

ISSN:1835-2707
Aust J Crop Sci. 18(08):471-478 (2024)
https://doi.org/10.21475/ajcs.24.18.08.pne68
Micropropagation of three ginger cultivars and the subsequent growth and rhizome yield assessment and phytochemical composition analysis
Guochen Yang*1, Julia C Robinson1, William E. Lashley IV1, and Radiah C Minor1
College of Agriculture and Environmental Sciences, North Carolina Agricultural and Technical State University, Greensboro, NC 27411, USA
Abstract
Farmers have turned to biotechnology techniques to produce ginger, due to disease issues. Micropropagation is a proven and effective means for mass production of disease-free plants. Our research focused on ginger cultivar specific reactions (from culture initiation to rhizome harvest) to plant growth regulators (PGRs) to determine if plant response to PGRs is cultivar specific. Ginger (Zingiber officinale Rosc.) cultivars studied were: Chinese White (CW), Hawaii Yellow (HY), and Khing Yai (KY) under benzylaminopurine (BA) versus kinetin (KT) treatments using MS basal medium to micropropagate ginger seedlings and subsequent growth and yield performance and phytochemical composition analysis. In vitro culture data were collected every month for number of buds and shoots (and shoot length) produced per initiating bud and shoot growth. Mature seedlings were subsequently transplanted in a greenhouse with a randomized complete block design (RCBD) with 4 replications, 18 plants per replication (Main Plot = Cultivar, Sub Plot = PGR) for a total of 72 pots/plants. Growth data included number of stems per seedling, stem length, and stem diameter. Rhizome yield data included number of pieces of rhizome per seedling, and rhizome weights (biological, edible, and total rhizome weights respectively). Phytochemical composition analysis of ginger rhizome/PGR treatment included average amount of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, 8-shogoal, and 10-shogoal. Data was analyzed using SAS OnDemand for Academics with PROC GLM at α=0.05 level of significance. As ginger tissue culture seedlings progressed in culture, KT was more suitable for multiplication of buds, BA more suitable for proliferation of shoots, and KT matured ginger tissue culture seedlings at a higher rate than BA. CW had the highest rhizome yield and least amount of biological roots in comparison to other cultivars. CW produced the least amount of 8-gingerol, 10-gingerol, 8-shogoal and 10-shogoal of all three cultivars and with no significant change between two PGR treatments. In comparison, HY produced the highest amount of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, and 8-shogoal, followed by KY and CW, when micropropagated with BA. KY produced the highest amount of 10-shogoal when micropropagated with BA and the highest amount of 6-gingerol, 6-shogoal, 8-shogoal, and 10-shogoal when micropropagated with KT.
Keywords: Zingiber officinale Rosc.; Ginger; BA; KT; ginger cultivars; micropropagation; plant growth regulator
Abbreviations: BA_6-Benzylaminopurine; NAA_1-Naphthaleneacetic acid; KT_Kinetin 6-Furfurylaminopurine; TDZ_Thidiazuron 1-Phenyl-3-(1,2,3-thiadiazol-5-yl) urea; PGR_plant growth regulator; LSD_least significant difference.
Introduction
Ginger (Zingiber officinale Rosc.) is one of the most important spice crops worldwide and has been categorized by the U.S. Food and Drug Administration as a food additive primarily used for flavoring food products like tomato sauce, ketchup, salad dressings, meat sausages, gravies, pickles, and curry dishes (Hicks and Sharma 2001, Valenzuela 2011). Ginger can be processed into value-added and often high-priced products, such as dehydrated ginger, candy, powder, oil, and oleoresins. In addition to its culinary purposes, ginger has been used to treat a variety of medical conditions, such as rheumatoid arthritis, neurological diseases, nausea, cancer, diabetes, asthma, and allergy (de Lima et al., 2018, Grzanna 2005, Semwal et al., 2015, Mashhadi et al., 2013, Ali 2020, Zitek et al., 2020). The entrepreneurship opportunities for such value-added and medicinally important products further increases the global demand for ginger (Datta et al., 2015).
