

Aust J Crop Sci. 18(12):847-857 (2024) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.24.18.12.p134
Role of rice bacterial endophytes from organic and conventional paddy fields as biofertilizers and bio-resistance inducers against insecticide toxicity
Vita Ratri Cahyani*1, Sudadi1, Hadiwiyono2, Laily Shofiyah3
1Department of Soil Science, Faculty of Agriculture, Sebelas Maret University, Indonesia
2Department of Agrotechnology, Faculty of Agriculture, Sebelas Maret University, Indonesia
3Master Study Program of Soil Science, Faculty of Agriculture, Sebelas Maret University, Indonesia
*Corresponding author: Vita Ratri Cahyani 
Abstract: Different management methods of paddy fields affects plant growth and associated microbiota, including endophytes over long periods. This study aimed to isolate and compare the functional capabilities of endophytic bacteria from the roots and leaves of rice plants cultivated in organic and conventional paddy fields. After surface sterilization of the roots and leaves samples the endophytic bacteria were isolated on agar plates using NA, YEMA, and Pikovskaya media. Results showed the higher diversity and population density of endophytic bacteria in rice plants from organic fields compared to conventional fields. A total of 28 distinct bacterial colonies were purified, subcultured, and characterized for multifunctional abilities as biofertilizers and resistance to insecticides (chlorantraniliprole 100 g L-1 and thiamethoxam 200 g L-1). Each of the selected isolates of YEROI-1, PELOI-3, and NEROI-2 that were closely related to Burkholderia sp., Burkholderia seminalis and Bacillus paramycoides, respectively. The isolates and control were applied to rice seedling in agarose medium with and without insecticide (10 x) dose using completely randomized design with three replications. The result showed that isolate NEROI-2 significantly increased rice seedling growth and induced resistance to insecticide toxicity, followed by PELOI-3. In contrast, YEROI-1 inhibited rice seedling growth. The present finding revealed the functional capability of the selected isolates by in vitro and in vivo (soilless media) assessments. Further studies are needed to confirm the capability of the selected isolates in a greenhouse experiment by using soil media.
Keywords: in vivo assessment; multifunctional isolate; resistance test; rice cultivation system; rice seedling.
Abbreviations: DAP_days after planting; NA_nutrient agar; NB_nutrient broth; NFB_nitrogen fixing bacteria; KSI_ potassium solubilization index; PSB_phosphate solubilizing bacteria; PSI_phosphate solubilizing index; YEMA_yeast extract mannitol agar.
Introduction
The total area of organic rice land increased sharply from 1,401 ha in 2016 to 53,974 ha in 2018 or around 21% of the 251,631 ha total organic agricultural land in Indonesia (Organic Institute et al., 2019), meaning that it is only around 0.47% of the 11,377,934 ha total area of rice harvested land (BPS-Statistics Indonesia, 2022). This fact reveals that rice cultivation practices in Indonesia are still dominated by conventional farming systems, which rely on manufactured chemical inputs such as various chemical fertilizers and pesticides. The effects of the excessive use of chemical fertilizers are not visible immediately, but the pollution impact emerged over the time, including accumulation of nitrate and phosphate that results in water eutrophication, soil degradation, soil salinity (Chislock et al., 2013), and accumulation toxic heavy metal in agricultural topsoil (Hu et al., 2024). The toxic effects of accumulated of pesticide residues have broad impacts on ecosystems, including soil degradation or decreased soil health (Maggi et al., 2020), adverse effects on the biotic environments of non-target organism (Wandscheer et al., 2017) and decrease in biodiversity (Simarmata et al., 2023). These residues can also cause phytotoxic effect on the cultivated plants (Ajermoun et al., 2022) and impacts non-biotic environments (Abdullah et al., 2017). Consequently, this toxicity effect leads to reduced plant productivity and poses risks to human health (Hernández et al., 2013). Various efforts to overcome the toxic effects of pesticide residues on agricultural lands have been widely reported, either by applying various ameliorant materials such as such as compost (Marín-Benito et al., 2018), biochar (Arridho et al., 2019), plant residue and animal dung (Joshi et al.,
2019), using microbiota which act as bioremediation agents or pesticide degraders (Castro-Gutiérrez et al., 2019) or by using a plant-based approach in phytoremediation (Gao et al., 2023). Long term land use with different cultivation systems such as organic and conventional farming systems has been proven to affect the chemical, physical and biological characteristics of the soil, and also to the characteristics of the grown plants and the associated microbiota (Bai et al., 2020). This fact indicates that plants as the productive resources are influenced direct and indirectly by the given inputs to the cultivated land either in the form of healthy organic inputs or toxic chemical inputs, in which consequently affects the abundance, diversity, characteristics and functional abilities of their endophytic bacteria (Shofiyah et al., 2023; Sunaryo et al., 2019).
Bacterial endophytes are a class of endosymbiotic microbiota widespread among plants that live or spend at least parts of their life cycle inside plants or in plant tissues (Hardoim et al., 2015) or colonize intercellular and intracellular spaces of all plant compartments (Miliute et al., 2015). Previous studies reported that endophytic bacteria do not visibly harmful effects on the plants (Hallmann et al., 1997), do not cause plant disease or significant morphological changes (Miliute et al., 2015). However, some other studies reported a range of different lifestyles of endophytic bacteria with their host, the most common endophytes are typed as commensals, with unknown or yet unknown functions in plants, and less common ones are detected to have positive (symbiotic, mutualistic, trophobiotic) or negative (antagonistic, pathogenic) effects on plants (Brader et al., 2017; Hardoim et al., 2015).
Many studies have successfully isolated rice bacterial endophytes from leaves, roots, stems and seeds of several rice varieties (Kumar et al., 2020; Sun et al., 2008), or from rice plants grown in flooded conditions, dry conditions, phase changes after flooding (Bertani et al., 2016; Ferrando and Fernández Scavino, 2015), and wild rice plants (Koomnok et al., 2007), and organic cultivation (Phetcharat and Duangpaeng, 2012). Furthermore, previous studies reported that the endophytic bacteria of rice plants has the ability to produce IAA and siderophores, dissolve phosphate, fix nitrogen, antagonistic activity against disease and the overall effect of increasing plant growth (Ferrando and Fernández Scavino, 2015; Kumar et al., 2020). Rice endophytes were reported in some studies for their multifunctional abilities as biofertilizers coupled with a function as pesticide degraders, such chlorpyrifos, diazinon, and fungicides of etridiazole, metalaxyl, and tricyclazole (Shen et al., 2019).
There is limited information about the comparison of functional capabilities of rice endophytic bacteria from different cultivation system of organic dan conventional paddy fields. The present study aims to explore, characterize and assess the functional capabilities of endophytic bacteria of rice plants from organic and conventional paddy fields as biofertilizers with high resistance to insecticide toxicity that potentially support plant growth by supplying plant nutrients and inducing plant resistance to insecticide toxicity.
Results and Discussion
Diversity and population density of endophytic bacteria
The diversity and population density of the rice endophytic bacterial colonies on the respective media of YEMA, Pikovskaya, and NA are presented in Table 1. The representative endophytic bacterial colonies grown in each medium are presented in Supplementary Figure 1. As shown on Table 1, the diversity and population density of rice endophytic bacteria isolated from organic paddy field either for general bacteria (grown on NA medium ) or specific functional bacteria of PSB (grown on Pikovskaya medium) and NFB (grown on YEMA medium) were higher than those isolated from conventional paddy field. It is important to note that the total PSB presented in Table 1 refers to the total number of bacterial colonies grown on Pikovskaya medium, including both the isolates that were able to form a halo zone of phosphate solubilization and those that were not. The population density of general and specific functional endophytic bacteria isolated from the roots of rice plants was higher than those isolated from the leaves, both on organic and conventional paddy fields. Each morphologically distinct bacterial colony from each medium was chosen and sub-cultured for the further examination. In total we obtained 28 isolates, consisted of 4 isolates from YEMA medium, 13 isolates from NA medium, 11 isolates from Pikovskaya medium. The colony morphology is described in Supplementary Table 1. On YEMA medium, colony morphology of all isolates from rice roots and leaves either from organic or conventional paddy field showing the same type of morphology. On NA medium, there were two isolates from rice roots and leaves on the both paddy field which showed the same colony morphology. No isolate grown on Pikovskaya showed the same colony morphology. Several previous studies have reported the presence and functional capability of endophytic bacteria in rice plant in diverse conditions. Shofiyah et al. (2023) reported that the population density of endophytic bacteria on YEMA and Pikovskaya media isolated from the root of rice plants var. IR 64 grown on Inceptisol of conventional paddy field was higher than those in the leaves. Endophytic diazotrophic bacteria from various tissues (root, stem, and leaf) of six cultivar rice plant showed the highest population density in root tissue at nursery stage (Rangjaroen et al., 2015). Inoculation of endophytic bacteria isolated from the roots and leaves of rice plants on saline land to the rice plant in greenhouse experiment resulted in the higher population density of general endophytic bacteria in the roots compared to those in the shoots (Setiawati et al., 2021). The present study showed the similar results comparing with those three studies that the general and specific nitrogen fixing and phosphate solubilizing endophytic bacteria in the different rice cultivar, soil media, and cultivation system have the higher population density in the root tissues than in leaves. The fact was explained by Chi et al. (2005) that the root is the entrance of endophytic bacteria to enter the shoot plant tissue, so the population of endophytic bacteria in the root is higher than other plant tissues.
