

ISSN:1835-2707
Aust J Crop Sci. 18(08):486-492 (2024)
https://doi.org/10.21475/ajcs.24.18.08.pne71
In vitro germination and establishment of Platonia insignis Mart. from immature fruit and seed rescue
Karina da Silva Vieira1, Givago Lopes Alves1, Sérgio Heitor Sousa Felipe2, Marcos Vinícius Marques Pinheiro3, Irislene Souza Albuquerque1, Tácila Rayene Marinho Dutra1, Fábio Afonso Mazzei Moura de Assis Figueiredo2, Fabrício de Oliveira Reis1, Tiago Massi Ferraz1,2, Diego Silva Batista2,4, Thais Roseli Corrêa1,2*
1Programa de Pós-Graduação em Agroecologia, Universidade Estadual do Maranhão, São Luís, MA, 65055-310, Brazil
2Programa de Pós-Graduação em Agricultura e Ambiente, Universidade Estadual do Maranhão, São Luís, MA, 65055-310, Brazil
3Universidade Regional Integrada do Alto Uruguai e das Missões, Campus Frederico Westphalen (URI-FW), Frederico Westphalen, RS, 98400-000, Brazil
4Departamento de Agricultura, Universidade Federal da Paraíba, Campus III, PB, 58220-000, Brazil
Abstract
Platonia insignis Mart. is a species native to the Brazilian Amazon that has fruits of high commercial value and potential use in the cosmetic and pharmaceutical industries. However, the techniques for its propagation are limited. In the present study, we aimed to establish P. insignis in vitro by germinating immature seeds from the fruit. Immature fruits were collected, disinfected, and their seeds were placed in the following culture media (50 mL): agar + distilled water; MS0 (no growth regulators); MS + 1 μM BAP; MS + 2 μM BAP; MS + 3 μM BAP; MS + 1 μM GA3; MS + 2 μM GA3; and MS + 3 μM GA3 in a growth chamber at 24 ± 2 ºC in the dark, until shoot emergence. Callus formation, oxidation, germination, number of seedlings, shoot emergence, radicle, and shoot length were measured every 15 days after inoculation (DAI). At 60 DAI, seedlings grown without growth regulators had higher or equal values for all variables than those cultivated with growth regulators. The in vitro germination and establishment of P. insignis were efficient and feasible and could contribute to its domestication by reducing the germination time to approximately 60 days, producing homogeneous plants in a short period, and making it possible to explore other biotechnological techniques for this species.
Keywords: Amazonian fruit tree, In vitro propagation; Native fruit tree; Plant growth regulators.
Abbreviations:
BAP_ 6-benzylmonopurine; Ca_ Cataphylls; Eo, Eophylls; Ep, Epicotyl; GA3, Gibberellic acid; Lr, Lateral roots; Me, Metaphylls; Ox, Oxidation; Ra, radicle; Sh, Shoot; Sm, Spongy mass; Yc, Yellowish calluses.
Introduction
The “bacuri” tree (Platonia insignis Mart.; family Clusiaceae) is native to Brazil, with a center of dispersion in the Eastern Brazilian Amazon (Carvalho et al., 2022), and is also found in Guyana, Peru, Bolivia, Colombia and Ecuador (Jacomino et al., 2018). Amazonian fruits have gained prominence over the years, because of their multiple uses and forms of consumption (Rodrigues Lima et al., 2022). This fruit is highly consumed both in natura and industrialized in the form of pulp, yogurts, jams, jams, and ice creams (Lima et al., 2022; Pontes et al., 2017; Yamaguchi et al., 2021). It is a potential source of supporting phytochemical with health benefits, with several applications including in the composition of cosmetics, as analgesic, antidepressant, antidiabetic, anti-inflammatory, antioxidant, antifungal, anti-HIV, and antimicrobial (do Nascimento Cavalcante et al., 2020; Jacomino et al., 2018; Lima et al., 2022), which results in added economic, ecological and social value, especially in the North and Northeast regions of Brazil (Botelho et al., 2020; Mourão and Beltrati 1995b).
The main obstacles that hinder the expansion of the “bacuri” market is the rusticity of the species, and the absence of technologies for consolidation of the production chain, because fruits are procured directly from trees that exist in the producing regions, i.e., from management of natural populations, directly affecting the supply of products in the national and international market (Botelho et al., 2020; Lima et al., 2022). Furthermore, Platonia insignis has being domesticated because of the long duration required for the emergence of seedlings (with more than 500 days on average), which makes it difficult to produce seedlings and establishing orchards (Carvalho et al., 2022; Carvalho et al., 1998; Carvalho et al., 2002).
Under natural conditions, the seeds begin root protrusion at 12 days on average, however, from this stage on, there is a marked root growth, in which this primary root reaches lengths greater than 180 cm, and a diameter in the basal portion equal to 0.7 cm. This process takes a long-time which can vary from 180 to 900 days (Carvalho et al., 2022). Only after this long period of root growth, epicotyl emergence occurs to complete the formation of seedlings, and this delay and unevenness in the emergence of the epicotyl is related to dormancy of the plumule (Carvalho et al., 2022). This long time required to form seedlings is a major bottleneck for the propagation of the species, making it difficult to produce seedlings in nurseries.
The application of biotechnological techniques may contribute directly to the formation of seedlings in Platonia insignis, as well as to a better understanding of the morphophysiological processes involved (Marinho et al., 2022), and can be conserved in active germplasm banks to support future genetic improvement programs (Pontes et al., 2017). Additionally, these plants can be used in several other techniques, such as composing crossing materials in breeding programs for plant domestication, propagation by micrografting, and conventional grafting. Based on this, in vitro germination is a viable alternative for the propagation of many species, as it promotes a high rate of seed germination owing to the greater environmental control provided by in vitro conditions compared to field conditions (Hesami et al., 2021).
