

Aust J Crop Sci. 18(12):819-824 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p96
Use of clove basil extract (Ocimum gratissimum) as a nematode control strategy in cropping systems
Leonardo Cesar Pradebon1, Jaqueline Piesanti Sangiovo1, Ivan Ricardo Carvalho*1, Murilo Vieira Loro2, Larissa Alves de Castro Jocarelli Rossini3, Tuane Araldi da Silva3, Carlos Roberto Polaquini3, Isadora Amalfi de Souza Pinto3, Luiz Henrique da Silva Barros3, Luís Fernando Maranho Watanabe3, Lays Garcia Meireles3
1Regional University of the Northwest of the State of Rio Grande do Sul, Ijuí, Rio Grande do Sul, Brazil
2Federal University of Santa Maria, Santa Maria, Rio Grande do Sul, Brazil
3Biotrop - Biological and Natural Solutions, Vinhedo, São Paulo, Brazil
*Corresponding author: Ivan Ricardo Carvalho 
Abstract: Nematodes are responsible for significant losses in agriculture, especially in crops of great agronomic interest. Control alternatives, with an emphasis on reducing costs and minimizing environmental impacts, have been used, such as the use of biological products based on microorganisms and plant extracts. The objective of this work was to evaluate the efficiency of Ocimum gratissimum L. in controlling the nematodes Meloidogyne incognita and Meloidogyne javanica for lettuce and Pratylenchus brachyurus in bean crops. Experiments were carried out to evaluate biological nematicides in each culture individually. In each culture, experiments were carried out in multi-environments in the states of SC, PR, and SP in Brazil. The experimental design of randomized blocks and four replications was used across all experiments. Agronomic characters, nematode incidence and nematicide efficiency were evaluated for each crop. Analysis of variance was performed to verify the significance of variation effects through the F test at 5% probability. Subsequently, the Tukey mean multiple comparison test was used at a 5% probability level. The results showed that use of Ocimum gratissimum L. reduces the populations of nematodes Meloidogyne incognita and Meloidogyne javanica in lettuce and Pratylenchus brachyurus in beans in 47%.
Keywords: Nematicide efficiency; Agronomic characters; Meloidogyne javanica; Meloidogyne incognita; Pratylenchus brachyurus.
Introduction
Nematodes (Meloidogyne spp.) are one of the genera of pests with wide distribution in the national territory, and which cause economic damage to the main agricultural crops. Damage can be direct and indirect, resulting in reduced grain productivity and quality (Santos et al., 2019). Nematodes are responsible for significant losses in agriculture, especially in crops of great agronomic interest, such as soybeans, maze, rice, cotton and beans.
In Brazil, the most common nematodes in bean cultivation belong to the genera Pratylenchus and Meloidogyne (Ferraz, 2005). The symptoms caused are associated with rot and necrosis of the root system of parasitized plants, resulting in a reduction in radicelles, leading to the loss of pivoting roots. Lettuce is susceptible to attack by nematodes, the estimated damage caused by this pathogen can reach 100% in lettuce crops (Rabello et al., 2021). High populations of Pratylenchus brachyurus can cause reductions of between 21 and 30% in soybean grain productivity (Schimitt and Barker, 1981; Franchini et al., 2014). Damage from attack by nematodes can manifest itself in several ways, through interference with the root system, reducing the absorption of nutrients and water and its symptoms are confused with water stress and nutritional deficiency (Nicol et al., 2011).
Chemical control has mostly been used to minimize damage from nematodes, however, chemical control is associated with contamination of natural resources and risk of human toxicity (Santos et al., 2023), mainly due to the persistence of these molecules in the soil. Control alternatives, with an emphasis on reducing costs and minimizing environmental impacts, have been used, such as the use of biological products based on microorganisms and plant extracts (Mateus et al., 2014; Strom et al., 2020). Fungi and bacteria are the main organisms used in biological control, bacteria of the genus Bacillus have been reported to produce endotoxins, which interfere with the reproductive cycle of nematodes, through their antagonistic effect on the development of this pathogen (Sharma and Gomes, 1996).
Extracts obtained from plants, which contain nematicides compounds such as alkaloids, fatty acids, organic acids, isothiocyanates, phenolic compounds and tannins (Gardiano et al., 2011; Mateus et al., 2014) have also been used. The extract of clove basil (Ocimum gratissimum L.) known as basil has therapeutic, bactericidal, fungal and nematicide activity. Studies have shown the efficiency of Ocimum gratissimum L. extract in controlling root-knot nematodes in eggplant (Hasabo and Noweer, 2005), cowpea (Cole; Aminu; Fawole, 2010) and tomato (Otieno et al., 2020). Its effects can be attributed to the high levels of oxygenated compounds and their lipophilic properties, which dissolve the cytoplasmic membrane of nematodes, through interference in the structure of the enzymatic protein (Knoblock et al., 1989).
Identifying the effectiveness of biological nematicides is essential to adopting ecologically sustainable methods to control nematodes. In this sense, the objective of this work was to evaluate the efficiency of clove basil extract (Ocimum gratissimum L.) in controlling the nematodes Meloidogyne incognita and Meloidogyne javanica for lettuce and Pratylenchus brachyurus in bean crops.