As shown in Table 1 Shannon diversity index on all the isolated endophytic bacteria revealed that the diversity index of endophytic bacteria from organic paddy field was higher than that from conventional paddy field. Moreover, the Shannon diversity index of endophytic bacteria isolated from rice roots was higher than those isolated rice leaves in both paddy fields. Similar to the rice endophytes observed in conventional rice fields in this study, other research has also reported that the Shannon diversity index of rice endophytes in the root is the highest when compared to the index in the rice stem and leaf. Shannon diversity index (alpha) of endophytic bacteria isolated from Oryza officinalis in the flowering phase showed the highest diversity in the roots compared to stems and leaves (Tian et al., 2023). Borah et al. (2018) also reported that Shannon diversity index of endophytic bacteria was higher in cultivated rice compared to wild rice with the highest diversity in roots (H=2,718:H=1,946) compared to stems (H =2,659:H=1,609) and leaves (H=2,393:H=1,386) in both cultivated and wild rice.
The population of endophytic bacteria is influenced by many factors including plant age, species, soil tillage, and environmental conditions (Sulistiyani and Meliah, 2017). Paddy field cultivation with organic systems results in high diversity and colony density. Organic processing systems that use inputs of various types of organic materials provide more diverse nutrients and growth factors that are different from chemical inputs which concentrated for certain nutrients. Microbial composition is supported by the host genotype producing root exudates in the rhizosphere and agricultural practices such as fertilization, tillage and type of cropping system influence this (Sessitsch and Mitter, 2015). Long-term organic cultivation can improve soil structure and functions and increase microbial diversity and population. Sunaryo et al. (2019) reported that addition of liquid fertilizer from goat manure resulted in a high population density of endophytic bacteria in potato and black nightside plants, as well as in those plants that had been treated with cuttings compared to the control treatment. The functional contributions of endophytic colonization depends on many variables, including plant tissue type, plant genotype, plant biotic and abiotic conditions (Hardoim et al., 2015).
Cellular and physiological characteristic
The observation on the cellular and physiological characteristic of 28 isolates was summarized on Table 2. Four bacterial isolates from YEMA medium, 5 out of 13 from NA medium, and 5 out of 11 from Pikovskaya medium were characterized as gram negative bacteria. Kumar et al. (2020) recorded 19 isolates (59.3%) of gram-positive bacteria and 13 isolates (40.6%) gram negative bacteria of endophytic bacteria isolated from roots, stems, leaves and grains of 6 different rice varieties. The endophytic bacteria found in the present study showed higher proportion of gram negative (55.1%) bacteria compared to gram positive bacteria (44.8%) compared to previously reported (Kumar et al., 2020).
All bacterial isolate in three media showed motility activity. The shape of all bacterial isolate from YEMA was coccus, while the shape of bacterial cellular from NA and Pikovskaya media was diverse in coccus, bacillus, and coccobacillus. The diversity of physiological characteristic of endophytic bacterial isolate from YEMA, NA, and Pikovskaya media were as follows: the range of temperature 27-45◦C, the range of pH 5-9, and the range of NaCl tolerance of 2-6%, with the dominant member were found at temperature 27◦C, pH 7, and NaCl tolerance 2%.
Table 1. Population density and diversity index of endophytic bacteria from rice plant on various media.
| Sources of isolates | NA | Pikovskaya | YEMA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Population density of endophytic bacteria (Log 10 CFU g-1) | Number of distinct colony morphology | Shannon Diversity Index (H’) | Population density of endophytic bacteria (Log 10 CFU g-1) | Number of distinct colony morphology | Shannon Diversity Index (H’) | Population density of endophytic bacteria (Log 10 CFU g-1) | Number of distinct colony morphology | Shannon Diversity Index (H’) | |
| Organic paddy field | |||||||||
| Root | 5.86±0.00a | 4 | 1.31±0.16a | 4.83±0.05a | 4 | 1.25±0.36a | 5.84±0.00a | 1 | 0.00±0.00 |
| Leaf | 5.60±0.00b | 4 | 1.16±0.36a | 4.12±0.25b | 4 | 1.01±0.45a | 5.53±0.00b | 1 | 0.00±0.00 |
| Conventional paddy field | |||||||||
| Root | 4.99±0.02c | 4 | 0.87±0.34ab | 3.42±0.24c | 1 | 0.00±0.32c | 5.40±0.01c | 1 | 0.00±0.00 |
| Leaf | 4.95±0.03d | 4 | 0.57±0.47b | 3.21±0.32c | 2 | 0.49±0.00b | 2.56±0.21d | 1 | 0.00±0.00 |
| ANOVA | 0.00** | 0.00** | 0.00** | 0.00** | 0.00** | ||||
Remarks: Significant level: ns=no significant, * = p <0.05, ** = p < 0.01. Means followed by same letters are not significantly different at 5% level by DMRT; H’= Shannon-Wienner diversity index, H’<1 = low diversity, 1<H’<3 = medium diversity, H’>1 = high diversity.
Table 2. Cellular and physiological characteristic of endophytic bacteria from rice plant
| Isolate code | Cellular Characteristic | Physiological Characteristic | ||||||
|---|---|---|---|---|---|---|---|---|
| Shape | Size | Gram stain | Motility | Optimum temperature | Optimum pH | NaCl resistance | ||
| YEROI-1 | Coccus | 1.19 µm | Negative | Motile | 27◦C | 7 | 2% | |
| YELOI-1 | Coccus | 1.64 µm | Negative | Motile | 45◦C | 5 | 2% | |
| YERCI-1 | Coccus | 2.80 µm | Negative | Motile | 45◦C | 7 | 2% | |
| YELCI-1 | Coccus | 1.47 µm | Negative | Motile | 27◦C | 7 | 2% | |
| NEROI-1 | Coccus | 1.19 µm | Positive | Motile | 27◦C | 7 | 2% | |
| NEROI-2 | Bacillus | 1.67 µm | Positive | Motile | 27◦C | 7 | 2% | |
| NEROI-3 | Bacillus | 1.00 µm | Positive | Motile | 45◦C | 9 | 2% | |
| NEROI-4 | Bacillus | 1.26 µm | Positive | Motile | 27◦C | 7 | 2% | |
| NELOI-1 | Coccobacillus | 1.57 µm | Negative | Motile | 27◦C | 7 | 6% | |
| NELOI-2 | Coccus | 0.89 µm | Positive | Motile | 27◦C | 7 | 2% | |
| NELOI-3 | Coccobacillus | 1.44 µm | Negative | Motile | 45◦C | 9 | 2% | |
| NELOI-4 | Coccus | 1.35 µm | Positive | Motile | 27◦C | 9 | 2% | |
| NERCI-1 | Coccus | 1.32 µm | Positive | Motile | 27◦C | 9 | 6% | |
| NERCI-2 | Coccus | 0.77 µm | Negative | Motile | 27◦C | 7 | 2% | |
| NERCI-3 | Coccus | 1.10 µm | Negative | Motile | 45◦C | 9 | 2% | |
| NELCI-1 | Bacillus | 1.26 µm | Positive | Motile | 27◦C | 9 | 2% | |
| NELCI-2 | Coccobacillus | 0.76 µm | Negative | Motile | 27◦C | 9 | 6% | |
| PEROI-1 | Coccobacillus | 1.44 µm | Positive | Motile | 45◦C | 5 | 2% | |
| PEROI-2 | Coccus | 1.13 µm | Positive | Motile | 45◦C | 7 | 2% | |
| PEROI-3 | Coccus | 0.91 µm | Positive | Motile | 27◦C | 7 | 2% | |
| PEROI-4 | Bacillus | 2.23 µm | Positive | Motile | 27◦C | 5 | 2% | |
| PELOI-1 | Coccus | 0.55 µm | Positive | Motile | 27◦C | 5 | 2% | |
| PELOI-2 | Coccus | 0.55 µm | Negative | Motile | 27◦C | 9 | 2% | |
| PELOI-3 | Coccus | 0.89 µm | Negative | Motile | 27◦C | 5 | 2% | |
| PELOI-4 | Coccus | 0.78 µm | Negative | Motile | 27◦C | 5 | 2% | |
| PERCI-1 | Coccus | 1.55 µm | Negative | Motile | 27◦C | 5 | 2% | |
| PELCI-1 | Coccus | 0.89 µm | Positive | Motile | 27◦C | 7 | 2% | |
| PELCI-2 | Coccus | 0.74 µm | Negative | Motile | 27◦C | 9 | 2% | |
Remarks: Y = YEMA medium, N = NA medium, P = Pikovskaya medium, ER = Endophytic bacteria from root of rice plant, EL = Endophytic bacteria from leave of rice plant, OI = Organic paddy field, CI = Conventional paddy field.