Multiple crosstalk levels in hormonal networks control seed germination and are essential for embryo growth and development (Carrera-Castaño et al., 2020). For example, gibberellins are directly involved in seed germination, where they influence dormancy breaking (e.g., resumption of embryo growth) and induce the mobilization of endosperm reserves through hydrolytic enzymes (e.g., ά-amylase) (Gupta and Chakrabarty 2013); while cytokinins, especially with their negative interaction with abscisic acid (ABA), can positively regulate seed germination by controlling cell division (Miransari and Smith 2014).
Considering that in vitro propagation techniques can be (i) an excellent alternative to accelerate the propagation of P. insignis; (ii) provide large-scale production of healthy plants; and (iii) contribute to the management and domestication strategies of the species; the present study aimed to evaluate the in vitro germination and establishment of P. insignis based on the rescue of seeds from immature fruits under different concentrations of the growth regulators 6-benzylmonopurine (BAP) and gibberellic acid (GA3).
Results
The in vitro germination of P. insignis was successfully obtained in five of the tested treatments, which in turn showed significant differences (P ≤ 0.05). Seeds placed on agar + distilled water (control treatment), MS0 (no growth regulators) and MS + 2 µM GA3 showed 100% germination, being preceded by MS with different BAP concentrations (1, 2, and 3 µM) and 1 µM GA3, with germination between 25 and 50%. In contrast, germination was inhibited in MS + 3 µM GA3 (Fig. 2a).
Seeds on MS + 2 µM GA3 showed the best results for percentage of seedlings (e.g., 100% of seedlings formed), differing from MS at concentrations of 3 µM BAP or GA3, in which there was no seedling development (P ≤ 0.05; Fig. 2b). taken together, MS + 2 µM GA3 not only provides a high percentage of germination, but also promotes a higher percentage of seedling in vitro (a plant with desirable characteristics: presence of shoots and roots). However, this treatment did not differ from the agar medium + distilled water (control treatment), which is inexpensive because it does not use minerals or growth regulators.
In all treatments, callus formation was observed in the seeds; however, there was no significant difference, with a variation of 78–100% in callus formation (Fig. 3a). However, callus oxidation was observed, mainly in MS + 1 µM GA3, which differed only from MS + 2 µM BAP, the latter with less oxidation (Fig. 3b).
No significant differences in shoot length between treatments (P > 0.05; Fig. 4) were observed; however, seedlings in agar + distilled water and MS + 2 µM of GA3 showed higher values of radicle length, 6.2 and 5.9 cm, respectively (P ≤ 0.05; Fig. 4).
Initially, we observed that the seeds had a white color with yellowish regions; however, as the days passed after inoculation, the color changed to brown, indicating possible oxidation. Additionally, morphological changes were observed in the seeds throughout the experiment, such as the formation of a spongy mass (Fig. 5a-h; 6a) in all the treatments tested and yellowish callus formation, as observed in the agar + distilled water treatment, which was also observed in all treatments (Fig. 6a). Also, we observed radicle, lateral roots, epicotyl and shoot formation (Fig. 5; 6b, 6c), proving seedling formation, less in MS + 3 μM BAP, MS + 1 μM GA3, and MS + 3 μM GA3 (Fig. 2b). Overall, seedling formation occurs initially through the emission of eophylls (the first leaves produced by plants), followed by cataphylls (reduced, small, and opposite leaves) and metaphylls (showing the first pair of adult leaves formed after cataphylls) (Fig. 6c).
Discussion
Platonia insignis is a tree native to South America with high potential for tropical fruit growth owing to its bittersweet pulp and nutraceutical characteristics (Yamaguchi et al., 2021). However, domestication of this species is still incipient, with the need for biotechnological studies that provide greater characterization (Marinho et al., 2022). Here, for the first time, seeds of immature fruits were rescued for in vitro germination and establishment of Platonia insignis. In addition, the treatment without MS medium (e.g., agar + distilled water) resulted in high germination and seedling formation, in line with greater shoot and root lengths.
When comparing the seedling emergence time in vitro with that under natural conditions, the seeds germinated in vitro showed a reduction in seedling formation time (~ 60 days). Although it is unknown to us which factors in the in vitro cultivation have the greatest influence on this rapid epicotyl emission, there is possibly a greater activity of enzymes related to cell expansion and division, facilitating the rupture of the cortical ground meristem. In parallel, we cannot neglect the fact that seeds without seed coats were used in this study, which in turn may have facilitated the water absorption and growth induction stages (Bentsink and Koornneef, 2008). Based on these results, other applications may be adopted, such as micrografting, conventional grafting, or propagation by somatic embryogenesis, which guarantee the clonal fidelity of the genetic material that may be selected as superior in terms of the organoleptic qualities of fruits and small trees among other characteristics of interest to producers and the consumer market.
Platonia insignis seeds are recalcitrant, and under ideal germination conditions (e.g., light, water, oxygen, and temperature), they exhibit rapid radicle emission, parallel to the slow and uneven emergence of the epicotyl (Carvalho et al., 2022; Carvalho et al., 2002). Root emergence is fast, occurring on an average of 18 days, whereas epicotyl emission is slow and uneven, and can occur at an average of 564 days after sowing (Carvalho et al., 1998). In our study, after 15 d in the dark and 60 d under light conditions, we observed seedlings with eophyll emissions, followed by cataphylls and metaphylls (Fig. 6c), which proved the complete development of P. insignis seedlings, as reported by Mourão and Beltrati (1995a).
Here, the reduction in the time for seedling formation by Platonia insignis in vitro may be related to conditions of greater environmental control, such as light intensity and temperature, indicating that tissue culture can be an excellent tool for the production of plants of this species in a shorter time. However, this response may also be associated with the rescue of seeds from immature fruits, thereby mitigating the potential effects of dormancy on epicotyl emissions.
Interestingly, the agar + distilled water treatment (control) resulted in higher germination rates. This differs from other woody and fruit species that require growth regulators to start germination, such Annona crassiflora, which obtained the best results in WPM medium supplemented with 72.18-92.39 μM GA3 and exhibited rooting and enhanced growth when inoculated with MS medium containing 75.06- 86.61 μM GA3 and 10.74 μM ANA (Ribeiro et al., 2009).
