: 104493.
Barbosa JZ, Hungria M, Sena JVS, Poggere G, Reis AR, Corrêa RS (2021) Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 163: 103913.
Bécquer CJ, Lazarovits G, Lalin I (2013). In vitro interaction between Trichoderma harzianum and plant growth promoter rhizosphere bacteria. Cuba. J. Agric Sci. 47: 97-102.
Benítez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 77(4): 249-260.
Braccini AL, Milléo MR, Alleoni B, Rondina ABL, Paulitsch F, Martins LM, Gravina F, Yoshida TH, Gravina F, Ávila VT, Figueiredo FMRS, Almeida R (2023) Influence of seed co-inoculation with Bradyrhizobium species and Azospirillum brasilense on soybean development in Southern and Southeastern Brazil. Afr. J. of Agric. Res. 19: 81-90.
Brignoli FM, Zampar EJO, Junior JHVA, Cassim BMAR, Inoue TT, Batista MA (2023) Effect of different methods of inoculation and co-inoculation of Bradyrhizobium spp. and Azospirillum brasilense on soybean agronomic performance in fields with a history of inoculation. Arch. Agron. Soil Sci. 69(14): 2925-2937.
Brito TS, Lima WF, Pan R, Porfirio MG, Canello KT Chaves EID (2018) Antagonism of diazotrophic bacteria isolated from maize plants in the biocontrol of phytopathogens. Revista cultivando o saber. 11: 78-88.
Cadore LS, Vey RT, Fresinghelli JCF, Dotto L, Mendes FB, Silva ACF (2020) Trichoderma and Bradyrhizobium japonicum bioformulates on soy initial growth. Ciência e Natura. 42: e22.
Carvalho DDC, Lobo Junior M, Martins I, Inglis PW, Mello SCM (2014). Biological control of Fusarium oxysporum f. sp. phaseoli by Trichoderma harzianum and its use for common bean seed treatment. Trop. Plant Pathol. 39(5): 384-391.
Chagas LFB, Junior AFC, Soares LP, Fidelis RR (2017) Trichoderma in promoting plant growth. ver. de Agric. Neotrop. 4(3): 97-102.
Conte ED, Magro TD, Dal Bem LC, Dalmina JC, Matté JA, Schenkel VO, Schwambach j (2022). Use of Trichoderma spp. in no-tillage system: Effect on soil and soybean crop. Biol. Control. 171: 104941.
Costa GS, Costa, EM, Rocha LB, Silva JKP, Santos JF (2020). Nodulation and phytomass production of cowpea under co-inoculation of Bradyrhizobium elkanii and doses of Trichoderma asperellum. In: Anais do XI Congresso Brasileiro de Agroecologia, São Cristóvão, Sergipe.
Duré, LMM, Galeano RMS, Viana TFC, Roque CG, Matias R, Paggi GM, Corrêa BO, Brasil MS 2022. Bacillus strains with potential for growth promotion and control of white mold in soybean. Biol. 77: 3305-3317.
El-Nahrawy S, Elbagory M, Omara AE (2020) Biocompatibility Effect of Bradyrhizobium japonicum and Trichoderma strains on growth, nodulation and physiological traits of Soybean (Glycine max L.) under water deficit conditions. J. Adv. Microbiol. 20(11): 52-66.
Ferri GC, Braccini AL, Anghinoni FBG, Pereira LC (2017) Effects of associated co-inoculation of Bradyrhizobium japonicum with Azospirillum brasilense on soybean yield and growth. Afr. J. of Agric. Res. 12: 6-11.
Fuga CAG, Vieira BS, Cunha WV (2016) Efficiency and compatibility of Trichoderma spp. and Bacillus spp. isolates on the inhibition of Sclerotium cepivorum. Científica. 44(4): 526–531.
Garcia MVC, Nogueira MA, Hungria M (2021) Combining microorganisms in inoculants is agronomically important but industrially challenging: case study of a composite inoculant containing Bradyrhizobium and Azospirillum for the soybean crop. AMB Express. 11(71):1-13.
