ISSN:1835-2707
Aust J Crop Sci 18(08):448-452 (2024)
https://doi.org/10.21475/ajcs.24.18.08.p1411
Application of mannitol as pre-treatment and sucrose supports callus induction through anther culture in Gossypium arboreum
Wiwik Indrawati1, Any Kusumastuti1, Muhammad Zahir Ahsan2, and Muhammad Tahir1, Jakty Kusuma1*
1Politeknik Negeri Lampung, Bandar Lampung, Indonesia
2Cotton Research Station, Sahiwal, Pakistan
Abstract
Diploid or tree cotton (Gossypium arboreum) species may provide a breeding source for fiber quality and disease resistance. Developing homozygous lines of Gossypium arboreum through anther culture can be a successful strategy for breeding the parental lines needed for cotton hybrids. This study describes callus induction on four G. arboreum genoytypes under various concentrations of sucrose as well as plant growth regulators. Explant materials were derived from immature anther and grew on modified Murashige and Skoog (MS) media. Prior to main treatment, mannitol application were applied to anthers with concentration of 0.7 M and modified MS media consisted of 3%, 5%, and 7% sucrose, as well as an addition of IAA, NAA, BAP, 2,4-D and Kin. Optimum callus formation was observed in Marvi genotype, in a medium containg IAA as well as sucrose between 5-7%. Furthermore, combination of IAA and NAA with sucrose 7% indicates highest number of embryogenic calli. However, Marvi genotype showed decreased rate of callus formation when the amount of sucrose increased on MS supplemented with NAA medium. The results indicated that the best formula on each genotypes were different. However MS media with IAA (1 mg/l) + kin (0.2 mg/l) + CM (70 ml/l) + sucrose 5% provided the best performance to produce embryogenic calli on each genotypes. Our report can be utilized as an opportunity and strategy to obtain double haploid lines as a parental source in hybrid production.
Keywords: Gossypium arboreum, embryogenic, callus, anther culture.
Abbreviations: 2,4-D_2,4-Dichlorophenoxyacetic acid, BAC_Beginning after culture, BAP_6-Benzylaminopurin, CM_Coconut milk, DAF_Days after flowering, DAP_Days after planting, IAA_Indole-3-acetic acid, MS_Murashige and Skoog (1962) medium, NAA_Napthaleneacetic acid.
Introduction
Cotton is the most important source for textile fiber, as the world demand projecting nearly 20 million tonnes in 2018 (Macdonald, 2018). Many researches proposed that Gossypium arboreum or tree cotton (2n = 2x = 26) is putatively donor species to tetraploid cotton. Tree cotton provides many favorable traits for cotton production, which is absent in upland cottons (G. hirsutum cultivars) (Liu et al., 2006) such as drought tolerance and pest resistance (Mehetre et al., 2003) as well as breeding source for low input cultivation (Liu et al., 2006). Utilization of G. arboreum as parental source had been conducted by Vij et al. (2016) and suggested that intercrossed progenies can enhance quality of fiber and pest resistance.
Development of hybrid cotton is an important effort to meet the needs of textile and fiber industries, by creating inbred lines of cotton plants to produce expected heterosis effect on selected progenies. Homozygous line production usually takes 5-6 generations to reach desirable homozygosity levels (Bohorova and Atanassov, 1990). This is usually done through multiple self-pollination processes, until the plant obtains the desired homozygosity.
It has also been reported that homozygosity of G. arboreum can be reached after 18 successive self-pollination process (Li et al., 2014), which is a very long and exhaustive process. By producing double haploid lines through anther culture, selfing process can be shortened. Anther culture is a rapid method to obtain homozygous cotton lines by biotechnological approach and is an important method in plant breeding due to its positive impact on the application of mutation, selection, genome sequencing, genetic analysis, and gene transformation (Germana and Lambardi, 2016). In this process, stress is given to microspore induction so that immature male gametes undergo differentiation of growth from gametophytes to sporophytes.