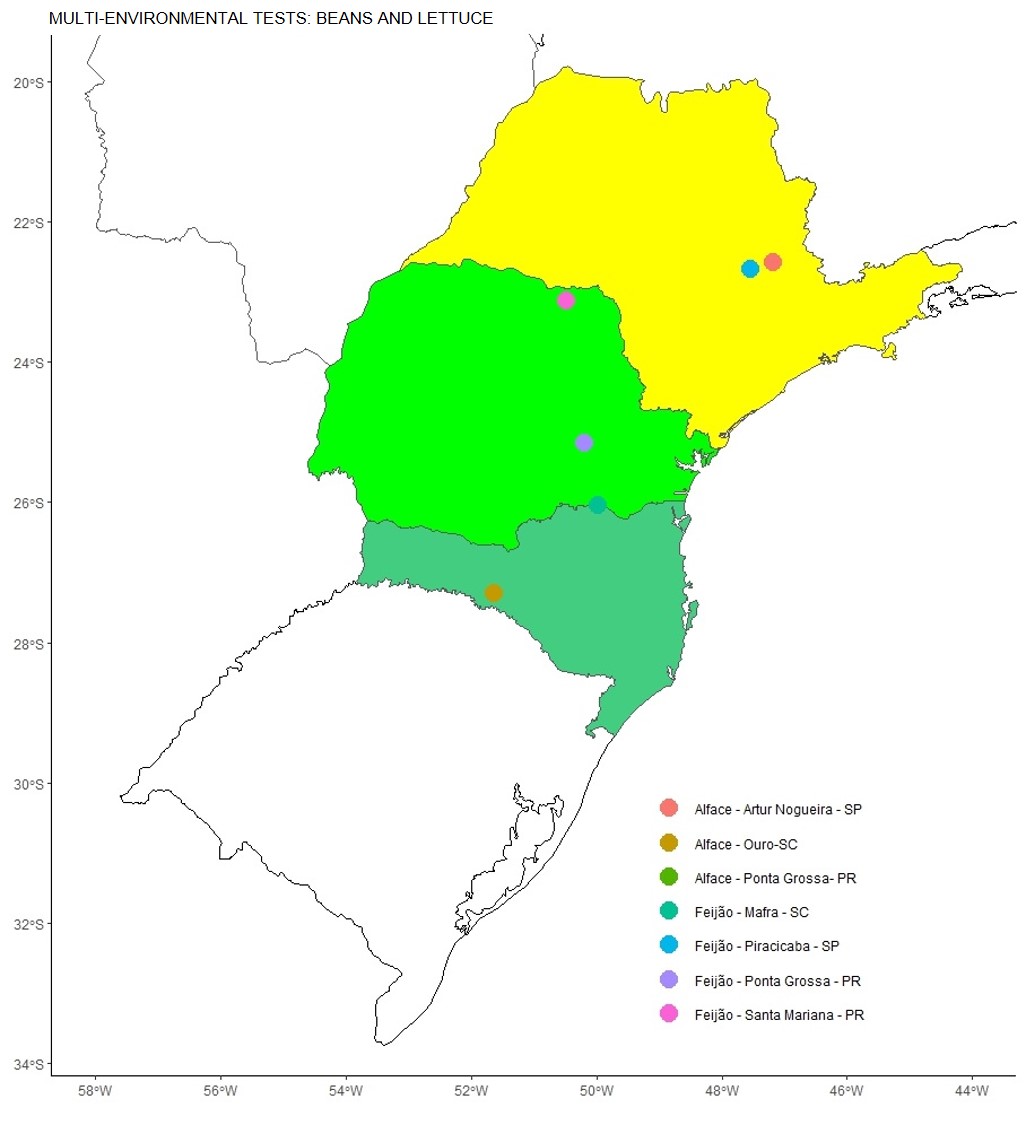
Figure 1. Geographic representation of the environments studied to control Meloidogyne javanica, Meloidogyne incognita in lettuce and Pratylenchus brachyurus in beans.
Results and discussion
Phase 1: Lettuce cultivation
Nematode control, in general, can be done through various methods, such as chemical, physical, cultural or biological, where viability is observed in each case. Therefore, for the percentage of hatching of M. incognita eggs, superior performance was observed with the use of Bacillus subtilis + Bacillus licheniformis and Abamectin, with percentages of 8.8 and 2.7% respectively (Table 2). The percentage of juvenile nematodes in the second stage (J2), superior performance was observed for the absence, with 14.9% and inferiority for the use of abamectin and intermediate performance was evidenced for the use of Ocimum gratissimum L. and Bacillus subtilis + Bacillus licheniformis. Almeida et al., (2017), observed superior results for the use of abamectin in hatching nematode eggs, confirming the results of the present study. However, these same authors found greater control of juvenile nematodes using abamectin, disagreeing with the present study.
Variations in temperature and precipitation were observed between growing environments for lettuce and beans (Figure 2). In lettuce, temperatures above 30°C, recorded in Ouro-SC, can negatively affect the quality of the product (Souza et al., 2013), inducing its early flowering. As for beans, Santa Mariana- PR presented the highest temperatures, reaching up to 32°C, while Ponta Grossa- PR recorded the lowest minimum temperature, at 14°C. Rainfall averages varied according to the months, being suitable for the growth of lettuce (Cavalheiro et al., 2015) and beans (Castro et al., 2008).