Multi-functional capability of endophytic bacteria isolates
Of the four isolates obtained from YEMA medium, two demonstrated the ability to solubilize phosphorus (P) and potassium (K) as well as fix nitrogen, one isolate exhibited the ability to solubilize phosphorus and fix nitrogen, while the remaining isolate was capable only of nitrogen fixation. All isolates from NA medium (13 isolates) had functional capability of nitrogen fixation except one isolate of NELOI-1, and only two isolates showed the functional capability in the solubilization of P namely isolates NEROI-1 and NEROI-2. A total of 11 isolates from Pikovskaya medium showed the functional capability for three nutrients, in the solubilization of P and K, and fixation of nitrogen. We suggest that utilization of general agar medium for isolation is not always yield the target specific microbes. The present study revealed that isolation procedure by using specific media directly resulted in the higher isolated target bacteria compared with using general medium. Phosphate solubilization index is assessed based on a scale formulated by Sati and Pant, (2019) values < 2.0 classified as low solver, values 2.0 - 3.0
classified as medium and values > 3.0 classified as high solver. The present study classified 8 isolates as medium-solver and 8 isolates as low-solver based on classification value of PSI (Sati and Pant, 2019). Sudewi et al. (2020) reported that 19 isolates of endophytic bacteria isolated from the roots of rice plants with 6 different locations using NA medium, then sub-cultured on Pikovskaya medium and showed clear zone formation with PSI between 2.00 - 2.53.
Among all the isolates from Pikovskaya medium showed the capability of K solubilization with KSI at the range of 2.3-3.12, while the highest KSI was obtained by isolate PELOI-3. Furthermore, two isolates from YEMA showed the value of KSI at 2.21 and 2.83. All isolates capable of K solubilization were also exhibited the P solubilization, whereas the isolates capable of P solubilization were not always indicated the capability in K solubilization. Azizah et al. (2020) reported isolated endophytic bacteria from the roots, stems, and leaves of maize and showed that 4 isolates grown on Pikovskaya medium were also able to form clear zone on Aleksandrow medium as an indication of the
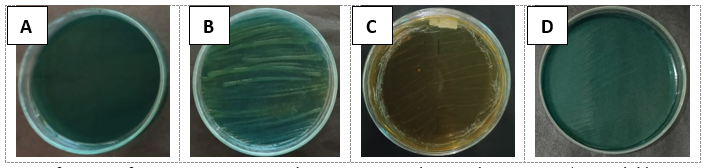
Figure 1. Representation of nitrogen fixation examination with BTB. A = control, B = isolate YEROI-1 (greenish blue to yellowish green and the color of colony became yellowish), C = Isolate PELOI-3 greenish blue to yellow, D = isolate NEROI-2 greenish blue to dark blue.
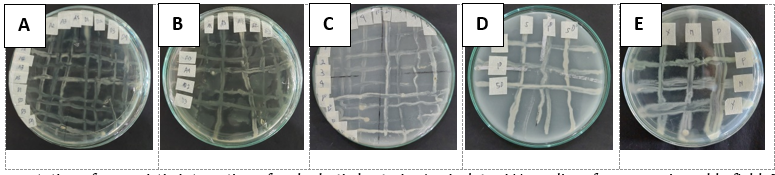
Figure 2. Representation of synergistic interaction of endophytic bacteria. A = isolates NA medium from organic paddy field, B = isolates NA medium from conventional paddy field, C = isolates Pikovskaya medium from organic paddy field, D = isolates Pikovskaya medium from conventional paddy field, E = 3 superior isolates from various media.
solubilization of potassium. The mechanism by which endophytic bacteria solubilize phosphate and potassium involves the secretion of organic acids, such as oxalic acid, tartaric acid, and gluconic acid, which are capable of dissolving insoluble minerals (Eid et al., 2021). To confirm the functional capability in nitrogen fixation for the three selected endophytic bacteria (isolate YEROI-1, PELOI-3, and NEROI-2), the continued examination was conducted by streaking each isolate to YEMA medium with the addition of Bromothymol blue (BTB). As shown in Figure 1, isolate YEROI-1 changed the color of media YEMA+BTB from greenish blue to yellowish green and the color of colony became yellowish. Isolate PELOI-3 affected the color alteration of YEMA+BTB medium and the colony to yellow, whereas the isolate NEROI-2 changed the color of YEMA+BTB medium to dark blue. According to (Howieson and Dilworth, 2016) the three isolates have proved a positive function in nitrogen fixation, by which isolates YEROI-1 and PELOI-3 showed the mechanism of organic acid production and mediated the acidification of the medium. In contrast, the isolate NEROI-2 showed the mechanism ammonium production and mediation of medium (Sulistiyani and Meliah, 2017). This interesting finding showed that endophytic bacteria isolated from the root and leave of rice plant using YEMA medium have functional capability of nitrogen fixation by in-vitro assessment. YEMA medium is designed for cultivation of nitrogen fixing bacteria which are symbiotically associated with legumes. Numerous previous studies have reported the presence of these symbiotic nitrogen-fixing bacteria (NFB) in non-leguminous plants, such as sugarcane (Muangthong et al., 2015), cassava (Zhang et al., 2022), and paddy rice (Alvarez et al., 2020). These bacteria contribute to plant growth by supplying nitrogen through nitrogen fixation in plant tissues (Mahmud et al., 2020) and producing phytohormones like IAA and ethylene (Rangjaroen et al., 2015).
Resistance of endophytic bacteria against insecticide
The result of resistance test of 28 endophytic bacterial against 5 x and 10 x doses insecticides are presented in Table 4. Among all isolates, isolate NEROI-2 showed the highest resistance capability to insecticide with 5x recommended dose. Isolate NEROI-2 (with insecticide) showed the highest growth with absorbance value of 2.322, thus it was resulted a cell density gap
or absorbance different by 0.374. In the second resistance test using 10x dose insecticide, although it did not show the higher absorbance value (the growth of cell density), but isolate NEROI-2 consistently showed the highest absorbance difference or cell density gap (the difference cell density in medium with and without 10x dose insecticide). Based on the high resistance to insecticide performed by isolate NEROI-2, and supported by the multifunctional capabilities in phosphate solubilization and nitrogen fixation (Table 3), the isolate NEROI-2 was selected as one as the superior isolates which then subjected for the further examination. The higher growth of cell density in the treatment with insecticide with high dose (5-10x) clearly revealed the high resistance of the bacterial isolate to insecticide. Moreover, it showed the capability of bacterial isolate to use insecticide in supporting their cellular growth. Some previous reported that some microbia isolate capable use insecticide as the carbon or energy sources. Chia et al. (2024) stated that microbes are capable to break down certain pesticide compounds that serve as carbon and energy sources, thus further paving the way for remediation processes. Growth of Sphingomonas sp. strain HJY from the leaves of Chinese chives on MSM medium including 20 mg L-1 chlorpyrifos showed an increased cell density on day 3 and then decreased slightly on day 6 to 15 (Feng et al., 2017). These results indicate that strain HJY can use chlorpyrifos as the only carbon source. The decrease of Sphingomonas sp. strain HJY after day 3 might be attributed to the fact that endophytic bacteria isolated from plant tissues usually do not experience high levels of stress compared to bacteria from polluted soils or water.
This finding explained that two crucial criteria should be considered to evaluate the resistance capability to insecticide of each bacterial isolate: the first is the growth capacity of each isolate in determined culture medium with insecticide and the second is the comparison of the growth bacteria cell density gap (absorbance different) between the treatment with and without insecticide. In addition, various biotic and abiotic factors affect the resistance capability of bacteria isolate to insecticide, such as the age of isolate, preservation condition of isolate, temperature during incubation, etc.