Production of ginger in the United States has been an ongoing struggle because it is typically propagated by “seeds,” the ginger roots and often requires a large quantity for commercial production. However, ginger roots are susceptible to diseases, such as ginger rhizome soft rot (Pythium myriotylum) and dry rot (Fusarium oxysporum f. sp. Zingiberi), that can cause severe yield losses (de Lange et al., 1987, Hossain et al., 2010, Meenu and Jebasingh 2019, Nair 2019, FAO 2020). High quality disease-free propagules ensure a successful ginger production (Lincy and Sasikumar 2010, Sathyagowri and Seran 2011). The main source of ginger production in the United States is Hawaii (Hawaii Ag. Statistics Services 2008). Purchasing of ginger “seed” and subsequent transport from Hawaiian producers to the U.S. mainland growers can be difficult due not only to ginger’s susceptibility to diseases but also because Hawaii cannot meet growers’ demands (NARI 2004, Kone et al, 2022). In fact, Hawaii can only meet 20% of the United States total demand, which includes demand for seed as well as fresh rhizome for consumption. As of 2020, the U.S. remains one of the top importers of ginger. In 2018, the U.S. imported 89,000 tons of fresh ginger worth about $125 million, an 11% increase from 2017. From 2007 to 2018, there was an increase in annual import price of more than 5.1% (Aleksandra 2019, Global Ginger Market 2019 and 2020).
The low proliferation rate of propagated ginger rhizome has resulted in a lack of ginger seed for farmers, and many of those seeds are susceptible to disease. Because of this, many farmers have turned to biotechnological techniques to produce ginger (Mosie, 2019). Micropropagation is a proven and effective means for mass production of disease-free plants (Murashige 1966 & 1974, Archana et al., 2013, Solanki et al., 2014). Our lab has previously developed a micropropagation protocol using two ginger cultivars, which can be modified for other cultivars (Yang et al., 2019).
Establishing aseptic cultures and proliferation of axillary shoots are two of the key steps in successfully producing tissue culture products, and understanding both the short- and long-term effects of plant growth regulators is essential to these steps. Mosie (2019) summarized in a review that KT was not as effective in stimulating bud sprout in ginger as BA. This has been supported by many other studies that have posited that BA (in conjunction with an auxin) will produce the highest number of shoots in comparison to other plant growth regulators (Miri, 2020) for ginger (Zingiber officinale Rosc.). During our study in 2019, however, we observed a different result when comparing BA, KT, and TDZ. Each PGR treatment had a consistently different effect on both ginger cultivars: TDZ induced good callus and shoot primordia (buds), but few shoots; BA produced reasonably good multiple shoot production; and KT produced the best in vitro multiple shoot production. Other researchers also have had recorded differences in growth compared to the established norm. Karyanti et al. (2021) observed that TDZ significantly increased the multiplication of shoots in red ginger (Zingiber officinale Rosc. var. rubrum) in comparison to all other plant growth regulators tested, including BA. The different growth patterns in culture, when exposed to plant growth regulators, could be due to ginger cultivar differences.
Shahrajabian et al. (2019) in a review broke down the classification and variation of the ginger species Zingiber officinale Rosc., listing out more than 13 different varieties within the species, most of which were investigated for their potential health benefits. With so much variation within the same species, it is possible that a micropropagation protocol that works for one ginger cultivar might not work as effectively for another ginger cultivar. In fact, our own research found that when exposed to the same plant growth regulator at the same concentration, Hawaii Yellow consistently produced a higher number of shoots than Kali Ma regardless of plant growth regulator treatment. Therefore, it became imperative to determine the most effective micropropagation protocol for specific ginger cultivars.
This study investigated ginger plant growth of three ginger cultivars from the very beginning of initiation of micropropagation until harvesting of subsequent ginger rhizome from the micropropagated ginger plants in a greenhouse (Figure 1) for their growth and rhizome yield performance and phytochemical composition profile. Our research aims to provide science-based data on cultivar specific ginger when micropropagated with two different plant growth regulator treatments to provide knowledge on: (1) which PGR is effective in stimulating bud and shoot growth in culture, (2) how PGR treatment affects seedling growth in the greenhouse, and/or (3) whether PGR affects gingerol and shogaol compounds in different ginger cultivar rhizomes. Therefore, the objective of this study was to measure the differences between ginger varieties when micropropagated with two different PGR treatments. This information can be used to determine the most effective protocol for initiation of ginger tissue culture seedlings by specific cultivar.