Many reports revealed that sucrose has significant role in embryogenesis, such as in maize (Ismaili and Mohammadi, 2016), cucumber (Abdollahi et al., 2016), as well as in citrus (Cardoso et al., 2016). According to Bajaj and Gill (2013), the treatment of dark conditions and starvation with the provision of some mannitol concentrations can induce embryos in cotton plants.
To our knowledge, embryogenesis through anther culture of G. arboreum has not been done in Indonesia. The purpose of this study is to accelerate the formation of pure strains that produce prospective elders superior inbred with homozygous agronomic characters in the formation of hybrid cotton plants, so that the effects of heterosis can be expressed in their progenies.
Results and discussion
Effects of pre-treatment on callus induction
The anther culture responses of cotton on nine different media are presented in Table 1. Callus was initiated 14-21 days after culturing the anthers in all genotypes. Influence of Mannitol on anther-derived callus was observed, and all samples performed callus formation above 50%. A previous report shows that using mannitol to pretreat anther has caused significantly higher callus induction rate compared no pre-mannitol treatment (Lijuan et al., 2016). A lower callus rate was reported by Bajaj and Gill (1998) on G. arboreum without mannitol pre-treatment. On the other hand, Bajaj and Gill (1989) performed cold treatment and enhanced callusing in G. arboreum. Addition of mannitol stimulates specific sugar sensors (Muñoz‐Amatriaín et al., 2006). This condition can enhance the cell division on certain tissue, since the treatment triggers a multidimensional stress.
Among tested genotypes, the highest percentage of callus formation was shown by Marvi 1 (Fig. 1), while the lowest was also appeared on the same genotype. This situation suggested that interaction between sugar and growth regulators can affect callus induction in genotypes. Maximo et al. (2018) stated that somatic embryos supplemented by cytokinin can significantly and
Table 1. LSI test on callus formation in culture tubes by using anther as explant material. * = significant on P ≤ 0.005.
| Code | Media | Variable | Varieties | |||
|---|---|---|---|---|---|---|
| FDH 1 | FDH 2 | Marvi 1 | Marvi 2 | |||
| A1 | MS+IAA (0.5 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+sucrose 3% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 37 | 40* | 36 | 44* | ||
| Contaminated | 13 | 10 | 14 | 6 | ||
| A2 | MS+IAA (1 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+ sucrose 5% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 38 | 42* | 45* | 40* | ||
| Contaminated | 12 | 8 | 5 | 10 | ||
| A3 | MS+IAA (1,5 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+ sucrose 7% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 31 | 28 | 25 | 45* | ||
| Contaminated | 19 | 22 | 25 | 5 | ||
| B1 | MS+2,4-D (1 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+sucrose 3% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 29 | 24 | 23 | 31 | ||
| Contaminated | 21 | 26 | 27 | 19 | ||
| B2 | MS+2,4-D (1,5 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+sucrose 5% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 38 | 42* | 28 | 25 | ||
| Contaminated | 12 | 8 | 22 | 25 | ||
| B3 | MS+2,4-D (2 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+sucrose 7% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 37 | 40* | 27 | 26 | ||
| Contaminated | 13 | 10 | 23 | 24 | ||
| C1 | MS+NAA (1 mg/l)+BAP (1 mg/l)+sucrose 3% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 28 | 25 | 38 | 42* | ||
| Contaminated | 22 | 25 | 12 | 8 | ||
| C2 | MS+NAA (1.5 mg/l)+BAP (1 mg/l)+sucrose 5% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 39 | 40* | 27 | 26 | ||
| Contaminated | 11 | 10 | 23 | 24 | ||
| C3 | MS+NAA (2 mg/l)+BAP (1 mg/l)+sucrose 7% | Num of anther cultured | 50 | 50 | 50 | 50 |
| Callus formation | 31 | 28 | 25 | 20 | ||
| Contaminated | 19 | 22 | 25 | 30 | ||
statistically different. Romanov et al. (2000) stated that KIN not only influences cell division, but also increases the number of microtube cells.