Table 3. Multi-functional capability of endophytic bacteria from rice plant.
| Isolate Code | PSI | KSI | Symbiotic Nitrogen Fixing Bacteria |
|---|---|---|---|
| YEROI-1 | 2.65 | 2.83 | Positive |
| YELOI-1 | 2.25 | nh | Positive |
| YERCI-1 | 2.75 | 2.21 | Positive |
| YELCI-1 | nh | nh | Positive |
| NEROI-1 | 2.39 | nh | Positive |
| NEROI-2 | 2.24 | nh | Positive |
| NEROI-3 | nh | nh | Positive |
| NEROI-4 | nh | nh | Positive |
| NELOI-1 | nh | nh | Negative |
| NELOI-2 | nh | nh | Positive |
| NELOI-3 | nh | nh | Positive |
| NELOI-4 | nh | nh | Positive |
| NERCI-1 | nh | nh | Positive |
| NERCI-2 | nh | nh | Positive |
| NERCI-3 | nh | nh | Positive |
| NELCI-1 | nh | nh | Positive |
| NELCI-2 | nh | nh | Positive |
| PEROI-1 | 1.07 | 2.84 | Positive |
| PEROI-2 | 1.43 | 2.45 | Positive |
| PEROI-3 | 1.00 | 3.03 | Positive |
| PEROI-4 | 1.00 | 3.05 | Positive |
| PELOI-1 | 1.60 | 3.1 | Positive |
| PELOI-2 | 2.52 | 2.3 | Positive |
| PELOI-3 | 2.14 | 3.12 | Positive |
| PELOI-4 | 2.00 | 3.02 | Positive |
| PERCI-1 | 1.51 | 3.08 | Positive |
| PELCI-1 | 1.00 | 2.74 | Positive |
| PELCI-2 | 1.56 | 3.08 | Positive |
Remarks: Y = YEMA medium, N = NA medium, P = Pikovskaya medium; ER = Endophytic bacteria from root of rice plant, EL = Endophytic bacteria from leave of rice plant; OI = Organic paddy field, CI = Conventional paddy field; nh = no halo zone; PSI values < 2.0 classified as low solver, values 2.0 - 3.0 classified as medium and values > 3.0 classified as high solver.
Synergism test
Before preparing a formula of bacterial culture as consortium consisted of several endophytic bacterial isolate, it is important to evaluate the synergic and antagonistic interaction among all the isolates by synergism test. If there is an inhibition zone that appears in the interaction of among the isolates, it indicates that one or more of individual isolate show antagonistic interaction among others (Ethica et al., 2019). The synergy streak method can evaluate the inhibitory activity of one bacterial isolate to produce antagonistic properties against several other more sensitive bacteria (Dinata et al., 2021).
The present study was arranged to prepare a consortium containing three superior isolates based on the functional capability as biofertilizer and as insecticide bio-resistance inducer. According to the functional capability as biofertilizer, isolate PELOI-3 and isolate YEROI-1 was selected as the superior of phosphate solubilizing and nitrogen fixing endophytic bacteria, respectively. As insecticide bio-resistance inducer, isolate NEROI-2 was selected for its superior endophytic bacteria activity. The three selected isolates were then subjected for the synergism test on NA medium. The results of the synergism test (Figure 2) of the three isolates on NA medium showed synergic interaction among other and there was no antagonistic interaction. The three isolates proved the capability to grow together in synergic interaction with no inhibitory activity among other. Synergism between two or more microbes occurs when they do not compete for the same nutrient source (Setiawati et al., 2021) and there is inhibition or antagonistic activity among the microbes (Ethica et al., 2019). Similarly, microbial consortia allow for synergistic interactions between microbes through physical or biochemical activities, thus increasing viability of each individual microbes (Wang et al., 2019). The ability of bacteria to synergize among each other is affected by several factors including type of bacteria tested, charge distribution, bacterial cell wall content, and diffusion of bacterial molecules into the growth medium (Gurunathan and Dhamotharan, 2014). In addition, component of bacterial
consortium was capable to synergize one another due to the collaboration activity to utilize nutrients or to obtain energy from the same media. Many interactions occur between different types of bacteria and result in either growth promotion or resistance in plants (Dinata et al., 2021).
Effect of endophytic bacteria inoculation on rice seedling growth
Table 5 and Figure 3 showed that the interaction between inoculation of endophytic bacteria and insecticide (10x dose) significantly affected the root length and shoot height of the rice seedling (7 DAP). The highest root length was yielded by the I3P0 treatment followed by I3P1, while the highest shoot height was observed by the I2P1 treatment followed by I2P0. These finding showed that isolate PELOI-3 from Pikovskaya medium has the functional capability to increase root development. The in-vivo assessment on rice seedling using Agarose medium was conducted according the method of Verma et al. (2018). In the present study, the basal nutrition was not added to agarose medium. Thus, the role of the isolate PELOI-3, that is characterized as effective PSB in increasing root length, was not related to phosphate solubilization since no P source exist in the medium. This phenomenal proposed the other mechanism that might played by isolate PELOI-3 in increasing root length. Verma et al. (2018) reported significant effects of 23 endophytic bacterial isolates from Leersia oryzoides seeds on rice grown on petri dish with 0.7% agarose media. After 5 days of incubation, 10 isolates showed activity to increase root and shoot length in rice seedlings, whereas 13 isolates showed inhibitory activity on rice seed growth. Krishnamoorthy et al. (2020) isolated 9 endophytic bacteria from in vitro grown callus of two aromatic rice cultivars and inoculated rice seeds of IR64. Only 4 isolates among them showed significant increases in root and shoot length compared to the control treatment. Endophytic bacteria can enhance seed germination efficiency by biosynthesizing hormones such as gibberellin that can stimulate the activity of specific enzymes
Table 4. Resistance test of endophytic bacterial isolates against insecticides (5-10x doses)
| Isolate code | Resistance to insecticide 5x dose | Resistance to insecticide 10x dose | ||
|---|---|---|---|---|
| Absorbance value (Cell density in medium with insecticide 5x dose) | Absorbance difference (Cell density gap = the difference cell density in medium with and without insecticide 5x dose) |
Absorbance value (Cell density in medium with insecticide 10x dose) | Absorbance difference (Cell density gap = the difference cell density in medium with and without insecticide 10x dose) |
|
| Control | 0.000±0.00n | 0.000±0.00bcde | 0.000±0.00l | 0.000±0.00fgh |
| YEROI-1 | 1.572±0.02fghij | -0.165±0.07cdefgh | 1.492±0.09bc | 0.115±0.00bcdef |
| YELOI-1 | 1.621±0.03efgh | -0.243±0.14efgh | 1.576±0.09abc | 0.289±0.00abcd |
| YERCI-1 | 1.492±0.05ghijk | -0.129±0.15cdefgh | 1.612±0.12ab | 0.388±0.21a |
| YELCI-1 | 1.580±0.05efghi | -0.910±0.22cdefgh | 1.472±0.08bcd | 0.404±0.18a |
| NEROI-1 | 1.584±0.10efghi | -0.101±0.17cdefgh | 1.018±0.09hi | -0.296±0.01ij |
| NEROI-2 | 2.322±0.32a | 0.374±0.27a | 1.095±0.11ghi | 0.432±0.23a |
| NEROI-3 | 1.344±0.10k | -0.304±0.09gh | 0.652±0.11j | 0.012±0.01efgh |
| NEROI-4 | 1.669±0.07def | 0.029±0.17bcd | 1.296±0.11def | -0.002±0.00fgh |
| NELOI-1 | 1.427±0.10ijk | 0.039±0.00bcd | 1.541±0.11abc | 0.253±0.00abcde |
| NELOI-2 | 0.621±0.10m | -1.404±0.19k | 1.191±0.10fgh | -0.148±0.00hi |
| NELOI-3 | 1.561±0.10fghij | 0.083±0.20bc | 0.988±0.11i | 0.352±0.00ab |
| NELOI-4 | 1.942±0.09b | -0.019±0.00cde | 1.193±0.07fgh | 0.098±0.16cdefg |
| NERCI-1 | 1.869±0.12bc | 0.349±0.19a | 0.733±0.11j | -0.691±0.01k |
| NERCI-2 | 1.918±0.10bc | 0.348±0.17a | 1.182±0.17fgh | 0.405±0.17a |
| NERCI-3 | 1.407±0.09jk | -0.054±0.00cdefg | 1.407±0.09cde | 0.325±0.10abc |
| NELCI-1 | 1.659±0.10defg | -0.104±0.09cdefgh | 1.136±0.09fghi | -0.261±0.00ij |
| NELCI-2 | 1.476±0.10hijk | -0.076±0.00cdefg | 1.256±0.13efg | 0.320±0.10abc |
| PEROI-1 | 1.436±0.10ijk | 0.048±0.00bcd | 1.537±0.10abc | 0.245±0.00abcde |
| PEROI-2 | 1.344±0.10k | -0.785±0.00i | 1.411±0.11cde | 0.247±0.20abcde |
| PEROI-3 | 1.506±0.10fghijk | 0.323±0.17a | 0.971±0.11i | -0.145±0.19ghi |
| PEROI-4 | 1.576±0.10fghij | -0.221±0.09efgh | 1.566±0.09abc | 0.032±0.19efgh |
| PELOI-1 | 1.366±0.07k | -0.170±0.10defgh | 1.701±0.10a | 0.278±0.00abcd |
| PELOI-2 | 1.794±0.10bcd | -0.327±0.17h | 0.484±0.11k | -0.402±0.01j |
| PELOI-3 | 1.746±0.10cde | -0.052±0.00cdef | 1.463±0.07bcd | -0.139±0.03ghi |
| PELOI-4 | 0.768±0.10l | -1.102±0.00j | 1.627±0.11ab | 0.089±0.17cdefgh |
| PERCI-1 | 1.560±0.10fghij | 0.230±0.00ab | 1.651±0.09ab | 0.050±0.09defgh |
| PELCI-1 | 1.555±0.10fghij | -0.080±0.16cdefgh | 1.706±0.01a | 0.345±0.08ab |
| PELCI-2 | 1.590±0.10efghi | -0.258±0.00fgh | 1.625±0.10ab | 0.121±0.19bcdef |
| ANOVA | 0.00** | 0.00** | 0.00** | 0.00** |
Remarks: Significant level: ns=no significant, * = p <0.05, ** = p < 0.01. Means followed by same letters are not significantly different at 5% level by DMRT. Y = YEMA medium, N = NA medium, P = Pikovskaya medium, ER = Endophytic bacteria from root of rice plant, EL = Endophytic bacteria from leave of rice plant, OI = Organic paddy field, CI = Conventional paddy field.