It is important to emphasize that the seeds of Platonia insignis are large and contain oils composed of saturated, monounsaturated, and polyunsaturated fatty acids, which, in turn, are indicative of a high concentration of reserves in the seed (Fasciotti et al., 2020). These reserves are mobilized during germination, providing energy for seedling formation. Considering this, seeds in the presence of water, temperature, and light probably already have the extrinsic factors necessary for germination to the detriment of regulators such as BAP and GA3. Therefore, the control medium (agar plus distilled water) was considered sufficient for seedling emergence.
Seeds inoculated in MS + 3 μM GA3 were strongly negatively affected. Rau et al. (2021) reported similar results for Psidium cattleyanum submitted to 144.35 μM of GA3, significantly reducing the germination index, rate, and average germination time, indicating the existence of hormonal changes that prevented or delayed germination. The effect of exogenous GA3 application on seed germination depends on other factors such as the endogenous concentration of abscisic acid (ABA) and other inhibitory compounds in the seed (Gilroy and Jones 1994). Platonia insignis seeds are likely to have high ABA concentrations, causing an antagonistic relationship between ABA and GA, which determines seed dormancy and germination. In the early stages of development, seeds are highly sensitive to ABA but minimally sensitive to GA, which favors dormancy over germination; however, in later stages, ABA sensitivity declines and GA sensitivity increases, favoring germination. Simultaneously, seeds become progressively more sensitive to environmental stimuli, such as light and temperature, which can stimulate or inhibit germination (Yan and Chen 2020). Therefore, it is possible that germination was inhibited.
The formation of spongy and yellowish calluses (Fig. 6c) in Platonia insignis was observed in vitro at 45 and 60 DAI. The same behavior was reported for other species, including Eugenia involucrata (Golle et al. 2020), Hevea spp. (Silva et al., 2021), and Satureja hortensis L. (Navroski et al., 2014). This high concentration of endogenous auxins, possibly present in the seeds, explains callogenesis induction, since spongy and yellowish calluses form even in culture medium without exogenous growth regulators.
Oxidation negatively affects Platonia insignis seedlings. Explant darkening caused by oxidation is an obstacle in the in vitro culture of certain woody species, likely because of the phenolic compounds released by tissues in response to injury, high growth regulator concentrations in the culture medium, and oxidation of polyphenols and quinines (Thomas and Ravindra 1997). However, phenolic oxidation did not compromise the establishment or germination of explants. Sousa et al. (2007) studied Cattleya walkeriana and Schomburgkia crispa seeds in culture media with and without activated charcoal and found a higher germination percentage in seeds inoculated with 0.1% activated charcoal, which inhibited necrosis; indicating that the activated charcoal in this study also contributed to reducing oxidation.
P. insignis seeds also exhibited morphological differences between explant responses, such as the formation of spongy and yellowish calluses. However, it has been argued that calluses formed by soft spongy tissue are incapable of plant regeneration or developing morphogenetic pathways (e.g., somatic embryogenesis or indirect organogenesis) (Nabors et al., 1983).
Amorphous areas formed in some explants (Fig. 6b) and were also observed in the in vitro germination of Byrsonima intermedia, where contact between cotyledonary leaves and the culture medium promoted cell multiplication, compromising normal seedling development (Nogueira et al., 2004). In Platonia insignis seeds, a hormonal balance is believed to occur between high endogenous auxin concentrations and the addition of cytokinins to the culture medium. This composition may have favored intense cell division, disorganized plant cell proliferation, and callus formation instead of multiple shoots.
Materials and methods
Plant material
Immature Platonia insignis fruits with an average diameter of 78 mm, with the green coloring and average weigh of 204.63 g (Fig 1a) were collected from different mother plants in Santa Luzia, in the municipality of Bacabeira, Maranhão state (MA), Brazil (2° 58' 14'' S and 44° 18' 32'' W). After collection, the samples were stored in polystyrene boxes and transferred to the Plant Tissue and Cell Culture Laboratory of Maranhão State University (LCT/UEMA) in São Luís, MA.
Disinfestation, in vitro seed inoculation, and treatments
The fruits were disinfected under laboratory conditions using running water and neutral detergent, followed by immersion in 70% (v/v) ethanol for 5 min and then 2% (v/v) commercial sodium hypochlorite (NaClO) with one drop of 0.1% Tween-20 (v/v) (Isofar Ltda®, Duque de Caxias, Rio de Janeiro, Brazil) for 20 min.
The fruits were sectioned in a laminar flow chamber to remove the pericarp (exocarp, mesocarp, and endocarp) and to rescue the seeds (Fig. 1b). Seeds were disinfected in 70% (v/v) ethanol for 1 min, followed by immersion in 2% (v/v) commercial NaOCl for 3 min for three washes in autoclaved distilled water for 1 min each.
The seeds were inoculated in glass flasks (350 mL) containing 50 mL of MS (Murashige and Skoog 1962); culture medium (Phytotechnology Laboratories, LLC, Shawnee Mission, KS, USA) supplemented with 30 g L-1 sucrose (Isofar® Ltda, Duque de Caxias, RJ, Brazil), 100 mg L-1 myo-inositol (Sigma-Aldrich Co, St Louis, MO, USA), 6.5 g L-1 agar (Agargel Indústria e Comércio LTDA, São Paulo, SP, Brazil), and 3 g L-1 of activated carbon (Sigma-Aldrich Co, St Louis, MO, USA). The treatments consisted of agar + distilled water (control treatment); MS0 (no growth regulators); MS + 1 μM BAP; MS + 2 μM BAP; MS + 3 μM BAP; MS + 1 μM GA3; MS + 2 μM GA3; and MS + 3 μM GA3. The pH of the media was adjusted to 5.7 ± 0.1 before autoclaving at 121 °C and 108 kPa for 15 min. After inoculation the seeds were placed in a growth chamber at 24 ±2 °C, initially in the dark until shoot emergence (approximately 7 to 15 days after inoculation (DAI)), and then under 5 µmol m-2 s-1 irradiance until 60 DAI, when the follow parameters were assessed: germination (%), seedling formations rate (%), shoot emergency (%), calluses formation (%), calluses oxidation (%), and shoot and radicle length (cm).