Effects of sucrose concentrations
MS media including various sucrose concentrations, supplemented with IAA as well as KIN increased callus formation. The highest callus formation appeared on Marvi 1 and Marvi 2 genotypes, both with 45 callus formation (on A2 and A3 media). The lowest formation was occurred in Marvi 1 and FDH 2 on B2 media, producing 23 and 24, respectively. The remaining anthers were failed to form a callus and contaminated with bacteria. Media with contaminated conditions act as a limited factors to propagated plant tissue. However, the use of biocides can eliminate contaminations.
It is noted that this combination have showed potential efficiency as a synthetic plant hormone to generate callus from anther explants. Sen et al. (2014) also reported an efficient combination of growth regulator on Achyranthes aspera. Effect of 2,4-D combined with KIN and sucrose on cotton anther was also observed. The highest callus formation was observed on FDH 2, while the lowest on Marvi 1, which valued 42 and 23, respectively. This observation clearly determined the role of auxin with addition of cytokinin that indispensable for efficient callus formation. Kumar et al. (2015) found that 2,4-D and KIN are the best combination for high frequency of callus induction in cotton.
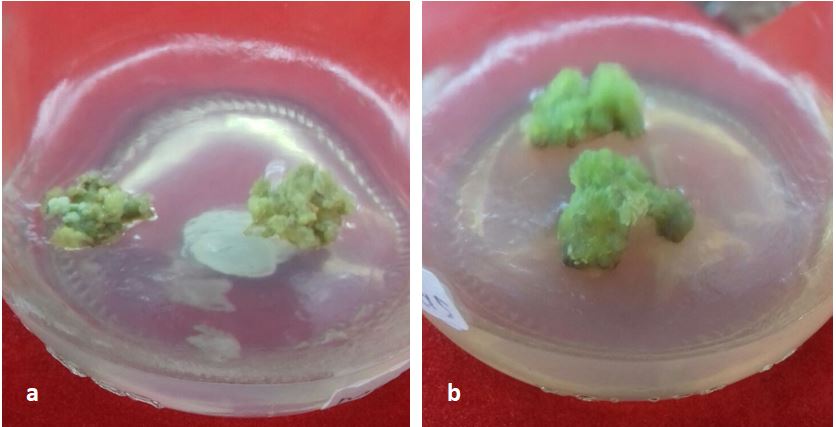
Figure 1. Compact embryogenic calli of (a) Marvi 2 grew on A3 media (MS+IAA (1,5 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+ sucrose 7%) and (b) FDH 2, grew on B2 media (MS+2,4-D (1,5 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+sucrose 5%).
MS with NAA and BAP, supplemented with sucrose showed highest formation of callus on Marvi 2, and the lowest were shown by FDH 2 and Marvi 1 (42 on highest, and 25 on both lowest, respectively).
Increasing the sucrose concentration of the culture media resulted increase of phenolics in willow (Julkunen-Tiitto, 1996). It is suggested that B1, B2, B3, C1, C2, and C3 media showed moderate number of failed callus formation, since cotton plants as well as their anthers are rich in phenolic compounds which act as limiting factors in culture medium and eventually result in their death (Kumar et al., 2015).
Based on the results, it is clear that effect of micronutrients on genotypes is different. Thus certain embryogenesis protocol on cotton anther should vary based on specific genotypes. Memon et al. (2010) reported that Reshmi variety (G. hirsutum) perform better callus response compare to other varieties. Similar findings were reported previously on species G. hrsutum, G. barbadense, and G. arboretum by Bajaj and Gill (1998). It is likely that G. arboreum used in this study can produce callus faster than G. hirsutum, compare to Bajaj (1982) report.