Harba M, Jawhar M, Arabi MIE (2020) Antagonistic comparative efficacy of Bacillus species against different soilborne fungal pathogens. J. Agroaliment. Processes Technol. 26(3): 111-116.
Hungria M, Nogueira MA, Araujo RS (2015) Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 6: 811-817.
Hungria M, Nogueira MA, Campos LJM, Menna P, Brandi F, Ramos YG (2020) Seed pre-inoculation with Bradyrhizobium as time-optimizing option for large-scale soybean cropping systems, Agron. J. 112(6), 5222-5236.
Iliäœić RM, Pivić RN, Dinić ZS, Latković DS, Vlajić AS, Joå Ić DL (2017). The Enhancement of Soybean Growth and Yield in a Field Trial through Introduction of Mixtures of Bradyrhizobium japonicum, Bacillus sp. and Pseudomonas chlororaphis. Not. Sci. Biol. 9(2): 274–279.
Iturralde ET, Stocco MC, Faura A, Mónaco CI, Cordo C, Pérez-Giménez J, Lodeiro AR (2020) Coinoculation of soybean plants with Bradyrhizobium japonicum and Trichoderma harzianum: Coexistence of both microbes and relief of nitrate inhibition of nodulation. Biotechnol. Rep. 26: e00461.
Junior AFC, Chagas LFB, Colonia BSO, Martins ALL (2022) Trichoderma asperellum (Samuels, Lieckf & Nirenberg) as a promoter of vegetative growth in soybeans. Ver. de Cienc. Agr. 39(E): 50-68.
Karuppiah V, Vallikkannu M, Li T, Chen J (2019) Simultaneous and sequential based co-fermentations of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841: a strategy to enhance the gene expression and metabolites to improve the bio-control and plant growth promoting activity. Microb. Cell Factories. 18(185): 1-16.
Kaschuk G, Nogueira MA, Luca MJ, Hungria M (2016) Response of determinate and indeterminate soybean cultivars to basal and topdressing N fertilization compared to sole inoculation with Bradyrhizobium. Field Crops Res. 195: 21-27.
Li L, Qu Q, Tian B, Zhang KQ (2005) Induction of Chlamydospores in Trichoderma harzianum and Gliocladium roseum by antifungal compounds produced by Bacillus subtilis C2. J. Phytopathol. 153: 686-693.
Mahmud K, Makaju S, Ibrahim R, Missaoui A (2020) Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants. 9: 97.
MAPA (2011). Instrução Normativa SDA nº 13, de 24 de março de 2011. Available in: https://www.gov.br/agricultura/pt-br/assuntos/sustentabilidade/organicos/arquivos-organicos/in-13-de-28-05-2015-cporg-e-stporg.pdf
Mattos MLT, Cocco, KLT, Oliveira ACB de, Valgas RA, Scivittaro WB, Hungria M (2019) para o incremento da produtividade de genótipos de soja em terras baixas. Embrapa, Pelotas-RS (Boletim de Pesquisa e Desenvolvimento, 330) Available in: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1117798/novas-estirpes-de-bradyrizobium-para-o-incremento-da-produtividade-de-genotipos-de-soja-em-terras-baixas
Mattos MLT, Martins JFS, Fillipi MCC (2020) Efeito de agentes de biocontrole sobre bactérias degradadoras de resíduos de agrotóxicos e fixadoras de nitrogênio (Comunicado técnico 376). Embrapa, Pelotas-RS. Available in: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/218217/1/COMUNICADO-TECNICO-376.pdf
Meyer MC, Mazaro SM, Silva JC (2022) Trichoderma: su uso en la agricultura. 1 ed. Embrapa, Brasília, DF.
Miljaković D, Marinković J, Tamindžić G, Đorđević V, Tintor B, Milošević D, Ignjatov M, Nikolić Z (2022) Bio-Priming of Soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to Improve Seed Germination and the Initial Seedling Growth. Plants. 11(15): 1927.