Significant effects of the treatment x environment interaction were observed for the variables NNA30AT and DL in lettuce. Significant main effects of environment on NNAT and PROD and of treatment on NNA60AT were also observed.
For beans, significance by the F test was observed for the environment factor for all variables measured, and the treatment factor only for number of nematodes at 30 days after application treatment, number of nematodes at 60 days after treatment, number of galls and grain productivity.
The highest incidence of nematodes before treatment was observed for the site Ponta Grossa- PR with 147.5 nematodes and the environment with the lowest incidence was Ouro- SC with 70.1 nematodes (Suppl Figure 3). This characterization before application allows studying the efficiency of treatments on nematode control. The incidence of nematodes in soils is associated with physical, chemical factors, pH, fertility and oxygen availability in the soil (Stuart et al., 2015). This explains
the difference in the number of nematodes at the sites before treatment.
At 30 days after treatment, no statistical difference was observed between treatments in the environments of Arthur Nogueira- SP and Ouro- SC (Suppl Figure 4). Although there were no significant differences, the lowest incidence of nematodes was observed when applying treatments with Ocimum gratissimum L., at doses of 1 L ha-1, 1.5 L ha-1 and Bacillus subtilis, isolated at a dose of 0.2 L ha-1 (Suppl Figure 4), this is due to the lower infestation in these locations. In Ponta Grossa-PR, the Ocimum gratissimum L. treatment, at a dose of 1 L ha-1, showed the best efficiency in controlling nematodes, exhibiting a control level of 82.17% in relation to the control. The essential oil of clove basil (Ocimum gratissimum L.), contains the eugenol component at a concentration of 43%, this compound showed 100% inhibition of gastrointestinal nematode eggs in small ruminants (Nery et al., 2009). The inhibitory effect of B. subtilis isolates on phytonematodes can be seen through reductions in the reproduction of Meloidogyne spp. in tomato and rice crops (Ludwig et al., 2013; Fernandes et al., 2014).
There was a higher incidence of nematodes in Ponta Grossa- PR for all treatments, with the exception of the Ocimum gratissimum L. treatment at a dose of 1 L ha-1, which did not differ from Ouro- SC and Arthur Nogueira- SP. At 60 days after treatment, it was shown that treatment with Ocimum gratissimum L at a dose of 1 L-1 ha-1 showed superiority in controlling nematodes, with an incidence of 58.7 units, an efficiency of 64% compared to the absence treatment, where an incidence of 166.5 units was found (Suppl Figure 5).
In the environment of Arthur Nogueira -SP, the absence of treatments resulted in a defoliation level of 70%, higher than the remaining treatments, which varied between 61 and 64% (Suppl Figure 6). In Ouro-SC, specific doses of Ocimum gratissimum L demonstrated superior control, while in Ponta Grossa-PR, the lowest defoliation occurred with a dose of 1 L ha-1 of the same extract. This extract, rich in eugenol, proved to be effective in controlling nematodes, proven by a previous study (Moreira et al., 2013). This effectiveness justifies the lower damage caused by defoliation, indicating the possible relevance of compounds absorbable by the leaves for the management of nematodes (Lopes et al., 2005).
Observing the environments within the treatments, it was inferred that only the Ocimum gratissimum L treatments at doses of 0.2 L ha-1 and 1 L ha-1, where the Ouro- SC environment presented a higher percentage of defoliation compared to Arthur Nogueira- SP and Ponta Grossa- PR. For productivity, the best environment was shown to be Ponta Grossa- PR with 24473 kg
Table 1. Description of treatments used in lettuce and bean crops.
| Phases | Culture | Treatments | Active ingrediente | Dose (L ha-1) | Commercial name |
|---|---|---|---|---|---|
| 1 | Lettuce | 1 | Absence | - | |
| 2 | Ocimum gratissimum L. | 0.1 | BTP 171 | ||
| 3 | Ocimum gratissimum L. | 0.2 | BTP 171 | ||
| 4 | Ocimum gratissimum L. | 0.5 | BTP 171 | ||
| 5 | Ocimum gratissimum L. | 1 | BTP 171 | ||
| 6 | Ocimum gratissimum L. | 1.5 | BTP 171 | ||
| 7 | Bacillus subtilis, isolate CNPSo 2657 | 0.2 | Furatrop | ||
| 2 | Beans | 1 | Absence | ||
| 2 | Ocimum gratissimum L | 0.05 | BTP 171 | ||
| 3 | Ocimum gratissimum L | 0.2 | BTP 171 | ||
| 4 | Ocimum gratissimum L | 0.5 | BTP 171 | ||
| 5 | Ocimum gratissimum L | 1 | BTP 171 | ||
| 6 | Bacillus subtilis, isolate CNPSo 2657 | 0.2 | Furatrop | ||
| 7 | Bacillus subtilis + Bacillus licheniformis | 0.2 | Quartzo |
Table 2. Multiple comparison test of means for Percentage of Hatching of M. incognita eggs and Percentage of Juvenile Nematodes (J2) of M. incognita.
| Product Validation | ||
|---|---|---|
| PH1 | ||
| Absence | 73.1 a | |
| Ocimum gratissimum L. | 44.0 b | |
| Bacillus subtilis + Bacillus licheniformis | 8.8 c | |
| ABAMECTIN | 2.7 c | |
| PJN2 | ||
| Absence | 14.9 c | |
| Ocimum gratissimum L. | 65.9 b | |
| Bacillus subtilis + Bacillus licheniformis | 71.4 b | |
| ABAMECTIN | 96.7 a | |
Means followed by the same lowercase letter in the column do not statistically differ from each other at 5% probability using the Tukey test. 1PH- Percentage of Hatching; 2PJN- Percentage of Juvenile Nematodes (J2)
of green weight ha-1, while the environments of Arthur Nogueira- SP and Ouro- SC revealed similar productivity of 18728 kg of green weight ha-1 (Suppl Figure 7).