Embryogenic calli
Percentage of embryogenic calli is presented in Figure 3. The present study indicates that callus induction and embryogenic callus formation is likely depend on genotypes. The amount of sucrose was likely influence the percentage of embryogenic calli on all tested media. Anatomical investigation suggested that embryogenic calli appeared on all media (Fig 2). Only A1, A2, and A3 media represented increased percentage of embryogenic calli in line with increasing of sucrose amount.
The other two B and C media reflects inconsistency compared to A media. Increasing sugar amount was associated with elevated phenolic levels (Curtis and Shetty, 1996). Also most of the genotypes failed to produce embryogenic callus from explants or regenerate since they are recalcitrant. The current observation revealed that proper amount of sucrose has critical role on percentage of embryogenic calli. Furthermore, proper amount of sucrose are related to the ability of each genotypes, meaning that ability of totipotency and plant regeneration were also affected
Figure 2. Embryogenic calli of Marvi 2 with (a) 100x magnification, with arrows pointed to ep = epidermis and (b) 400x magnification.
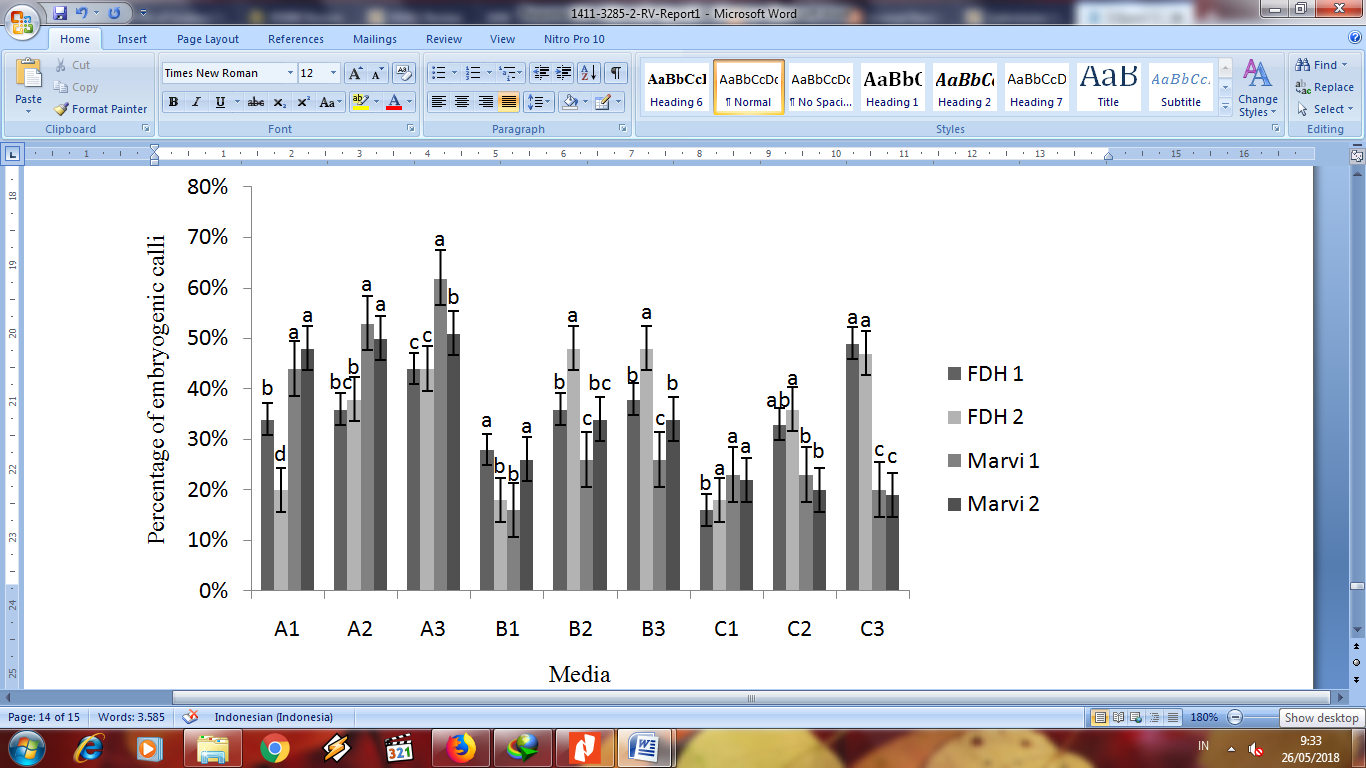
Figure 3. Percentage of embryogenic calli from in vitro anther culture in four genotypes of Gossypium arboreum. Means with the same letter do not differ significantly (at p ≤ 0.05) by Duncan’s multiple range test.