Variables analyzed
For the determination of germination (%) root protrusion greater or equal to 3 mm were considered, while for seedling rate (%) concomitant root and epicotyl with the presence of cataphyll, both without morphological anomalies (e.g., atrophied root and epicotyl) were considered.
The percentage of callus formation (%) was determined by counting the presence and absence of these amorphous structures in the seeds, whereas oxidation (%) was determined as follows: 0, no oxidized explants; 25%, up to ¼ oxidized; 50%, up to ½ oxidized; 75%, up to ¾ oxidized; and 100%, completely oxidized explants.
Shoot length was determined by measuring the distance from the collar region of the seedling to the apical bud, whereas radicle length was measured from the region below the seed to the apical bud.
Statistical analyses
The experiment was conducted following a completely randomized design consisting of eight treatments [agar + distilled water (control treatment); MS0 (no growth regulators); MS + 1 μM BAP; MS + 2 μM BAP; MS + 3 μM BAP; MS + 1 μM GA3; MS + 2 μM GA3; and MS + 3 μM GA3] with six replicates each and an experimental unit composed of one seed per flask. All variables were subjected to analysis of variance, and the means were compared using the SNK test (P < 0.05) using the SISVAR software (Ferreira, 2011).
Conclusions
The medium with agar + distilled water resulted in high germination, in line with longer shoots and roots. Thus, rescuing seeds from immature fruits and using this culture medium is effective for in vitro germination and establishment of Platonia insignis, in addition to having a low cost. Our results can be used for micropropagation and in vitro genetic conservation of this species, directly contributing to a better characterization of the native species.
Acknowledgments
The authors thank the Brazilian sponsoring agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), FAPEMA (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior) for financial support.
References
Bentsink L, Koornneef M (2008) Seed dormancy and germination. Arab. B. 6: e0119. https://doi.org/10.1199/tab.0119
Botelho MGL, Homma AKO, Furtado LG, Lima MCS and Costa MSS (2020) Productive and market potential of bacuri fruit (Platonia insignis Mart.) in Pará, Brazil. Res., Soc. Dev. 9(7): e989975124. https://doi.org/10.33448/rsd-v9i7.5124
Carrera-Castaño G, Calleja-Cabrera J, Pernas M, Gómez L, Oñate-Sánchez L (2020) An updated overview on the regulation of seed germination. Plants 9(6):703. https://doi.org/10.3390/plants9060703
Carvalho JEU, Homma AKO, Nascimento WMO (2022) Platonia insignis. In: Coradin, L., Camillo, J, and Vieira, I. C. G. (eds) Espécies nativas da flora brasileira de valor econômico atual ou potencial: Plantas para o futuro - Região Norte. Ministério do Meio Ambiente, Brasília, DF, p 424-449.
Carvalho JEU, Müller CH, Leão NVM (1998) Cronologia dos eventos morfológicos associados à germinação e sensibilidade ao dessecamento em sementes de bacuri (Platonia insignis Mart.-Clusiaceae). Rev. Bras. Sem. 20(2): 475-479.
Carvalho JEU, Müller CH, Nascimento WMO (2002) Métodos de propagação do bacurizeiro (Platonia insignis Mart.). Belém: Embrapa-CPATU, 12p.
Cavalcante AN, Lima LKF, Araújo CM, Santos FPS, Nascimento MO, Sousa JMC, Rai M, Feitosa CM (2020) Toxicity, cytotoxicity, mutagenicity and in vitro antioxidant models of 2-oleyl-1,3-dipalmitoyl-glycerol isolated from the hexane extract of Platonia insignis MART seeds. Toxicol. Rep. 7: 209-216. https://doi.org/10.1016/j.toxrep.2020.01.014
Fasciotti M, Monteiro TVC, Rocha WFC, Morais LRB, Sussulini A, Eberlin MN, Cunha VS (2020) Comprehensive Triacylglycerol Characterization of Oils and Butters of 15 Amazonian Oleaginous Species by ESI-HRMS/MS and Comparison with Common Edible Oils and Fats. Eur. J. Lipid. Sci. Technol. 122(9):2000019. https://doi.org/10.1002/ejlt.202000019
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc. Agrotec. 35(6):1039 1042. https://doi.org/10.1590/S1413-70542011000600001
Gilroy S and Jones RL (1994) Perception of gibberellin and abscisic acid at the external face of the plasma membrane of barley (Hordeum vulgare l.) aleurone protoplasts. Plant Physiol. 104(4):1185-1192. https://doi.org/10.1104/pp.104.4.1185
Golle DP, Reiniger LRS, Stefanel CM, Serrote CML (2020) Fitorreguladores na calogênese e rizogênese em Eugenia involucrata. Pesq. Flor. Bras. 40: 1-9. https://doi.org/10.4336/2020.pfb.40e201901908
Gupta, R and Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Signal. Behav. 8(9): e25504. https://doi.org/10.4161/psb.25504
Hesami M, Pepe M, Monthony AS, Baiton A, Jones AMP (2021) Modeling and optimizing in vitro seed germination of industrial hemp (Cannabis sativa L.). Ind Crops Prod . 170:113753. https://doi.org/10.1016/j.indcrop.2021.113753
Jacomino AP, Pinto PM, Gallon CZ (2018) Bacuri—Platonia insignis. In: Rodrigues S, de Oliveira Silva E & de Brito ES (eds) Exotic Fruits. Academic Press, p 49-52.