Phase 2: Bean cultivation
The number of nematodes before application for bean cultivation showed the highest number of individuals for the Mafra- SC environment with 69.1 units. Ponta Grossa- PR found 37.7 units, being the environment with the lowest number of individuals (Suppl Figure 8). Nematodes have a wide distribution, and as beans are a host plant, their cultivation in infested areas promotes an increase in their population, causing losses that can reach up to 90% (Baida et al., 2011) and can spread over a wide range variety of climatic conditions mainly in tropical areas (Caillauda et al., 2008). One of the main factors affected by the nematode population in the soil is the plant stand, where they parasitize the root system and affect the absorption of water and nutrients essential for plant growth and development (PERRY et al., 2009). It was observed that the plant stand in Mafra-SC was superior in relation to other environments with 12.6 plants per meter-1, while inferiority was found in Piracicaba- SP, an environment where the highest number of nematodes was found 30 days after applying the treatments (Suppl Figure 9).
For the environmental factor, the number of nematodes 30 days after application, it was observed that the environment of Piracicaba- SP and Mafra- SC showed a higher incidence of Pratylenchus brachyurus with 49.5 units and 44.9 units respectively (Suppl Figure 10). The interaction between treatments, it was observed that the absence and Ocimum gratissimum L at a dose of 0.05 L ha-1 resulted in a higher incidence of Pratylenchus brachyurus, with 73.9 units and 46.8 units, respectively. The other treatments were efficient, with an incidence of nematodes between 26.12 and 31.5 units. Therefore, the use of Ocimum gratissimum L, Bacillus subtilis, isolate and Bacillus subtilis + Bacillus licheniformis proved to be efficient in controlling nematodes. Study by Oliveira et al. (2017), with the use of Bacillus sp., in the control of nematodes in beans, reported a reduction in the nematode population in plants that received treatment with Bacillus subtilis, due to the ability to produce
nematicidal endotoxins and interfere in the life cycle of these parasites.
There was a lower incidence of P. brachyurus 60 days after application, in Santa Mariana-PR (57.2 units). In Piracicaba- SP, Ponta Grossa- PR and Mafra the incidence of nematodes varied from 79 to 83.7 units (Suppl Figure 12). The greatest efficiency in nematode control was observed when the Ocimum gratissimum L. treatment was applied at a dose of 1 L ha-1, with an efficiency of 64.78% compared to its absence (Suppl Figure 13). The greater efficiency of Ocimum gratissimum L. in controlling nematodes is attributed to the nematicidal action of the compound eugenol. This component alters the vital systems of nematodes Meloidogyne spp. and Pratylenchus spp., interfering with various phases of their cycle, such as cell multiplication, embryonic development and egg hatching, paralyzing these processes (ROCHA, 2003).
In Santa Mariana- PR and Piracicaba- SP the highest number of galls was observed (1.29 and 0.50 units, respectively) (Suppl Suppl Figure 14). There was less gall formation when using the treatments Ocimum gratissimum L. at doses of 0.2 L ha-1, 0.5 L ha-1 and 1 L ha-1 and Bacillus subtilis 0.2 L ha-1 and Bacillus subtilis + Bacillus licheniformis with values between 0.713 and 0.76 galls (Suppl Figure 15). Studies have reported the efficiency of clove basil extract in reducing the number of galls in beans (Martins and Santos, 2016; Onyeke and Akueshi; 2012) and tomatoes (Lopes et al., 2005).
The highest averages of bean grain productivity were observed in the environments of Mafra-SC, Ponta Grossa- PR and Santa Mariana- PR (2266 Kg ha-1 to 2366 Kg ha-1), not differing statistically (Suppl Figure 16). Treatments with Ocimum gratissimum L. at doses of 0.5 L ha-1 and 1 L-1, Bacillus subtilis 0.2 L-1 and Bacillus subtilis + Bacillus licheniformis promoted the highest average bean grain productivity (2336, 2377, 2354 and 2410 kg ha-1 of grains, respectively) (Suppl Figure 17).