by the genotypes. This was also reported by Ismaili (2016), suggesting that sucrose supports the production of embryo in maize anther culture.
Materials and methods
Plant materials
Four G. arboreum genotypes were used in this study, with accession number FDH 1, FDH 2, Marvi 1, and Marvi 2 were received through Central Cotton Research Institute, Sakrand, Pakistan to Lampung State Polytechnic via Material Transfer Agreement (Kusuma et al., 2016) and conserved with ex-situ method (Engelmann, 2011) on optimum condition.
Pre-treatment of anther
A 60 days after planting (DAP), flowers started to bloom and anthers were excised from 3-5 days after flowering (DAF). Inflorescence information was obtained by Kusuma and Tahir (2016). The first stage of this experiment was to provide a starvation treatment with mannitol. Isolation of anther as material explant was done in sterile condition by using stereoscopic microscope to facilitate the process of separation from flower to pre-treatment media in a Petri dish.
Cotton flower buds containing microspores at final uninucleate stage were disinfected for 1 minute in 70% alcohol then rinsed twice in sterile distilled water. The flower buds were subsequently disinfected in 2% NaOCl with addition of 0.05% (v/v) Tween-20 for 10 min, then rinsed three times in sterile distilled respectively for 1.5 and 10 min. Next flower bud opened with tweezers then anther separated from filaments and petals. A total of 20 anthers were collected from each flower bud and placed on pretreatment medium, and was supplemented with 0.2% (v/v) PPMTM (Plant Preservative Mixture) as a biocide that serves to prevent contamination in culture (Paul et al., 2001).
Pretreatment media contains 0.7 M mannitol, 40 mM CaCl2 and 8 g/L Agarosa SeaPlaqua composition. The mannitol composition was prepared by adding 5.88 g of CaCl2 x 2H2O and 8 g of agarose to 500 ml of water. After that, solutions were dissolved with 127.54 g mannitol on 500 ml of water heated to 380C and sterilized by a 0.22 μm filter. Both solutions were mixed and poured as much as 8 ml into a Petri dish. All samples were covered by parafilm and incubated for 4 days in dark at 240C.
Media culture
The anthers of each genotype were removed from the pre-treatment media after 4 days of incubation. They were cultured in nine different modified MS media, contained with 3-7% sucrose, IAA, NAA, BAP, 2,4-D, Kinetin, as well as coconut milk, which consisted A1 (MS+IAA (0,5 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+sucrose 3%), A2 (MS+IAA (1 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+ sucrose 5%), A3 (MS+IAA (1,5 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+ sucrose 7%), B1 (MS+2,4-D (1 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+sucrose 3%), B2 (MS+2,4-D (1,5 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+sucrose 5%), B3 (MS+2,4-D (2 mg/l)+kin (0,2 mg/l)+CM (70 ml/l)+sucrose 7%), C1 (MS+NAA (1 mg/l)+BAP (1 mg/l)+sucrose 3%), C2 (MS+NAA (1,5 mg/l)+BAP (1 mg/l)+sucrose 5%), and C3 (MS+NAA (2 mg/l)+BAP (1 mg/l)+sucrose 7%). All samples were subjected to statistical analysis.