such as α-amination. In this condition, endophytic bacteria are transmitted vertically through seeds. Endophytic bacteria as PGPB live in the vital organs of plants that are obtained from seeds (vertically from the host plant) or soil (horizontally from the environment) (Frank et al., 2017). Seed endophytic bacteria play important roles in germination such as hormone production, seedling resistance, maintaining plant health, nutrient mobilization, phytopathogen control, and antioxidant activity (Pal et al., 2019).
Molecular identification of endophytic bacteria
The sequences of the 16 rDNA of the three superior isolates of YEROI-1, PELOI-3, and NEROI-2 were submitted to DNA Data Bank of Japan (DDBJ) and recorded with the accession number LC790726, LC790728, and LC790727, respectively. The length of sequence of DNA fragments of the three isolates was 1375 – 1405 bp.
Two isolates were closely related with the genus Burkholderia but different species, and one isolate closely related with Bacillus (Table 6). Many members of genus Burkholderia are detected as beneficial e.g. Burkholderia cepacia from root of rice (Singh et al., 2013) and sugarcane (Luvizotto et al., 2010), Burkholderia vietnamensis from rice and shoot of rice (Govindarajan et al., 2008). The authors demonstrated their functions in nitrogen fixation, production of phytohormone (IAA production and acetylene reduction), phosphate solubilization, siderophore production. Many members of genus Bacillus were also recorded as beneficial bacteria. Shahzad et al. (2016) reported Bacillus amyloliquefaciens as an endophyte derived from rice seeds that plays a role in the regulation of endogenous phytohormones
(salicylic acid, abscisic acid, and jasmonic acid) and gibberellin production. Thus, based on the characteristic of closest relatives of the three superior isolates in the present study, it is known that the three isolates have potential to be used as beneficial member of Burkholderia (for isolate YEROI-1 and PELOI-3) and Bacillus (for isolate NEROI-2). Further studies of in-vivo test are needed to confirm the beneficial function of these three isolates.
Materials and Methods
Sampling of rice plant and rhizosphere soils
Healthy rice plant cultivar Inpari 32 were grown at maximum vegetative phase on 45 DAP and their rhizosphere soils were collected from organic paddy field in Kuto Village (7º32.998’S; 111°01.760’E) and from conventional paddy field in Tamansari Village (S7º33’47.8’S; 111º03’07.7’E), Kerjo District, Karanganyar Regency, Central Java, Indonesia in May 2023. The root and leaf of the rice plants were used as the sources of endophytic bacteria. Both of the organic and conventional paddy fields were classified as Alfisols with chemical characteristics as follows: pH H2O 5.32 and 4.76, organic-C 3.39 % and 1.37 %, total-N 0.60 % and 0.75 %, C/N ratio 5.67 and 1.84, available-P 5.49 ppm and 4.64 ppm, available-K 0.08 cmol(+) kg-1 and 0.19 cmol(+) kg-1, respectively.
Isolation, colony observation, and purification
All steps of the laboratory research were conducted in the Laboratory of Biology and Biotechnology, Faculty of Agriculture, Universitas Sebelas Maret, Surakarta, Indonesia from May to August 2023. The samples of leaf and root of rice plants were
Table 5. Effect of inoculation of endophytic bacterial and insecticide to root length and shoot height of rice seedling.
| Combination treatment | Root length of rice seedling (cm) | Root hairs (*) | Shoot height of rice seedling (cm) |
|---|---|---|---|
| I0P0 | 2.96±0.67cd | +++ | 4.16±0.30bc |
| I0P1 | 3.26±0.25bcd | +++ | 4.43±0.37ab |
| I1P0 | 3.29±0.25bc | + | 3.29±0.20bcd |
| I1P1 | 3.76±0.83abc | +++ | 3.71±0.62bcd |
| I2P0 | 3.66±0.26abc | +++ | 5.36±0.57a |
| I2P1 | 3.21±0.42bcd | +++ | 5.37±0.54a |
| I3P0 | 4.42±0.76a | +++++ | 4.16±1.01bc |
| I3P1 | 4.01±0.32ab | +++++ | 4.20±0.68bc |
| I4P0 | 3.06±0.45bcd | +++ | 3.24±0.95cd |
| I4P1 | 2.27±0.62d | +++ | 2.98±0.31d |
| F-Values | |||
| I | 0.00** | 0.00** | |
| P | 0.39ns | 0.65ns | |
| I x P | 0.00** | 0.00** |
Remarks: Significant level: ns=no significant, * = p <0.05, ** = p < 0.01. Means followed by same letters are not significantly different at 5% level by DMRT. I0P0 = Control, I0P1 = Without isolates, add insecticide, I1P0 = Isolate YEROI-1, I1P1 = Isolate YEROI-1+insecticide, I2P0 = Isolate NEROI-2, I2P1 = Isolate NEROI-2+insecticide, I3P0 = Isolate PELOI-3, I3P1 = Isolate PELOI-3+insecticide, I4P0 = Combination of three isolates, I4P1 = Combination of three isolates + insecticide. (*) + = short root hairs, +++ = moderate root hairs, +++++ = long and abundant root hairs.

Figure 3. The growth of rice seedling as affected by the combination treatment of endophytic bacteria and insecticide.
subjected for the isolation of bacterial endophytes. At first, the leaves and roots were washed under running tap water, then their surfaces were sterilized by a consecutive soaking in 70% ethanol (1 min), 3.25% NaOCl (6 min), 70% ethanol (1 min), and rinsed with sterile water (1 min). The sterilization procedure was repeated five times (Yan et al., 2018). After drying, the samples were cut and mashed using a sterile mortar and pestle, then weighed and subjected for a serial dilution of spread plate method with six replications (Sanders, 2012).Three types of media were prepared, namely YEMA for symbiotic NFB (Birhan et al., 2018), Pikovskaya for PSB (Naik et al., 2008), and NA for general bacteria (Pathmanathan et al., 2016). After incubation for two days at room temperature, the colony morphology and population density were observed (Sousa et al., 2013). Morphologically distinct bacterial colonies were selected and purified using the same media in the slant agar for the stock culture and in the new petri dish to be used for further examination.
Physiological and cellular characterization of bacterial isolate
Each bacterial isolate was tested for NaCl resistance, temperature, and pH tolerance. NaCl resistance was assessed in nutrient broth (NB) with 2%, 6%, and 10% NaCl (Hena et al., 2022). Temperature tolerance was tested at 10°C, 27°C, and 45°C (Katipoglu-Yazan et al., 2023). pH tolerance was examined in NB media at pH 5, 7, and 9 (Katipoglu-Yazan et al., 2023). All cultures
were incubated for 4 days at room temperature, and growth was measured at 590 nm with a spectrophotometer. Cell shape, size, and wall characteristics were observed using gram staining (Kandi, 2015).
Assessment of capability for phosphate and potassium solubilization
All isolates from the three media of YEMA, Pikovskaya, and NA were examined for their multifunctional capability as biofertilizer by cross culturing each isolate to Pikovskaya and Aleksandrov media to evaluate the capability in the solubilization phosphate and potassium. The formation of halo zone on the Pikovskaya and Aleksandrow media was measured on day 5 of the incubation for the PSI and KSI (Bouizgarne et al., 2023).