Results
Bud and shoot data in culture
Our hypothesis postulates that ginger cultivars, when grown in culture, will have different growth responses when exposed to plant growth regulators. In Table 1, the average number of buds per initial bud explant (Stage 1 new growth) and the average number of shoots for different shoot lengths were measured once a month for seven months (seven culture transfers). For each stage of growth of the ginger tissue culture seedlings, the interaction between BA and KT was determined for each ginger cultivar. The results indicated that for Stage 0 (new growth) the average number of buds was highest when using KT for all cultivars. Because no interaction occurred, KY consistently had the highest number of new buds, followed by HY and then CW. Stage 1 (<0.5 < 1 cm), Stage 2 (<1.0 < 2.0 cm) and Stage 3 (<2.0 < 3.0 cm) consistently had the highest number of shoots when using BA, with some non-significant interaction occurring for Stages 1 and 3. For Stage 1, CW would consistently produce fewer shoots than both KY and HY, and KY would produce more shoots when using BA, but less when using KT. Stage 2 shoots would consistently produce the highest number for CW, and KY would produce more shoots than HY when using BA, but significantly less when using KT. When BA was used, HY consistently produced the highest number of Stage 3 shoots, followed by CW and then KY. However, when KT was used, CW produced the greatest number of shoots, followed by HY and then KY. Stage 4 seedlings (>3.0 cm) were mature seedlings that were ready to transplant from in vitro culture to the acclimatization stage in the greenhouse. The average number of Stage 4 seedlings grown each month was highest when using KT. HY would consistently produce more Stage 4 seedlings each month than both CW and KY. There was slight interaction indicating that CW would produce more shoots than KY when using KT, but less when using BA, although this was not considered significant. When we counted the total number of buds and shoots, on average, within a single culture, BA produced the highest number of combined buds and shoots for all three cultivars. CW consistently produced the lowest number of buds and shoots. KY produced more buds and shoots than HY when using BA and less than HY when using KT.
Plant growth and yield in greenhouse
In Table 2 stem diameter, on average, was thickest when ginger cultivars were micropropagated with BA than KT. However, stem diameter thickness did not significantly differ among cultivars. When examining the interaction data, CW produced significantly thicker stems when micropropagated with BA than when they were micropropagated with KT. The average stem length did not significantly differ between cultivars or PGRs. However, CW, when micropropagated with BA, produced significantly longer stems than both HY and KY, but produced significantly shorter stems than HY and KY when micropropagated with KT. Number of stems tended to produce more when ginger cultivars were micropropagated with KT, however only HY demonstrated a significant increase in stem number when micropropagated with KT than with BA.
When observing the different parts of the rhizome, neither BA nor KT had any long-term effects on the growth of cultivars (Supplementary Figures 2 and 3). For this reason, Table 3 compared the different cultivars within each PGR treatment group. HY consistently had the heaviest biological root weight for both PGRs, followed by KY and then CW. Both edible root weight, the part of the rhizome that is usually sold for consumption, and total rhizome weight were the heaviest for CW, followed by KY and then HY. Finally, the number of fingers (pieces) that rhizomes produced were the highest on average for HY followed by KY and then CW.