Statistical analysis
The percentage of embryogenic calli per Petri dish was determined 90-110 days after BAC and the proportion relative to number of cultivated explants (10 explants) was calculated. To determine differences within treatments, two ways analysis of variance (ANOVA) were performed with four replications on each genotype. All significant probabilities were subjected to LSI (Least Significant Increase) to indicate the best culture media. Generalized linear model were fitted to evaluate the efficiency of induction and regeneration as a function of the proportion of embryogenic calli and regenerated plants, using the genotypes as fixed effects. The variable percentage of embryogenic calli was calculated via a binomial distribution. Means between genotypes were compared with a Duncan’s test.
Conclusion
These results revealed that sugar concentration and mannitol had positive effect for callus induction. Our findings determined that the best modification of MS media on each genotypes were likely to be different. MS media with IAA IAA (1 mg/l)+kin (0.2 mg/l)+CM (70 ml/l)+ sucrose 5% provide best performance to produce embryogenic calli on each genotypes. Furthermore, additional doses of sugars and mannitol with 5% and 0.7 M, respectively, appeared to improve the embryogenesis protocol for cotton. This study serves a good step to produce haploid materials of diploid cotton. Callus-derived anther provided reliable source to produce double haploid population in diploid cotton. By determining specific protocol for G. arboreum embryogenesis from anther explant, strategies to achieved homozigosity can be more time-saving.
Acknowledgement
This study was funded under Fundamental Research Scheme by Ministry of Research, Technology, and Higher Education of the Republic of Indonesia with grant number 159.3/PL15.8/LT/2017 through first and corresponding authors.
References
Abdollahi MR, Najafi S, Sarikhani H, & Moosavi SS (2016) Induction and development of anther-derived gametic embryos in cucumber (Cucumis sativus L.) by optimizing the macronutrient and agar concentrations in culture medium. Turk J Biol. 40(3), 571-579.
Bajaj YPS (1982) Survival of anther-, and ovule-derived cotton callus frozen in liquid nitrogen. Curr Sci. 51:139-140.
Bajaj YPS, Gill MS (1989) Pollen-embryogenesis and chromosomal variation in anther culture of a diploid cotton (Gossypium arboreum L.). Sabrao J. 21:57-63
Bajaj YPS, Gill MS (1998) Anther culture studies and pollen embryogenesis in cotton. In: Bajaj Y.P.S. (eds) Cotton. Biotechnology in Agriculture and Forestry, vol 42. Springer, Berlin, Heidelberg.
Bohorova NE, and Atanassov AI (1990) Sunflower (Helianthus annuus L.): In vitro Production of Haploids. In Bajaj, Y. P S. (eds) Haploids in Crop Improvement I: From Fundamentals to Quantum Computing. Springer-Verlag, Berlin Heidelberg p. 428-441.
Cardoso JC, Abdelgalel AM, Chiancone B, Latado RR, Lain O, Testolin R, & Germanà, MA (2016) Gametic and somatic embryogenesis through in vitro anther culture of different Citrus genotypes. Plant Biosyst. 150(2), 304-312.
Curtis OF, Shetty K (1996) Growth medium effects on vitrification, total phenolics, chlorophyll, and water content of in vitro propagated oregano clones. Acta Hortic. 426:489–504.
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev-Pl. 47(1), 5-16.
Germana MA, and Lambardi M (2016) In vitro embryogenesis in higher plants. Springer, New York.
Ismaili A, and Mohammadi PP (2016) Effect of genotype, induction medium, carbohydrate source, and polyethylene glycol on embryogenesis in maize (Zea mays L.) anther culture. Acta Physiol Plant. 38(3), 74.
Julkunen-Tiitto R (2016) Defensive efforts of Salix myrsinifolia plantlets in photomixotrophic culture conditions: The effect of sucrose, nitrogen and pH on the phytomass and secondary phenolic accumulation. Écoscience. 3:3, 297-303.