The use of Ocimum gratissimum L. and Bacillus subtilis proved to be efficient in reducing the nematode population in both crops. A correlation was observed between the application of these treatments and the reduction in the number of nematodes,
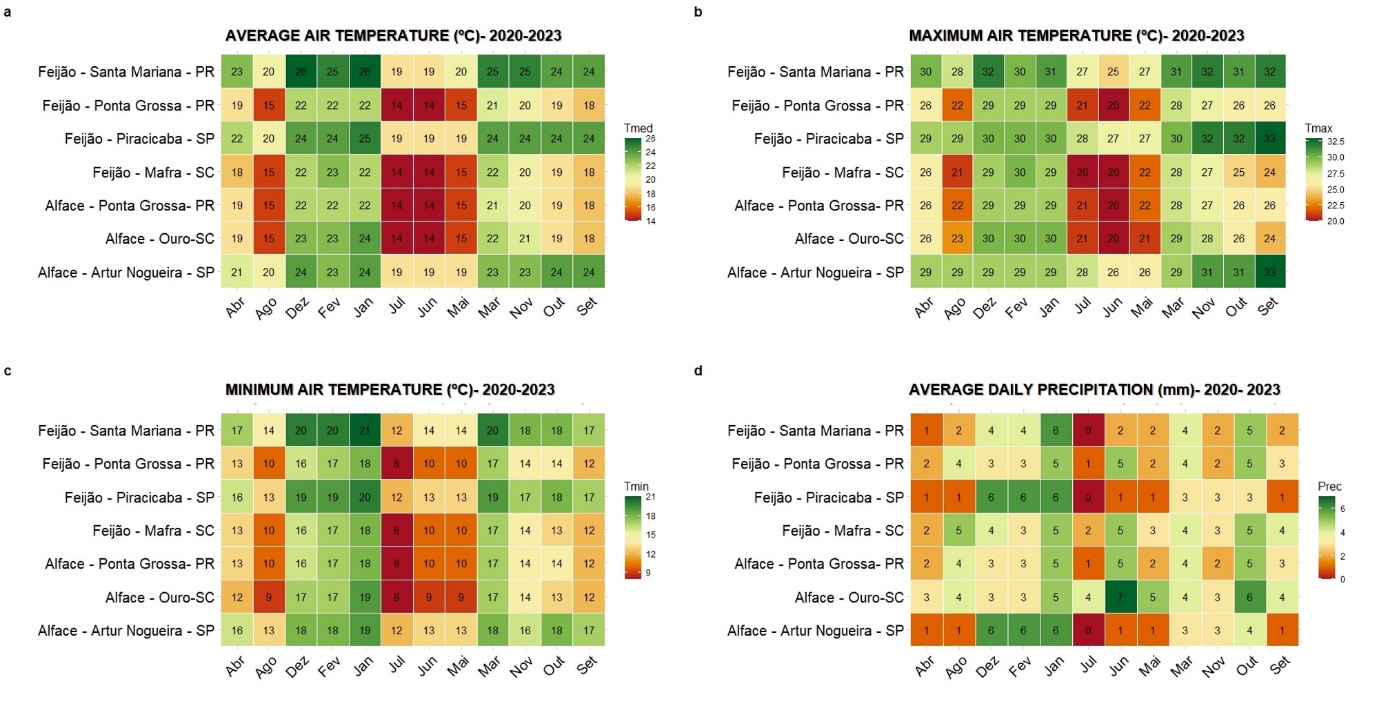
Figure 2. Representation of meteorological conditions relating to lettuce and bean growing periods in different environments.
Table 3. Summary of the analysis of variance for variables relating to lettuce and bean crops evaluated in different environments.
| FV | DF | Lettuce | |||||
|---|---|---|---|---|---|---|---|
| MS | |||||||
| NNAT | NNA30AT | NNA60AT | DL | FW | |||
| Environment | 2 | 42680.583* | 93044.86* | 4861.904 | 6.333 | 307970305* | |
| Treatment | 6 | 735.916 | 13901.762* | 16700.634* | 74.797* | 2924511 | |
| Block | 3 | 4129.98 | 3149.73 | 688.329 | 0.857 | 13235208 | |
| Env X Treat | 12 | 2037.625 | 3108.494* | 1313.557 | 4.583* | 1399830 | |
| Residue | 60 | 1929.692 | 1280.947 | 1897.504 | 1.698 | 6555838 | |
| Beans | |||||||
| NNAT | NNA30AT | NNA60AT | STD | NG | PROD | ||
| Environment | 3 | 5203.952* | 3385.291* | 4258.190* | 0.765* | 3.460* | 282934.674* |
| Treatment | 6 | 309.226 | 4799.320* | 19249.666* | 0.253 | 3.516* | 240038.925* |
| Block | 3 | 96.523 | 75.043 | 183.809 | 0.031 | 0.075 | 81.439 |
| Env X Treat | 18 | 179.48 | 207.646 | 266.746 | 0.050 | 0.128 | 6519.934 |
| Residue | 81 | 428.499 | 196.387 | 354.994 | 0.216 | 0.077 | 20477.056 |
Factor of variation (FV); degrees of freedom (DF); mean square (MS); number of nematodes in pre-application (NNAT); number of nematodes 30 days after application (NN30DAT, units); number of nematodes 60 days after application (NN60DAT); defoliation (DL, %); fresh weight productivity (FW, kg ha-1); grain productivity (kg ha-1); and number of galls (NG, units).
especially for Ocimum gratissimum L., whose eugenol component proved to be effective in controlling nematodes, as confirmed by previous studies (Moreira et al., 2013). The treatment x environment interaction was also relevant, highlighting the influence of local conditions on the effectiveness of these treatments. Environments with different climatic and soil characteristics showed varied responses to the treatments applied, indicating the importance of customizing these management strategies according to local conditions.
The results point to the relevance of integrated nematode management, considering not only specific treatments, but also environmental conditions and the interaction between different variables. The use of leaf-absorbable compounds, such as eugenol, and the application of beneficial bacteria, such as Bacillus subtilis, have shown promise in controlling nematodes, offering potential for sustainable management strategies.