Lima SKR., Coêlho AG, Lucarini M, Durazzo A, Arcanjo DDR (2022) The Platonia insignis Mart. as the promising brazilian ‘Amazon Gold’: The state-of-the-art and prospects. Agriculture 12(11):1827. https://doi.org/10.3390/agriculture12111827
Marinho TRS, Corrêa TR, Vieira KS, Albuquerque IS, Alve GL, Pinheiro MVM, Reis FO, Figueiredo FAMMA, Araújo JRG, Ferraz TM (2022) Genetic variability during in vitro establishment of bacurizeiro (Platonia insignis Mart.): an Amazon species. Aust. J. Crop Sci. 16(6):819-825. https://doi.org/10.21475/ajcs.22.16.06.p3575
Miransari M and Smith DL (2014) Plant hormones and seed germination. Environ. Exp. Bot. 99: 110-121. https://doi.org/10.1016/j.envexpbot.2013.11.005
Mourão KSM. and Beltrati CM (1995a) Morfologia dos frutos, sementes e plântulas de Platonia insignis Mart. (Clusiaceae). III germinação e plântulas. Acta Amazon. 25:47-53. https://doi.org/10.1590/1809-43921995252053
Mourão KSM and Beltrati CM (1995b) Morfologia dos frutos, sementes e plântulas de Platonia insigns MART. (Clusiaceae). I. Aspectos anatômicos dos frutos e sementes em desenvolvimento. Acta Amazon. 25:11-31. https://doi.org/10.1590/1809-43921995252032
Murashige T and Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473-497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nabors MW, Heyser JW, Dykes TA, Demottand KJ (1983) Long-duration, high-frequency plant regeneration from cereal tissue cultures. Planta 157(5):385-91. https://doi.org/10.1007/bf00397195
Navroski MCI, Waldow DAG, Reiniger LRS, Golle DP, Curti AR, Pereira MO (2014) Multiplicação in vitro de segmentos apicais caulinares de segurelha (Satureja hortensis L.). Rev. Bras. Pl. Med. 16(1):117-121. https://doi.org/10.1590/S1516-05722014000100017
Nogueira RC, Paiva R, Castro AH, Vieira CV, Abbade LC, Alvarenga AA (2004) Germinação in vitro de murici-pequeno (Byrsonima intermedia A. Juss.). Cienc. Agrotec. 28(5): 1053-1059. https://doi.org/10.1590/S1413-70542004000500012
Pontes LCG, Moura EF, Moura MF, Rodrigues SM, Oliveira MSP, Carvalho JEU, Therrier J (2017) Molecular characterization of progenies of bacurizeiro (Platonia insignis ) from Marajó Island, northeastern Amazon. Acta Amazon. 47(4):293-300. https://doi.org/10.1590/1809-4392201701302
Rau TG, Radaelli JC, Wagner Júnior A, Citadin I, Moura GC, Mazaro SM (2021) Benzylaminopurine and gibereline in the germinate process of yellow araça. Res., Soc. Dev. 10(5) :e49110515124. https://doi.org/10.33448/rsd-v10i5.15124
Ribeiro MNO, Pasqual M, Villa F, Pio LAS, Hilhorst HWM (2009) In vitro seed germination and seedling development of Annona crassiflora Mart. Sci. Agric. 66(3): 410-413. https://doi.org/10.1590/S0103-90162009000300017
Rodrigues-Lima SK, Pereira EJAL, Machado GO, Silva RA, Lucarini M, Durazzo A, Diele-Viegas LM, Arcanjo DDR (2022) Systematic mapping of the production chain of “bacuri” (Platonia insignis Mart.) in Brazil. Sustainability 14(22):15051. https://doi.org/10.3390/su142215051
Silva ACL, Manfio CE, Leão JRA, Carvalho JC, Gonçalves JFC, Raposo A (2021) Induction of calogenesis in leaf segment of rubber (Hevea spp.) in the South-Western Amazon. Res., Soc. Dev. 10(9): e17410917639. https://doi.org/10.33448/rsd-v10i9.17639
Sousa GC, Clemente PL, Isaac VLR, Faria SP, Ferreira AS, Campos MRC (2007) Contaminação Microbiana na Propagação in vitro de Cattleya walkeriana e Schomburgkia crispa. Rev. Bras. Bioc. 5(1):405-407.
Thomas P and Ravindra MB (1997) Effect of pruning or removal of in vitro formed roots on ex vitro root regeneration and growth in micropropagated grapes. Plant Cell Tissue Organ Cult. 51:177-180. https://doi.org/10.1023/A:1005928615179
Yamaguchi KKL, Dias DS, Lamarão CV, Castelo KFA, Lima MS, Antonio AS, Converti A, Lima ES, Veiga-Junior VF (2021) Amazonian bacuri (Platonia insignis Mart.) fruit waste valorisation using response surface methodology. Biomolecules 11(12): 1767. https://doi.org/10.3390/biom11121767
Yan A and Chen Z (2020) The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 86:39-75. https://doi.org/10.1007/s12229-020-09220-4
.
Figures
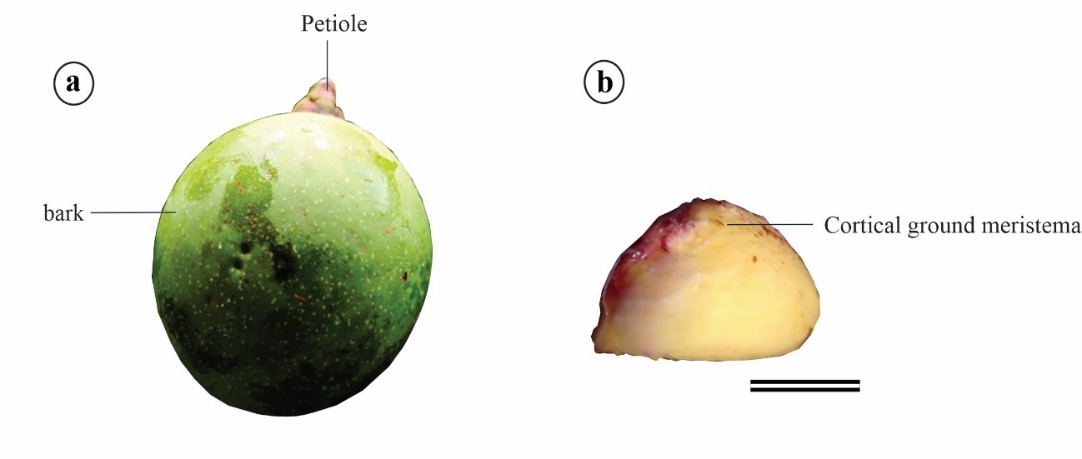
Figure 1. Immature fruits (a) and rescued seeds (b) of Platonia insignis. Bar: 1 cm.