Resistance test of endophytic bacteria grown on media with and without insecticide addition
Testing of resistance capability of endophytic bacteria to insecticide with active ingredients chlorantraniliprole 100 g L-1 and thiamethoxam 200 g L-1 was conducted by growing each isolate on the NB medium with and without insecticide addition. The resistance test was conducted in two steps, the first step used 5x dose and the second step used 10x dose. In each step, the experiment was performed in a completely randomized design with single factor of isolates treatment, consisting of 29 levels including the control (treatment with no isolate) with 3
Table 6. Identity of three superior endophytic bacterial isolates.
| The code and molecular identity of the three selected 3 isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
Isolate Code |
Source of isolation | Sequence bp | Accession Number | Closest Relatives | Similarity | ||||
| Microorganism | Phylogenetic affiliations | Accession Number | Source | ||||||
| YEROI-1 | Rice root tissue on organic paddy field | 1375 | LC790726 | Burkholderia sp. | Phylum: Pseudomonadota Class: Betaproteobacteria |
KY357354.1 | heavy metal-contaminated soils | 100% | |
| Burkholderia seminalis | Phylum: Pseudomonadota Class: Betaproteobacteria |
CP072521.1 | surface-sterilized vetiver roots | 100% | |||||
| PELOI-3 | Rice root tissue on organic paddy field | 1379 | LC790728 | Burkholderia cepacia | Phylum: Pseudomonadota Class: Betaproteobacteria |
OL469786.1 | Cd-contaminated soil | 100% | |
| Burkholderia seminalis | Phylum: Pseudomonadota Class: Betaproteobacteria |
MH748603.1 | the root nodules of the vegetable cowpea | 100% | |||||
| NEROI-2 | Rice leaf tissue on organic paddy field | 1405 | LC790727 | Bacillus paramycoides | Phylum: Bacillota Class: Bacilli |
OQ970651.1 | Salt polluted-soil | 99.93% | |
replications. Each isolate was cultured in 5 mL NB medium per tube with 3 replicates. In first step, 0.5 µL of insecticide (concentration 0.1 ppm) was added to each tube and the other tube was without insecticide (as positive control). One loopful of endophytic bacterial colony was inoculated to each tube. All those cultured NB medium were incubated at room temperature and shaken at 150 rpm for 4 days, then the cell density was observed using a spectrophotometer at a wave length of 590 nm (Andriani et al., 2017). The length of incubation (4 days) was determined based on preliminary test by culturing several isolates on NB medium in the tubes, until the cell density of all the isolates reached 108 cells m L-1 after 4 days.
The second step of the testing resistance capability was conducted after the isolate was sub-cultured three times during three months. This testing is important to evaluate the consistency of the resistance capability of each isolate after six months preservation in the laboratory condition. In second step, insecticide was applied with 10x of recommended usage dose (concentration 0.2 ppm). The design and procedure of the second strep of resistance test was the same as conducted in the first step. The resistance bacterial isolate against insecticide was indicated by the capability to grow in the insecticide treatment as demonstrated by the increase of cell density as determinate by absorbance value. To compare the resistance level among all the isolates, it was needed to measure the cell density gap (the difference cell density in medium with and without insecticide) as detected from the absorbance difference. The obtained data then was analyzed statistically using Statistical Analysis of Variance (ANOVA) and continued with Duncan Multiple Range Test (DMRT) with significance of 5%. Based on the resistance capability, one isolate was selected to be used for the greenhouse experiment.
Synergy test between endophytic bacterial isolates
All of the isolates from NA (13) and from Pikovskaya (11) were examined for the synergy interaction on the respective media. The four isolates from YEMA, which obtained from rice leaves and roots in both organic and conventional paddy fields and exhibited similar colony morphology, were not tested for synergistic interactions. Synergy test was conducted by the cross streak method on the respective media. In first step, each isolate was streaked in straight line at 1 cm of the distance between the line. After incubation, 3x24 hours at room temperature, the second streak from each isolate was made across the first line, and the incubation was continued for 3x24 hours. Then, the presence or the absence of the clear zone among the streaked line was observed. The synergistic interaction among the isolates
was analysed by the absence of an inhibition zone (clear zone) around the cross between colony streak lines (Ethica et al., 2019). The presence of clear zones is a sign that some bacteria are antagonistic to each other.
Selection of isolates for in-vivo assessment
Out of the 28 rice endophytic bacterial isolates from organic and conventional paddy fields, obtained from YEMA, Pikovskaya, and NA media, one isolate from each medium was selected based on its superior functional capability. Isolate YEROI-1 from YEMA, isolate PELOI-3 from Pikovskaya, and isolate NEROI-2 from NA were selected based on the multifunctional capability. The three selected isolates were evaluated for the synergistic interaction by streaking on NA medium.
The examination of the capability for the nitrogen fixation
Additional examination was performed to assess capability of nitrogen fixation for the three selected isolates. The examination was conducted by cross culturing to YEMA medium with the addition of Bromothymol Blue (BTB) to evaluate the capability of growth of isolates in the media with the absence of N source (Begom et al., 2021). As explained by Sulistiyani and Meliah, (2017) the color changes of YEMA+BTB medium from greenish blue to dark blue indicated the increase of medium pH (alkaline reaction) due to the ammonium production by the isolates which indicate the activity of nitrogen fixation. On the other hand, the color alteration of media YEMA+BTB from greenish blue to yellow indicate acid production by the fast grown rhizobia (Somasegaran and Hoben, 1994).
In vivo assessment to examine the effect of endophytic bacteria inoculation on rice seedling growth
In vivo assesment was designed using a completely randomized design with two factors: first factor was inoculation of isolates consisting of 5 levels: I0=no isolate; I1=Isolate YEROI-1; I2=Isolate NEROI-2; I3=Isolate PELOI-3; I4=mixture of three isolates. The second factor was insecticide application (P) consisting of 2 levels: P0=no insecticide; P1= with insecticide 10x dose (active ingredient chlorantraniliprole 100 g L-1 and thiamethoxam 200 g L-1). There were 10 combination treatments with 3 replications.
For in vivo assessment, first rice seeds var. Inpari 32 were sterilised with 4% NaOCl for 5 minutes with constant stirring, 70% ethanol for 3 minutes, then the seeds were washed with sterile water 5 times to remove the remaining NaOCl (Ferreira et al., 2021). Then isolate inoculation was performed by soaking rice seed into the treatment of endophytic bacterial culture (control, selected endophytic bacterial isolates of YEROI-1, PELOI-3, NEROI-2 and a mixture of the three isolates, respectively) with cell density 108 cells m L-1 and incubated for 24 hours. The agarose medium was prepared with and without insecticide. For the insecticide treatment, the insecticide was added to agarose medium just before plating (the temperature at 35-38◦C) in petri dish. The rice seeds were sown on agarose medium (5 seeds in each petri dish) according to respective treatment. The variables of rice seedling development consisted of root and shoot length that were observed 7 days after sowing (Verma et al., 2018). The obtained data was analyzed for Statistical Analysis of Variance (ANOVA) and continued with Duncan Multiple Range Test (DMRT) with significance of 5%.
Molecular identification of endophytic bacteria
Among 28 endophytic bacterial isolates, 3 isolates which indicated superior functional ability were selected to be identified by 16S rDNA sequencing. Total genomic bacterial DNA was extracted using Quick-DNA Magbead Plus Kit (Zymo Research, D4081) and the 16s rDNA was amplified using the eubacterial set of two primers, 27F primer (5’–AGAGTTTGATCMTGGCTCAG–3’) and 1492R primer (5’–TACGGYTACCTTGTTACGACTT–3’). PCR was performed in the GeneAmp@PCR system 9700 (Applied Biosystems, Foster City, CA). The component of PCR master mix (total volume 25 µL) contained of 12.5 µL of My Taq HS Red Mix (2x), 1 µL of 27F Primer (10 µM), 1 µL of 1492R Primer (10 µM), 1 µL DNA template (about 25 ng), and 9.5 µL of dd H2O. The PCR conditions was set up as follows: initial denaturation at 95◦C for 3 min, 35 cycles of denaturation at 95◦C for 15 sec, annealing at 52◦C for 30 sec, extension at 72◦C for 45 sec, and final extension at 72◦C for 2 min. After amplification, the PCR products were assessed by electrophoresis with 1% TBE Agarose. The PCR product was then subjected for Bi-directional Sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) with 27F and 1492R Primers according to the manufacturer’s instructions (Cahyani et al., 2009). Sequencing was performed using ABI PRISM® 3100-Avant™ Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequences of the DNA fragments of the selected isolates were compared to NCBI database (www.ncbi.nlm.nih.gov) with the BLAST search to determine closest relatives of the isolates, and then the sequences were submitted to DNA Data Bank of Japan (DDBJ) (https://www.ddbj.nig.ac.jp/submission-navigation-e.html). The 16S rDNA sequences of the selected isolates obtained in this study are available in the DDBJ database with the accession number LC790726-LC790728.