However, it is essential to continue research to improve these control techniques and better understand the interaction between treatments, environments and the affected crops. Investing in studies that evaluate the long-term effectiveness, environmental impacts and sustainability of these approaches will allow the development of efficient and ecologically appropriate methods for nematode control.
Due to the potential for damage caused by nematodes in annual crops and their wide distribution, new control strategies must be adopted, such as the use of biological nematicides, aiming to reduce dependence on synthetic molecules. The use of Ocimum gratissimum L. and Bacillus subtilis efficiently reduced nematodes in both cultures. Especially eugenol from Ocimum gratissimum L.
proved to be effective, corroborating previous studies (Moreira et al., 2013). The treatment x environment interaction was notable,
highlighting the need to adapt management strategies according to the environment. The use of leaf-absorbable compounds and beneficial bacteria offers the potential for sustainable strategies. Soon. It is essential that studies be developed related to the efficiency of biological nematicides, ensuring effective and ecologically viable approaches to nematode control.
Materials and methods
Study area and experimental design
Phase 1: Lettuce Cultivation
The experiment was conducted in the municipalities of Artur Nogueira - SP (Latitude 22°31'45.24"S and Longitude: 47°7'0.87"W, 613m), Ouro - SC Latitude (27°18'14.13"S and Longitude: 51 °37'55.88"W, 525m) and Ponta Grossa- PR (Latitude 25°09'45.68"S and Longitude 50°11'24.69"W, 806m). A randomized block experimental design was used with seven treatments (Table 1) and four replications. The plots consisted of an area of 3.4 m². The lettuce cultivar used was Elisa, transplanted in the first half of February 2023, with a spacing of 0.40 m between rows and 2 plants per linear meter -1. Base fertilization took place with 300 kg ha-1 of formula 04-14-08 NPK. Cultural treatments took place in order to minimize biotic effects in the experiment. The treatments were applied after transplanting, at a dose of 150 L ha-1, applied with a knapsack sprayer with constant CO2 pressure, with a pressure of 35 lb in-2. In each plot, a soil sample was taken to evaluate the percentage of hatching of nematode eggs (PH), percentage of juvenile nematodes in the second stage (PJN), number of nematodes before application treatment (NNAT, units); number of nematodes 30 days after application (NNA30AT, units); number of nematodes 60 days after application (NNA60AT, units). The evaluation of the number of nematodes in each of the periods was carried out by counting the number of live nematodes, in 100 cm³ of soil sample and per gram of root, and counted under an optical microscope, with the aid of a Peters counting slide.
Lettuce fresh weight productivity (PROD, Ton ha-1) was evaluated by evaluating plants at 2.0 m2 per experimental unit, transforming the values into kg ha-1.
Phase 2: Beans cultivation
The experiment was conducted in the municipalities of Mafra - SC (Latitude 26°2'0.76" S and Longitude 49°58'53.14" W, 794m), Piracicaba - SP (Latitude 22º`40´18.87"S and Longitude 47º32´20.96"W, 578m), Ponta Grossa - PR (Latitude 25°16'38.66"S and Longitude 50°14'35.83"W, 876m) and Santa Mariana - PR (Latitude 23 °7'38.03” S and Longitude 50°28'42.56” W, 418m). A randomized block experimental design was used with seven treatments (Table 1) and four replications. The bean cultivar used was IAC Veloz, sown in the first half of October 2022, with a sowing density of 13 seeds per meter-1. The experimental unit consisted of six sowing lines spaced 0.5m and five meters in length, with a useful area of 4 m2. The treatments were applied via the sowing furrow, using a knapsack sprayer with a constant pressure of 1 bar, pressurized by CO2, with a spray volume of 60 L ha-1. Five plants were evaluated per experimental unit, where the following were measured: the number of nematodes before application treatment (NNAT, units); number of nematodes 30 days after application (NNA30AT, units); and the number of nematodes 60 days after application (NNA60AT, units). The evaluation of the number of nematodes in each of the periods was carried out by counting the number of live nematodes, in 100 cm³ of soil sample and per gram of root, and counted under an optical microscope, with the aid of a Peters counting slide. To extract specimens from root samples (10 g), the method of grinding in a blender and sieving was used. Counting the extracted nematodes, both from the soil and roots, was carried out using a Peters chamber and an optical microscope.
The evaluation of the plant stand (STD, units) was carried out by counting the number of plants in one meter in the two central lines of each experimental unit. Number of galls (NG, units), by counting the galls on the root system of five plants per plot; and grain productivity (PROD, kg ha-1), by harvesting four m2, in each experimental unit, correcting the sample humidity to 13%.
Statistical analysis and multiple linear regression
Meteorological information on average air temperature (Tmed, °C), minimum air temperature (Tmin, °C) and maximum air temperature (Tmax, °C), precipitation (Prec, mm), were expressed with the purpose to better understand the results obtained (NASA POWER, 2023).
Analyzes of normality of errors were carried out using the Shapiro Wilk test and homogeneity of residual variances using the Bartlett test at 5% significance. Analysis of variance was performed to verify the significance of variation effects through the F test at 5% probability. Subsequently, the Tukey mean multiple comparison test was used at a 5% probability level. To carry out the statistical analyzes the Exp.Des.pt, agricolae and metan packages were used through the R Core Team 2023 Software (R CORE TEAM, 2023).