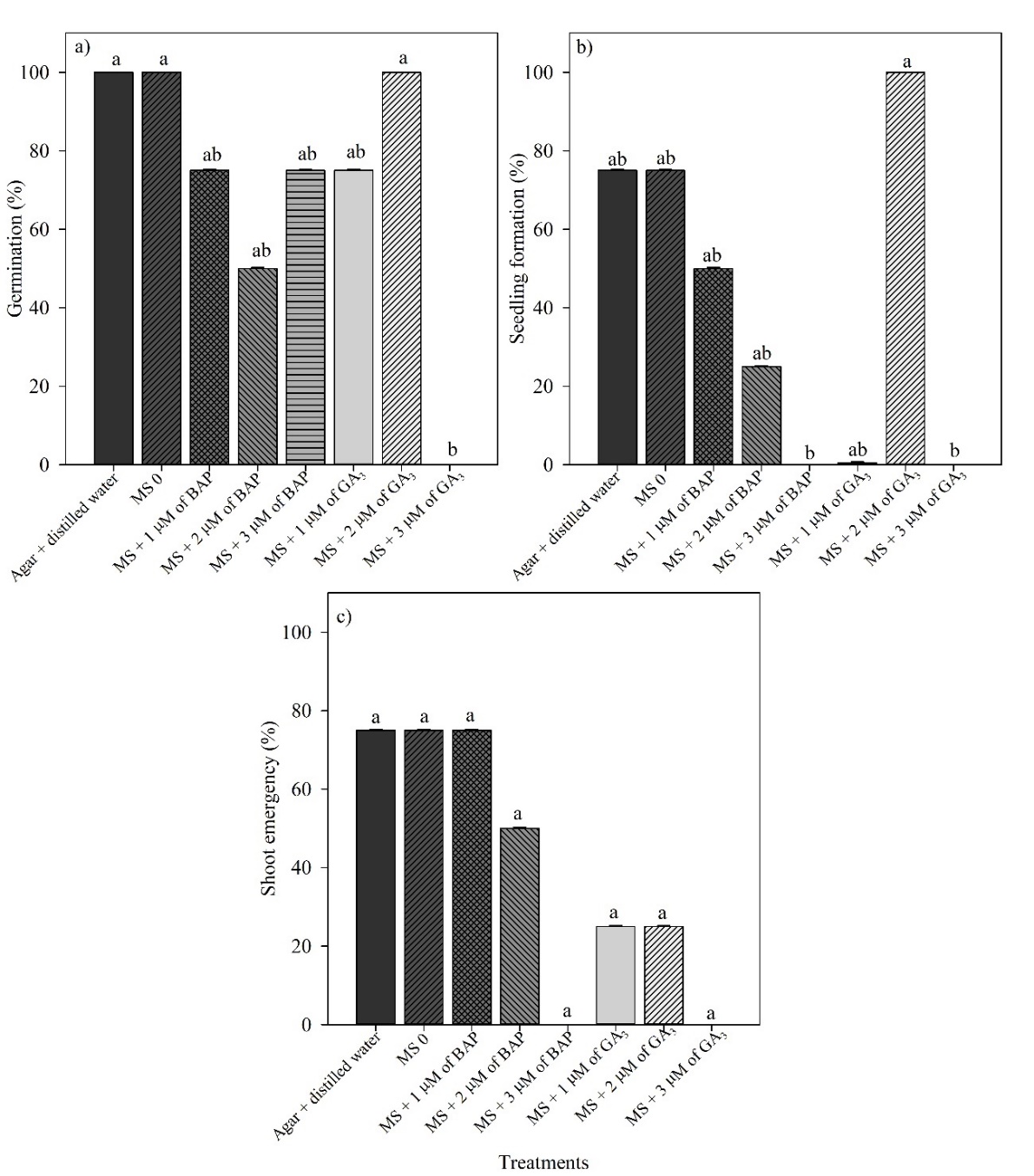
Figure 2. Germination (a), seedling formation (b), shoot emergency (c) of seedlings from seeds of immature fruits of Platonia insignis under different treatments at 60 days in vitro.