Kumar GP, Subiramani S, Govindarajan S, Sadasivam V, Manickam V, Mogilicherla K, Narayanasamy J. (2015) Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Biotechnol Rep. 7, 72–80.
Kusuma J and Tahir M (2016) Evaluasi karakter pertumbuhan dan inflorescence plasma nutfah kapas introduksi dan lokal pada iklim tropis. Jurnal Penelitian Pertanian Terapan. 16 (3): 205-211.
Kusuma J, Gusta AR, Abdullah K, Ahsan MZ, and Tahir M (2016) karakteristik keragaman morfologi dan deskripsi sifat vegetatif pada beberapa plasma nutfah kapas hasil introduksi. Paper presented at the 5th seminar nasional pengembangan teknologi pertanian, Politeknik Negeri Lampung, Indonesia ISBN 978-602-70530-4-5 p 112.
Li F, Fan G, Wang K, Sun F, Yuan Y, Song G, and Chen W (2014) Genome sequence of the cultivated cotton Gossypium arboreum. Nat Genet. 46(6), 567.
Lijuan Y, Shengqun P, Haixin M, Bin W, and Xinmin F (2016) The effect of mannitol pretreatment on callus induction rate of different genotypes processing tomato's anther. Mol Plant Breed. 3, 030.
Liu D, Guo X, Lin Z, Nie Y, and Zhang X (2006) Genetic diversity of Asian cotton (Gossypium arboreum L.) in China evaluated by microsatellite analysis. Genet Resour Crop Ev. 53(6), 1145-1152.
Macdonald S (2018) Cotton. In USDA (eds) World agricultural supply and demand estimates. ISSN: 1554 – 9089.
Máximo WPF, Santos PAA, Martins GS, Mendonça EG, and Paiva LV (2018) In vitro multiplication of Eucalyptus hybrid via temporary immersion bioreactor: culture media and cytokinin effects. Crop Breed Appl Biot. 18: 131-138.
Mehetre SS, Aher AR, Gawande VL, Patil VR, and Mokate AS (2003) Induced polyploidy in Gossypium: a tool to overcome interspecific incompatibility of cultivated tetraploid and diploid cottons. Curr Sci. 84(12), 1510-1512.
Memon S, Mari SN, Mari AK, and Gaddi NH (2010) Induction of callus through anther and ovule culture in upland Cotton (Gossypium hirsutum L.). World Applied Sciences, (Special Issue of Biotechnology & Genetic Engineering), 8, 76-79.
Muñoz‐Amatriaín M, Svensson JT, Castillo AM, Cistué L, Close TJ, and Vallés MP (2006) Transcriptome analysis of barley anthers: effect of mannitol treatment on microspore embryogenesis. Physiol Plantarum. 127(4), 551-560.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant. 15:473–497
Paul AL, Semer C, Kucharek T, Ferl RJ (2001) The fungicidal and phytotoxic properties of benomyl and PPM in suplemented agar media supporting transgenic Arabidopsis plants for a space shuttle flight experiment. J Appl Microbiol Biotech. 55 : 480-485.
Romanov GA, Aksenova NP, Konstantinova TN, Golyanovskaya SA, Kossmann J and Willmitzer L (2000) Effect of indole-3-acetic acid and kinetin on tuberisation parameters of different cultivars and transgenic lines of potato in vitro. Plant Growth Regul. 32:245-251.
Sen MK, Nasrin S, Rahman S, and Jamal AHM (2014) In vitro callus induction and plantlet regeneration of Achyranthes aspera L., a high value medicinal plant. Asian Pac J Trop Med. 4(1), 40–46.
Vij S, Pathak D, Kaur N, Pahwa K, and Gill MS (2016) Development and molecular confirmation of interspecific hybrids between Gossypium hirsutum and Gossypium arboreum. Agr Res J. 53(2), 169-172.