Conclusion
The differences in cultivation system affected the diversity and population density of endophytic bacteria of rice plants. On organic paddy field, the diversity and population density of rice endophytic bacteria was higher than those on conventional paddy field. The general endophytic bacteria in rice leaves showed higher population than those in rice roots. On the contrary, the specific endophytic bacteria of NFB and PSB in rice leaves indicated lower population density compared to those in rice roots. Among a total of 28 isolates from YEMA, Pikovskaya, and NA media, three isolates of YEROI-1, PELOI-3, and NEROI-2 were closely related to Burkholderia sp., Burkholderia seminalis and Bacillus paramycoides, respectively. They were selected based on their superior functional capability as biofertilizer and bio-resistance inducer to insecticide in in vitro assessment. Isolate NEROI-2 yielded the highest effect in increasing the growth of rice seedling as indicated the highest shoot height and root length either with and without insecticide treatments, followed by isolate PELOI-3. On the contrary, isolate YEROI-1 showed inhibition effect toward the growth of rice seedling in the treatments with and without insecticide. Further assessments are required to elucidate the comprehensive characteristics of isolate YEROI-1 or any other factors, causing inhibition effect for the plant growth. Evaluation study is needed to confirm the effect of these endophytic bacteria in supporting plant growth, inducing insecticide resistance, and increasing soil fertility and health in a greenhouse experiment.
Acknowledgements
The research was financially supported by Funding of The Ministry of Education, Culture, Research, and Technology, Republic of Indonesia, FY 2023. Directorate of Research, Technology, and Community Services for the Regular Fundamental Research Grants (Hibah Penelitian Fundamental Reguler) with the Decree number 0536/E5/PG.02.00/2023 (30 May 2023) and Contract number 160/E5/PG.02.00.PL/2023 (19 June 2023) and 1280.1/UN27.22/PT.01.03/2023 (20 June 2023).
References
Abdullah MP, Naban KJ, Al-Qaim FF, Ishak A, Othman MR, Afiq WM (2017) Analysis of pesticide residues in water sample: occurrence of pesticides in paddy field. Journal of Chemical and Pharmaceutical Sciences. 10:1159-1166.
Ajermoun N, Aghris S, Ettadili F, Alaoui OT, Laghrib F, Farahi A, Lahrich S, Bakasse M, Saqrane S, El Mhammedi MA (2022) Phytotoxic effect of the insecticide imidacloprid in Phaseolus vulgaris L. plant and evaluation of its bioaccumulation and translocation by electrochemical methods. Environmental Research. 214:113794.
Alvarez C, Navarro JA, Molina-Heredia FP, Mariscal V (2020) Endophytic colonization of rice (Oryza sativa L.) by the symbiotic strain nostoc punctiforme PCC 73102. Molecular Plant-Microbe Interactions. 33:1040–1045.
Andriani LT, Aini LQ, Hadiastono T (2017) Glyphosate biodegradation by plant growth promoting bacteria and their effect to paddy germination in glyphosate contaminated soil. Journal of Degraded and Mining Lands Management. 5:995–1000.
Arridho S, Ulpah S, Sabli TE (2019) The effectiveness of rice husk biochar application to metsulfuron methyl persistence. Presented at the 2nd international conference on science, engineering and technology, Riau Islamic University, Indonesia, 5-7 September 2019.
Azizah H, Rahajeng SM, Jatmiko YD (2020). Isolation and Screening of P and K Solubilizing Endophytic Bacteria Isolation and Screening of Phosphate and Potassium Solubilizing Endophytic Bacteria in Maize (Zea mays L.). Life Sci. 10:165-170.
Bai YC, Chang YY, Hussain M, Lu B, Zhang JP, Song XB, Lei XS, Pei D (2020) Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms. 8.
Begom MF, Ahmed MGU, Sultana R, Akter F (2021) Culture optimization for mass production of rhizobium using bioreactor made of readily available materials and agitated by air flow. Agricultural Sciences.12.
Bertani I, Abbruscato P, Piffanelli P, Subramoni S, Venturi V (2016) Rice bacterial endophytes: Isolation of a collection, identification of beneficial strains and microbiome analysis. Environmental Microbiology Reports. 8:388–398.
Birhan A, Tadele T, Yilkal B, Seble WY (2018) Phenotypic characterization and symbiotic effectiveness test of chickpea (Cicer arietinum L.) rhizobia isolated from Dejen and Aneded Districts, East Gojjam Zone, Amahara Region, Ethiopia. African Journal of Biotechnology. 17:730–738
Borah M, Das S, Baruah H, Boro RC, Barooah M, Boron RC (2018) Diversity of culturable endophytic bacteria from wild and cultivated rice showed 1 potential plant growth promoting activities 2. BioRxiv. 310797.
Bouizgarne B, Bakki M, Boutasknit A, Banane B, El Ouarrat H, Ait El MS, Amenzou A, Ghousmi A, Meddich A (2023) Phosphate and potash solubilizing bacteria from Moroccan phosphate mine showing antagonism to bacterial canker agent and inducing effective tomato growth promotion. Frontiers in Plant Science. 14
BPS-Statistics Indonesia (2022) Statistical yearbook of Indonesia 2022. Jakarta, Indonesia.
Brader G, Compant S, Vescio K, Mitter B, Trognitz F, Ma LJ, Sessitsch A (2017) Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Annual Review of Phytopathology. 55:61–83.
Cahyani VR, Murase J, Ishibashi E, Asakawa S, Kimura M (2009) Phylogenetic positions of Mn2+-oxidizing bacteria and fungi isolated from Mn nodules in rice field subsoils. Biology and Fertility of Soils. 45:337–346.
Castro-Gutiérrez V, Masís-Mora M, Carazo-Rojas E, Mora-López M, Rodríguez-Rodríguez CE (2019) Fungal and bacterial co-bioaugmentation of a pesticide-degrading biomixture: pesticide removal and community structure variations during different treatments. Water, Air, and Soil Pollution. 230.
Chi F, Shen SH, Cheng, HP, Jing YX, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Applied and Environmental Microbiology. 71:7271–7278.
Chia XK, Hadibarata T, Kristanti RA, Jusoh MNH, Tan IS, Foo HCY (2024). The function of microbial enzymes in breaking down soil contaminated with pesticides: a review. Bioprocess and Biosystems Engineering, 47:597–620.
Chislock MF, Doster E, Zitomer RA, Wilson AE (2013) Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nature Education Knowledge. 4:1-8.
Dinata GF, Aini LQ, Abadi AL (2021) The synergy between several bacteria isolated from the biodiversity of ub forest coffee litter in vitro. in: nusantara science and technology proceedings. the 1st Bioinformatics and Biodiversity conference, Indonesia, February 2021.
Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SED (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plant. 10:935.
Ethica SN, Muslim R, Widyawardhana RMBI, Firmansyah A, Muchlissin SI, Darmawati S (2019) Synergism and antagonism among indigenous hydrolytic bacteria from biomedical wastes for the generation of bacterial consortium used as bioremediation agent. International Journal of Environmental Science and Development.10:440–444.
Feng F, Li Y, Ge J, Chen J, Jiang W, He S, Liu X, Yu X (2017). Degradation of chlorpyrifos by an endophytic bacterium of the Sphingomonas genus (strain HJY) isolated from Chinese chives ( Allium tuberosum ). Journal of Environmental Science and Health, Part B. 52:736–744
Ferrando L, Fernández-Scavino A (2015) Strong shift in the diazotrophic endophytic bacterial community inhabiting rice (Oryza sativa) plants after flooding. FEMS Microbiology Ecology. 91.
Ferreira SC, Nakasone AK, Nascimento SMC, de Oliveira DA, Siqueira AS, Cunha EFM, de Castro GLS, de Souza CRB. (2021). Isolation and characterization of cassava root endophytic bacteria with the ability to promote plant growth and control the in vitro and in vivo growth of Phytopythium sp. Physiological and Molecular Plant Pathology. 116:101709.
Frank AC, Guzmán JPS, Shay JE (2017) Transmission of bacterial endophytes. Microorganisms. 5.
Gao W, Zhang Y, Lin M, Mao J, Xing B, Li Y, Hou R (2023) Capability of phytoremediation of glyphosate in environment by Vulpia myuros. Ecotoxicology and Environmental Safety. 265
Govindarajan, M, Balandreau, J, Kwon SW, Weon HY, Lakshminarasimhan C (2008) Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microbial Ecology. 55:21–37.
Gurunathan S, Dhamotharan R (2014) 16s rDNA based molecular identification of Bacteriocin-like inhibitory substance (BLIS/BIS) producing indigenous phytopathogenic bacteria isolated from various diseased plant materials. Int J of Current and Research. 11:105–119.
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW, Quadt-Hallmann A, Klepper J.W (1997) Bacterial endophytes in agricultural crops. NRC Canada Can. J. Microbiol. 43.
Hardoim, PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiology and Molecular Biology Reviews. 79:293–320.