Minamisawa K, Ogawa K-I, Fukuhara H, Koga J (1996) Indolepyruvate Pathway for Indole-3-Acetic Acid Biosynthesis in Bradyrhizobium elkanii. Plant Cell Physiol. 37(4): 449-453.
Mirriam A, Mugwe J, Raza MA, Seleiman MF, Maitra S, Gitari HH (2022) Aggrandizing soybean yield, phosphorus use efficiency and economic returns under phosphatic fertilizer application and inoculation with Bradyrhizobium. J. Soil Sci. Plant Nutr. 22(4): 5086-5098.
Moretti LG, Crusciol CAC, Bossolani JW, Momesso L, Garcia A, Kuramae EE, Hungria M (2020) Bacterial consortium and microbial metabolites increase grain quality and soybean yield. J. Soil Sci. Plant Nutr. 20: 1923-1934.
Moretti LG, Crusciol CAC, Kuramae EE, Bossolani JW, Moreira A, Costa NR, Alves CJ, Pascoaloto IM, Rondina ABL, Hungria M (2019) Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron. J. 112: 418-428.
Muniz PHPC, Peixoto GHS, Teixeira MPM, Mello SCM, Carvalho DDC (2018) Production of conidia on solid substrate and surface colonization by Trichoderma harzianum. Rev. Agric. Neotrop. 5(4): 40-44.
NCCLS (2003) Performance standards for antimicrobial disk susceptibility tests; approved standard – 8 ed. Wayne, Pennsylvania, USA.
Niu B, Wang W, Yuan Z, Sederoff RR, Sederoff H, Chiang VL, Borriss R (2020) Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 11:585404.
Öğüt M, Akdağ C, Düzdemir O, Sakin MA (2005) Single and double inoculation with Azospirillum/Trichoderma: The effects on dry bean and wheat. Biol. Fertil. Soils. 41(4): 262-272.
Öğüt M, Er F (2006). Micronutrient composition of field-grown dry bean and wheat inoculated with Azospirillum and Trichoderma. J. Plant Nutr. Soil Sci. 169(5): 699-703.
Prasad AA, Babu S (2017) Compatibility of Azospirillum brasilense and Pseudomonas fluorescens in growth promotion of groundnut (Arachis hypogea L.). An. Acad. Bras. Cienc. 89(2): 1027-1040.
R Development Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rabanal RM, Arias A, Prado B, Hernández-Pérez M, Sánchez-Mateo CC (2002) Antimicrobial studies on three species of Hypericum from the Canary Islands. J. Ethnopharmacol. 81: 287-292.
Santos MS, Rodrigues TF, Nogueira MA, Hungria M (2021) The Challenge of Combining High Yields with Environmentally Friendly Bioproducts: A Review on the Compatibility of Pesticides with Microbial Inoculants. Agronomy. 11(5): 870.
Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes-Giannini, MJS (2007) Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp. and Cryptococcus sp. Rev. Ciênc. Farm. Básica Apl. 28: 25-34.
Silva IWD, Goergen PCH, Viau LVM, Fernandes SBV, Silva JAGD, Bianchi CAM (2018) Growth Promoting Microorganisms for Treatment of Soybean Seeds. J.Agric. Sci. 10(6): 198.
Sibponkrung S, Kondo T, Tanaka K, Tittabutr P, Boonkerd N, Yoshida K-I, Teaumroong N (2020) Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms. 8(5): 678.
Thomloudi EE, Tsalgatidou PC, Douka D, Spantidos T-N, Dimou M, Venieraki A, Katinakis P (2019) Multistrain versus single-strain plant growth promoting microbial inoculants - The compatibility issue. Hell. Plant Prot. J. 12(2): 61-77.
Tyśkiewicz R, Nowak A, Ozimek E, Jaroszuk-Ściseł J (2022) Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 23(4): 2329.
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 52: 487–511.