Conclusion
The use of Ocimum gratissimum L. reduces the populations of nematodes Meloidogyne incognita and Meloidogyne javanica in lettuce and Pratylenchus brachyurus in beans, in 47%.
Acknowledgements
The authors thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES), the Brazilian National Council for Scientific and Technological Development (CNPq), the Regional University of the Northwest of the State of Rio Grande do Sul (UNIJUÍ), and the Biotrop Biological and Natural Solutions Company for financial support and for granting Scholarship for Scientific and Technological Initiation, Graduate Studies, and Research Yield; and the UNIJUÍ Graduate Programs in Environmental Systems and Sustainability for making resources available for the development of this research.
References
Almeida AA, Abe VHF, Gonçalves RM, Peña MIB, Santiago DC (2017) Seed treatment for management of Meloidogyne javanica in soybean. Semina: Agricultural Sciences. 38(5): 2995-3005.
Baida FC, Santiago DC, Takashi LSA, Athanázio JC, Cadioll MC, Levy RM (2011) Reaction of snap bean lines to Meloidogyne javanica and M. paranaensis in greenhouse conditions. Acta Scientiarum. Agronomy. 33: 237-241.
Caillaud MC, Dubreuil G, Quentin M, Perfus-Barbeoch L, Lecomte P, Engler JA, Abad P, Rosso MN, Favery B (2008) Root-knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 165: 104-113.
Castro PRC, Kluge RA, Sestari I (2008) Plant physiology manual: crop physiology.
Cavalcanti ESB, Morais SM, Lima MA, Santana EWP (2004) Larvicidal Activity of essential oils from Brazilian plants against Aedes aegypti L. Memories of the Osvaldo Cruz Institute.
Cavalheiro DB, Klosowski ES, Henkemeier NP, Gonçalves Junior AC, Vasconcelos ES, Chibiaqui E (2015) Production of lettuce (Lactuca sativa L.) cv. Vanda, grown under different environments and levels of mineral and organic fertilizer. Cultivating Knowledge. 8(1): 107-122.
Coimbra JL, Soares ACF, Garrido MS, Sousa CS, Ribeiro LB (2006) Toxicity of plant extracts to Scutellonema bradys. Brazilian Agricultural Research. 41(7): 1209-1211.
Cole AOC, Aminu AE, Fawole B (2012) Evaluation of plant extracts in the management of root-knot nematode Meloidogyne incognita on cowpea [Vigna unguiculata (L) Walp]. Mycopath. 8(2).
Dias CR (2000) Effect of aqueous extracts of medicinal plants on the survival of Meloidogyne incognita juveniles. Brazilian Nematology. 24(2): 203-210.
Fernades RF, Lopes EA, Vieira BS, Bontempo AF (2013) Control of Meloidogyne javanica in bean crops with isolates of Bacillus spp. Tropica Journal: Agricultural and Biological Sciences. 7, n.1.
Fernandes RH, Vieira BS, Fuga CAG, Lopes EA (2014) Pochonia chlamydosporia and Bacillus subtilis in the control of Meloidogyne incognita and M. javanica in tomato seedlings. Bioscience Journal. 30:194-200.
Franchini JC, Debiasi H, Dias WP, Ramos Júnior EU, Silva JFV (2014) Loss of soybean productivity in an area infested by root lesion nematode in the mid-northern region of Mato Grosso, In: Bernardi ACC, Naime JM, Resende AV, Bassoi LH, (Ed.) Precision agriculture: results from a new look. São Carlos: Embrapa Instrumentação. p.274-278.
Gardiano CG, Muramoto SP, Kryzanowiski AA, Almeida WP, Saab OJG (2011) A. Effect of aqueous extracts of plant species on the multiplication of Rotylenchulus reniformis Linford & Oliveira. Archives of the Biological Institute. 78(4): 553-556.
Guimarães NN, Silva RV, Guimarães LN, Oliveira BS, Santos BKS, Oliveira YA (2020) Effect of adding plant residues on the control of Meloidogyne javanica in lettuce. Brazilian journal of development, Curitiba. 6(1): 2350-2357.
Hasabo SA, Noweer EMA (2005) Management of Root-Knot Nematode Meloidogyne incognita on Eggplant with some Plant Extracts. Egypt J. Phytopathol. 33(2): 65-72.
Heinemann AB, Villegas JR, Souza TLP, Didonet AD, Stefano JG, Boote KJ, Jarvis A JLH, Ribeiro ND, Rosa SS, Jost E, Poersch NL, Medeiros SLP (2007) Response of bean cultivars to high air temperature during the reproductive period. Rural Science. 37(6); 1543-1548.
Knobloch K, Pauli A, Iberl B, Weigand H, Weis N (1989) Antibacterial and antifungal properties of essential oil components. Journal of Essential Oil Research. 1(3): 119-128.
Lopes EA, Ferraz S, Freitas LG, Ferreira PA, Amora DX (2005) Effect of aqueous extracts of black mucuna and basil on Meloidogyne incognita and M. javanica. Brazilian Nematology. 29: 67-74.
Ludwig J, Moura AB, Gomes CB (2013) Potential of microbiolization of rice seeds with rhizobacteria for the biocontrol of root-knot nematodes. Tropical Plant Pathology. 38:264-268.