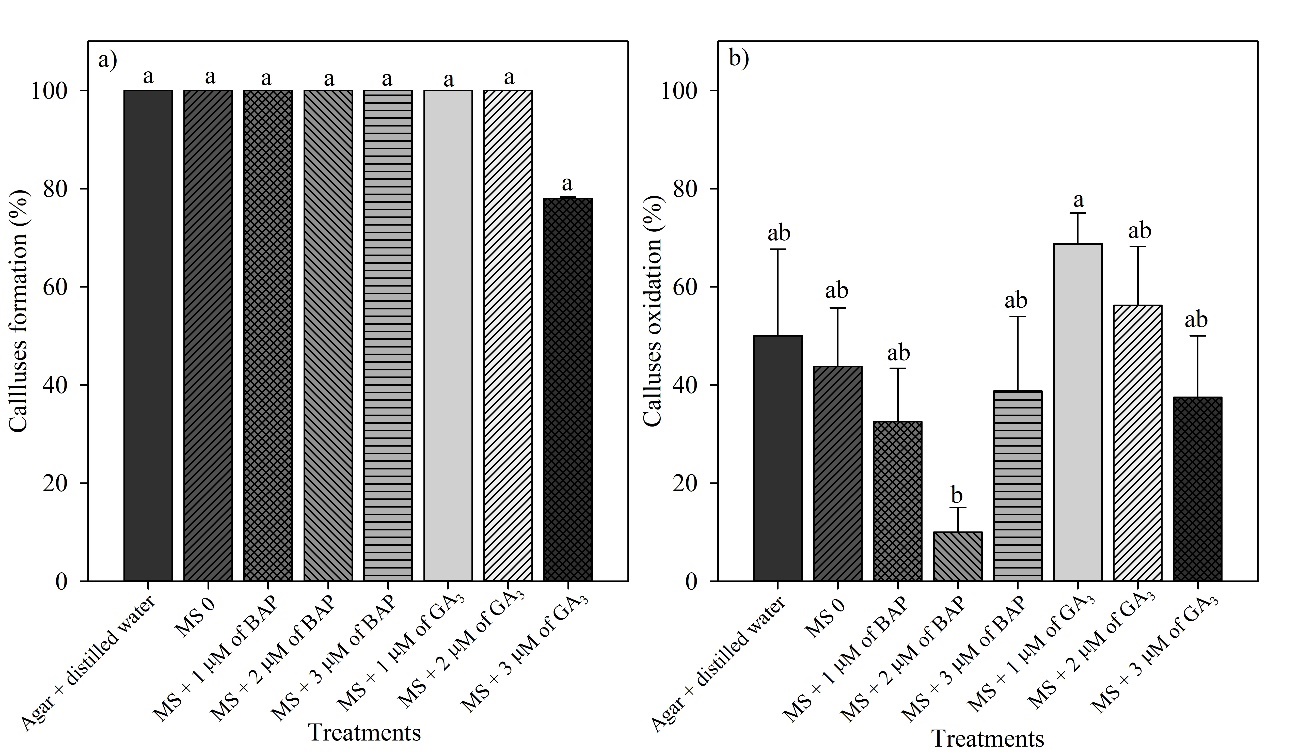
Figure 3. Calluses formation (a) and oxidation (b) in seeds of immature fruits of Platonia insignis under different treatments at 60 days in vitro.
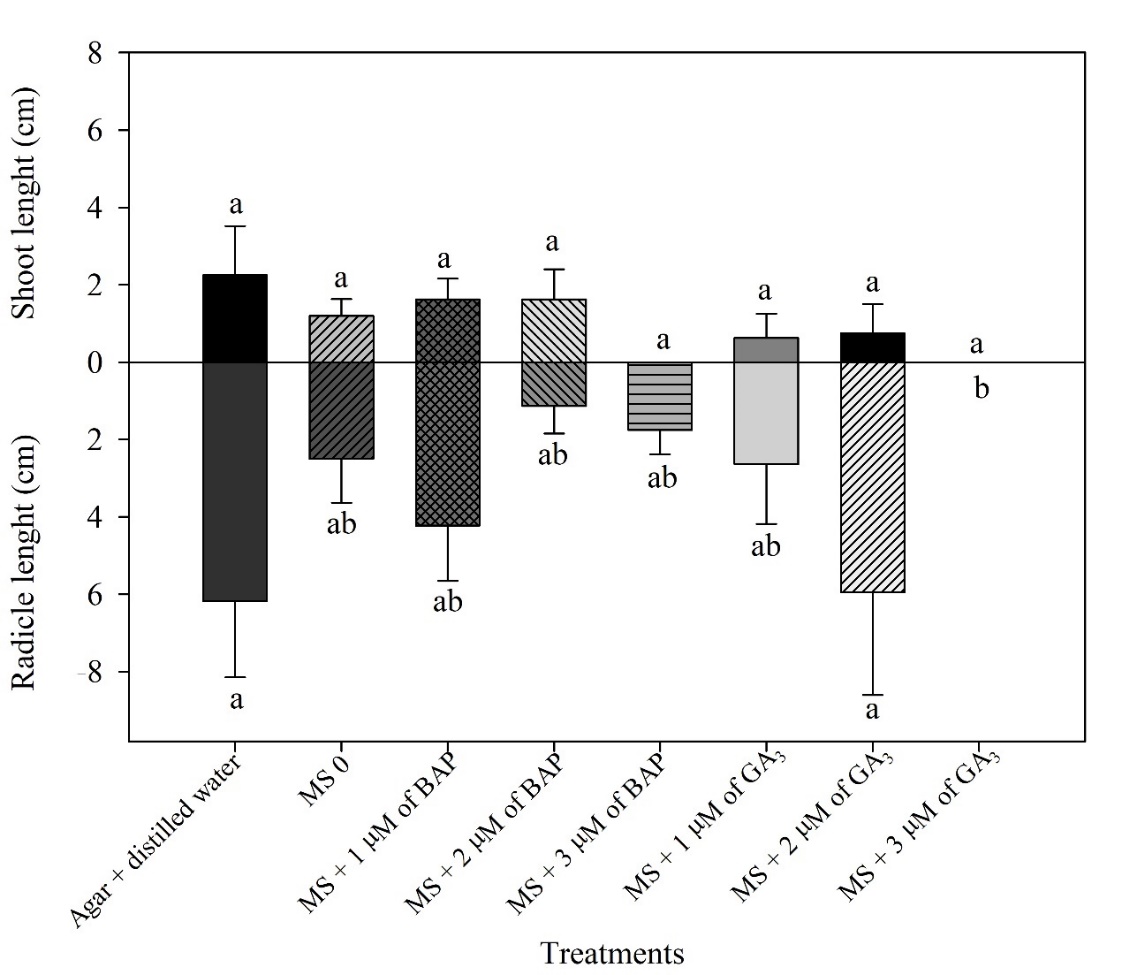
Figure 4. Shoot and radicle length of seedlings from seeds of immature fruits of Platonia insignis under different treatments at 60 days in vitro.