Hena H, Khanam M, Rahman GKMM, Afrad MSI, Alam MS (2022) Isolation and characterization of salt tolerant bacteria from saline soils of Bangladesh. Eurasian Journal of Soil Science. 11:284–294.
Hernández AF, Parrón T, Tsatsakis AM, Requena M, Alarcón R, López-Guarnido O (2013) Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology. 307:136–145.
Howieson JG, Dilworth MJ (2016) Working with Rhizobi. In: K Langford (ed) Australian centre for international agricultural research. Canberra.
Hu J, Wan Z, Williams GDZ, Dwyer GS, Gatiboni L, Duckworth OW, Vengosh A (2024) Evidence for the accumulation of toxic metal(loid)s in agricultural soils impacted from long-term application of phosphate fertilizer. Science of the Total Environment. 907
Joshi V, Suyal A, Srivastava A, Srivastava PC (2019) Role of organic amendments in reducing leaching of sulfosulfuron through wheat crop cultivated soil. Emerging Contaminants. 5:4–8
Kandi V (2015) Bacterial colony: first report of donut colony morphology among diphtheroids isolated in blood. Cureus. 7.
Katipoglu-Yazan T, Dev S, Desmond-Le QE, Bouchez T (2023) Data on the influence of temperature on the growth of Escherichia coli in a minimal medium containing glucose as the sole carbon source for the joint computation of growth yields and rates at each temperature from 27 to 45°C. Data in Brief. 48.
Koomnok C, Teaumroong N, Rerkasem B, Lumyong S (2007) Diazotroph endophytic bacteria in cultivated and wild rice in Thailand. Science Asia. 33:429–435.
Krishnamoorthy A, Agarwal T, Kotamreddy JNR, Bhattacharya R, Mitra A, Maiti TK, Maiti MK (2020) Impact of seed-transmitted endophytic bacteria on intra- and inter-cultivar plant growth promotion modulated by certain sets of metabolites in rice crop. Microbiological Research. 241.
Kumar V, Jain L, Jain SK, Chaturvedi S, Kaushal P (2020) Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. South African Journal of Botany. 134:50–63.
Luvizotto DM, Marcon J, Andreote FD, Dini-Andreote F, Neves AAC, Araújo WL, Pizzirani-Kleiner, AA (2010) Genetic diversity and plant-growth related features of Burkholderia spp. from sugarcane roots. World Journal of Microbiology and Biotechnology, 26:1829–1836.
Maggi F, la Cecilia D, Tang FHM, McBratney A (2020) The global environmental hazard of glyphosate use. Science of the Total Environment. 717.
Mahmud K, Makaju S, Ibrahim R, Missaoui A (2020) Current progress in nitrogen fixing plants and microbiome research. Plants. 9.
Marín-Benito JM, Barba V, Ordax JM, Andrades MS, Sánchez-Martín MJ, Rodríguez-Cruz MS (2018) Application of green compost as amendment in an agricultural soil: Effect on the behaviour of triasulfuron and prosulfocarb under field conditions. Journal of Environmental Management. 207:180–191.
Miliute I, Buzaite O, Baniulis D, Stanys V (2015) Bacterial endophytes in agricultural crops and their rolein stress tolerance: a review. Zemdirbyste Lithuanian Institute of Agriculture. 102:465-478.
Muangthong A, Youpensuk S, Rerkasem B (2015) Isolation and characterisation of endophytic nitrogen fixing bacteria in sugarcane. Tropical Life Sciences Research. 26:41–51.
Naik PR, Raman G, Narayanan KB, Sakthivel N (2008) Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiology. 8.
Organic Institute (2019). Statistik Pertanian Organik Indonesia 2019. In: Firman AR, David W (eds). Jakarta.
Pal G, Kumar K, Verma A, White JF, Verma SK (2019) Functional roles of seed-inhabiting endophytes of rice. In seed endophytes: biology and biotechnology springer international publishing. 11.
Pathmanathan S, Ravimannan N, Sathyaruban S (2016) Formulation of alternative culture media for bacterial and fungal growth. Scholars Research Library. 8:431–436.
Phetcharat P, Duangpaeng A (2012) Screening of endophytic bacteria from organic rice tissue for indole acetic acid production. Procedia Engineering. 32:177–183.
Rangjaroen C, Rerkasem B, Teaumroong N, Noisangiam R, Lumyong S (2015) Promoting plant growth in a commercial rice cultivar by endophytic diazotrophic bacteria isolated from rice landraces. Annals of Microbiology. 65:253–266.
Sanders, ER (2012) Aseptic laboratory techniques: plating methods. Journal of Visualized Experiments. 63:1–18.
Sati SC, Pant P (2019) Evaluation of phosphate solubilization by root endophytic aquatic hyphomycete tetracladium setigerum. Symbiosis. 77:141–145.
Sessitsch A, Mitter B (2015) 21st century agriculture: Integration of plant microbiomes for improved crop production and food security. Microbial Biotechnology. 8:32–33.
Setiawati MR, Sugiyono L, Kamaluddin NN, Simarmata T (2021) The use of endophytic growth-promoting bacteria to alleviate salinity impact and enhance the chlorophyll, N uptake, and growth of rice seedling. Open Agriculture. 6:798–806.
Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang SM, Yun BW, Lee IJ (2016) Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiology and Biochemistry. 106:236–243.
Shen FT, Yen JH, Liao CS, Chen WC, Chao YT (2019) Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability (Switzerland). 11.
Shofiyah L, Sudadi, Dewi WS, Cahyani VR (2023) Endophytic phosphate solubilization and potential nitrogen-fixing bacteria in the leaf and root of rice planted on the conventional wetland. In: IOP conference series: earth and environmental science. The 1st international conference of SAINS tanah, Indonesia, July 2022.
Simarmata R, Widowati T, FWP R, Christita M, Khairina Y, Erdayani E, Khumairah FH (2023). Rhizosphere bacteriome of Allium cepa after the application of chemical and endophyte-based fertilizer. Acta Ecologica Sinica. 43:1138-1148.
Singh RK, Malik N, Singh S (2013) Improved nutrient use efficiency increases plant growth of rice with the use of IAA-overproducing strains of endophytic burkholderia cepacia strain RRE25. Microbial Ecology. 66:375–384.
Somasegaran P, Hoben HJ (1994). Handbook for rhizobia. In Handbook for rhizobia. New York.
Sousa AM, Machado I, Nicolau A, Pereira MO (2013) Improvements on colony morphology identification towards bacterial profiling. Journal of Microbiological Methods, 95:327–335.
Sudewi S, Ala A, Baharuddin, Farid M (2020) The isolation, characterization endophytic bacteria from roots of local rice plant kamba in, central sulawesi, indonesia. Biodiversitas. 21:1614–1624.
Sulistiyani TR, Meliah S (2017) Isolation and Characterization of Nitrogen Fixing Endophytic Bacteria Associated with Sweet Sorghum (Sorghum bicolor). Paper presented at the 1st SATREPS conference, the indonesian institute of science, Bogor, 14 November 2016. 110–117.
Sun L, Qiu F, Zhang X, Dai X, Dong X, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microbial Ecology. 55:415–424.
Sunaryo Y, Purnomo D, Darini MT, Cahyani VR (2019) Tuber formation and endophyte dynamic in potato black nightshade grafting with application of goat manure liquid fertilizer. Asian Journal of Agriculture and Biology. 7:244–250.
Tian Q, Gong Y, Liu S, Ji M, Tang R, Kong D, Xue Z, Wang L, Hu F, Huang L, Qin S (2023) Endophytic bacterial communities in wild rice (Oryza officinalis) and their plant growth-promoting effects on perennial rice. Frontiers in Plant Science. 14
Verma SK, Kingsley K, Bergen M, English C, Elmore M, Kharwar RN, White JF (2018) Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant and Soil, 422:223–238.
Wandscheer ACD, Marchesan E, da Silva MF, Aramburu BB, de David R, Trivisiol VS, da Silva AL (2017) Impact of fungicide and insecticide use on non-target aquatic organisms in rice paddy fields. Ciencia Rural. 47.
Wang Z, Chen Z, Fu X (2019) Integrated effects of co-inoculation with phosphate-solubilizing bacteria and N2-fixing bacteria on microbial population and soil amendment under C deficiency. Int J of Envi Research and Public Health. 16.
Yan X, Wang Z, Mei Y, Wang L, Wang X, Xu Q, Peng S, Zhou Y, Wei C (2018) Isolation, diversity, and growth-promoting activities of endophytic bacteria from tea cultivars of Zijuan and Yunkang-10. Frontiers in Microbiology. 9.
Zhang X, Tong J, Dong M, Akhtar K, He B (2022) Isolation, identification and characterization of nitrogen fixing endophytic bacteria and their effects on cassava production. PeerJ. 10:1-21.