Martins MCB, Santos CDG (2016) Action of medicinal plant extracts on juveniles of Meloidogyne incognita race 2. Agricultural Science Journal. 47: 135-142.
Mateus MAF, Faria CMDR, Botelho RV, Dallemole-Giaretta R, Ferreira SGM, Zaluski, WL (2014) Aqueous extracts of medicinal plants in the control of Meloidogyne incognita (Kofoid e White, 1919) Chitwood, 1949. Bioscience Journal. Uberlândia, 30(3): 730-736.
Morais RA, Koga PS, Noetzold R, Silva JD, Costa RC (2018) Lettuce cultivation in different spatial arrangements of plants. Cultivating Knowledge Journal. 11(2): 20-30.
Moreira LCB, Vieira BS, Mota Júnior CV, Lopes EA, Canedo EJ (2013) Nematicidal effect of eugenol on tomato plants. Tropical Agricultural Research. 43(3).
Nery PS, Duarte ER, Martins ER (2009) Efficacy of plants for the control of gastrointestinal nematodes in small ruminants: review of published studies. Brazilian Journal of Medicinal Plants. 11:330-338.
Nicol JM, Turner SJ, Coyne DL, Nijs LD, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. Genomics and molecular genetics of plant-nematode interactions. p. 21-43, 2011.
Oliveira GRF, Silva MS, Proença SL, Bossolani JW, Camargo JA, Franco FS, Sá ME (2017) Influence of Bacillus subtilis on the biological control of nematodes and productive aspects of bean. Brazilian Journal of Biosystems Engineering. 11(1): 47-58.
Onyeke CC, Akueshi CO (2012) Infectivity and reproduction of Meloidogyne incognita (Kofoid and White) Chitwood on African yam bean, Sphenostylis stenocarpa (Hochst Ex. A. Rich) Harms accessions as influenced by botanical soil amendments. African Journal of Biotechnology. 11(67): 13095-13103.
Oparaocha ET, Iwu I, Ahanaku JE (2010) African Journal of Science and Technology (AJST) Science and Engineering Series. 9(2): 82 – 89.
Otieno PC, Mulwa RMS, Ogweno JO (2020) Management of root knot nematodes (Meloidogyne sp.) and enhancing growth yield of greenhouse produced tomatoes by using fresh plant derived soil amendments. Advances in Horticultural Science. 34(4): 357-372.
Owusu EO, Akutse KS, Afreh NK (2008) Effect of some traditional plant components on the control of termites, macrotermes spp. (Isoptera:Termitidae). African Journal of Science and Technology (AJST) Science and Engineering Series. 9(2); 82 – 89.
Perry RN, Moens M, Starr JL (2009) Root Knot Nematodes. CABI International. Wallingford. 488 p.
Pinheiro JB, Pereira RB, Carvalho ADF, Rodrigues CS, Suinaga FA (2013) Nematode management in lettuce. Brazilian Agricultural Research Company-EMBRAPA. Brasília-DF.
Rabello LKC, Gonçalves AO, Cruz TP, Zinger FD, Jesus Junior WC, Rodrigues LL, Souza AF, Moraes WB, Alvez FR (2021) Quantification of damage and yield losses caused by Root-knot nematode in lettuce in Brazil. IDESIA (Chile) 39(2): 121-130.
Rocha FS, Campos VP, Dutra MR, Nunes AS, Silva JRC (2005) Action of plant root exudates on hatching, motility, mortality and penetration of Meloidogyne incognita juveniles. Summa Phytopathologica. 31(2): 187-193.
Santos ARB, Almeida FA, Fonseca WL, Carvalho RM, Silva LMA, Simeão M, Santos TS, Fonseca KRL (2023) Biocontrol agents in the management of Meloidogyne incognita in soybean crops. DELOS: Desarrollo Local Sostenible, Curitiba. 16(45): 1615-1631.
Santos MFA, Mattos VS, Monteiro JMS, Almeida MRA, JUNIOR AJ, Cuidados JE, Sereno PC, Coyne D, Carneiro RMDG (2019) Diversity of Meloidogyne spp. from peri-urban areas of sub-Saharan Africa and their genetic similarity with populations from the Latin America. Physiological and Molecular Plant Pathology. 110-118.
Schmitt DP, Barker KR (1981) Damage and reproductive potentials of Pratylenchus brachyurus and P. penetrans on soybean. Journal of Nematology. 13: 327-332.
Sharma RD, Gomes AC (1996) Effect of bacillus spp. Toxins on oviposition and juvenile hatching of Heterodera glycines. Brazilian Nematology 20:53-62.
Souza AL, Júnior SS, Diamante MS, Souza LHC, Nunes MCM (2013) Behavior of iceberg lettuce cultivars under tropical climate. Caatinga Journal, Mossoró. 26(4):123–129.
Strom N, HU W, Haarith D, Chen S, Bushley K (2020) Interactions between soil properties, fungal communities, the soybean cyst nematode, and crop yield under continuous maze and soybean monoculture. Applied Soil Ecology. 147:103388.
Stuart RJ, Barbercheck ME, Grewal PS (2015) Entomopathogenic nematodes in the soil environment: distributions, interactions and the influence of biotic and abiotic factors. Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection. 97-137.