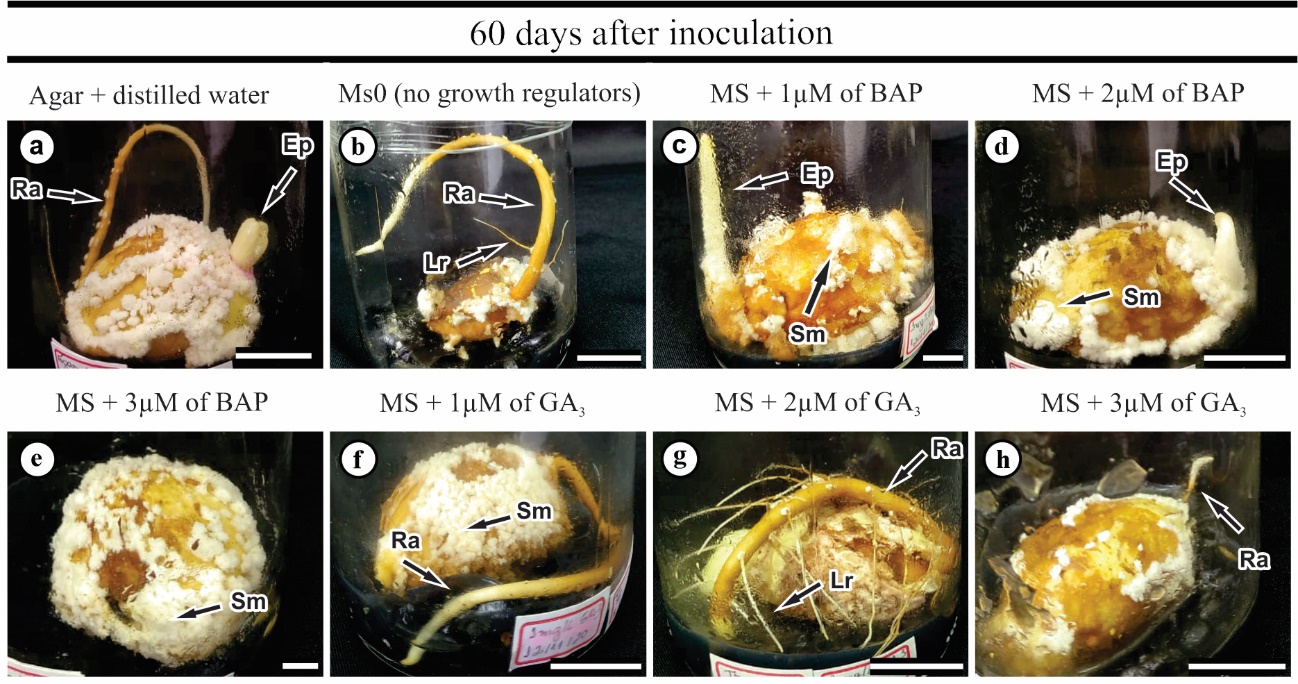
Figure 5. Platonia insignis explants 60 days after inoculation in vitro in the different treatments. Agar + distilled water (a); MS0 – no growth regulator (b); MS + 1 μM of BAP (c); MS + 2 μM of BAP (d); MS + 3 μM of BAP (e); MS + 1 μM of GA3 (f); MS + 2 μM of GA3 (g) and MS + 3 μM of GA3 (h). Abbreviations: Ep – Epicotyl; Lr – Lateral roots; Sm – spongy mass; Ra - radicle. Bars: 1 cm.
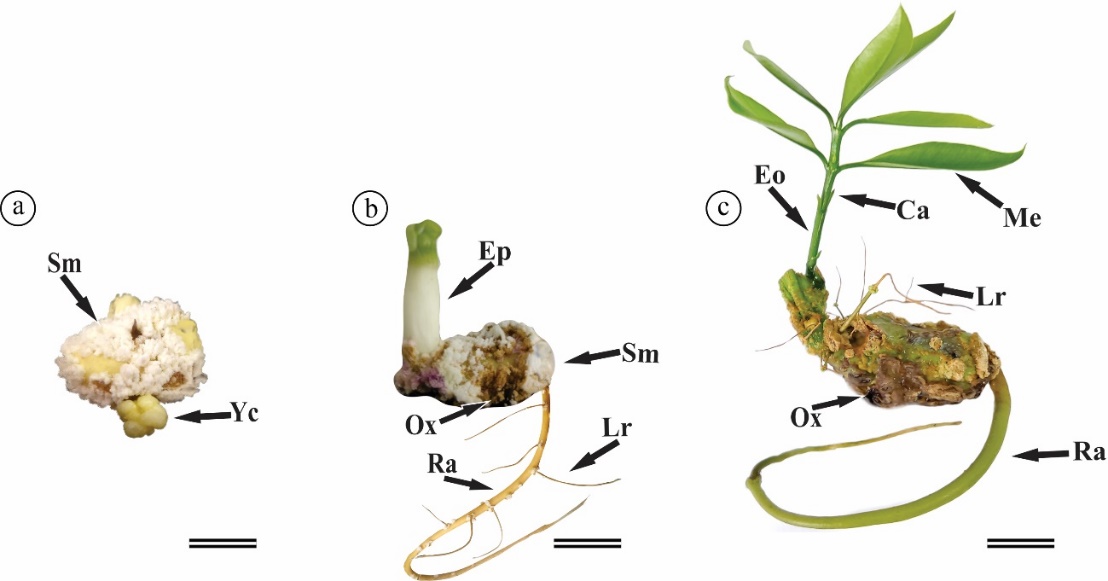
Figure 6. Morphological characteristics during the permanence of seeds of immature fruits of Platonia insignis in vitro in the different treatments at 60 (a, b) and 75 DAI (c). Abbreviations: Ca – Cataphylls; Eo – Eophylls; Ep – Epicotyl; Lr – Lateral roots; Me – Metaphylls; Ox – Oxidation; Ra - radicle; Sh – Shoot; Sm – Spongy mass; Yc – Yellowish calluses. Bars: 1 cm.