Composition analysis of ginger rhizomes
Our data indicated noticeable effects of PGR used during micropropagation process on subsequent phytochemical composition analysis of micropropagated ginger plants in terms of amount of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, 8-shogoal, and 10-shogoal compounds in ginger rhizomes. Our data in Table 4, as well as the interaction plots run in SAS, indicated that most of the phytochemicals analyzed demonstrated cultivar-specific reactions to PGR used during micropropagation process, with only 8-gingerol production demonstrating a significant overall trend, as all cultivars produced more 8-gingerol when ginger was micropropagated with KT in comparison to BA. HY produced the most amount of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, and 8-shogoal, followed by both KY and CW, when micropropagated with BA. KY produced the highest amount of 10-shogoal when micropropagated with BA, and the greatest amount of 6-gingerol, 6-shogoal, 8-shogoal, and 10-shogoal when micropropagated with KT. In comparison, CW produced the least amount of 8-gingerol, 10-gingerol, 8-shogoal and 10-shogoal of all three cultivars and with no significant change between the two PGR treatments. Cultivar specific interactions were noticeable with HY and KY, with HY producing more 6-gingerol, 6-shogoal, and 8-shogoal when micropropagated with BA versus KY, which would produce more when micropropagated with KT.
Discussion
Bud and shoot data in culture
A pattern emerged when looking at the different stages of ginger tissue culture seedling growth, occurring across all cultivars. This pattern is documented in Figure 2, highlighting which cytokine PGR, BA or KT, was most effective at different periods of ginger growth in culture. Based on our data, we suggest that two different plant growth regulators should be utilized at different times in the micropropagation process for all ginger cultivars tested. Overall, both BA and KT are effective for ginger micropropagation. However, KT is more suitable for multiplication of buds and maturation of seedlings, while BA is more suitable for multiplication of shoots. This is supported by other studies, such as Zahid et al. (2021), who reported that multiple shoot induction was highest when using BA in comparison to KT as well as the study by Miri (2020), who reported BA having the highest number of multiple shoot formation. However, our research observed different results on bud formation, as KT was more effective at inducing bud formation than BA for all ginger cultivars. When reviewing the literature, this contrasted with other ginger micropropagation research, such as Edison et al. (1996) that reported that KT was not effective in inducing bud sprouting as compared to BA. A more recent study though, by Alqadasil et al., 2021 posited that the concentration of cytokines BA and KT have a drastic effect on the growth of ginger seedlings in culture, and that at higher concentrations (2.0 mg∙L-1 or higher), KT is more effective than BA at producing the most number of shoots and greater shoot lengths. Our research measured BA and KT at 3.0 mg∙L-1 concentration, and so our results are consistent with result trends from other scientists on ginger micropropagation. Limited literature is available on the effect of these cytokines, at higher concentrations, when comparing different ginger cultivars. Our data suggests that different cultivars follow these same trends, but the rate of proliferation and maturation are cultivar specific.
Plant growth and yield in a greenhouse
Ginger is typically propagated by “seeds,” the ginger roots, and often requires a large quantity for commercial production. However, industry faces challenges with low quality planting material due to disease and pests (Kone et al. 2022). High quality disease-free propagules ensure a successful ginger production (Lincy and Sasikumar 2010, Sathyagowri and Seran 2011). Research on micropropagation of ginger as a source of planting material have consistently found that, when grown in a greenhouse, micropropagated ginger will produce more shoots then seed propagated ginger (Tamang et al. 2022). However, not much research has been conducted on different cultivars of micropropagated ginger, and their subsequent plant growth and yield in a greenhouse. Furthermore, research on the long-term growth effects (plant growth and rhizome formation and yield) of in vitro media conditions, specifically PGR types and their concentrations has not been studied extensively for micropropagated ginger.
We investigated subsequent long-term effects of PGR used during ginger micropropagation on ginger plant growth when micropropagated ginger seedlings for each cultivar and PGR treatment were transplanted in the greenhouse by measuring the stem diameter, stem length, and number of stems per ginger seedling as well as subsequent yield data. Our research found that plant growth did not significantly differ between ginger cultivars grown, but that there was a noticeable effect on plant growth depending on what cytokine PGR the plants were originally cultured in for the cultivar “Chinese White.” In contrast, there appeared to be no long-term effects of KT or BA on ginger rhizome morphological development, with Chinese White having the most edible root weight and total root weight on average, Hawaii Yellow produced the most number of pieces (fingers) and biological roots, and Khing Yai was solidly in the middle for each plant measurement. While not much research has been conducted on the long-term effects of PGR on ginger plant growth and rhizome development, other research has been conducted on long-term effects of biotechnological techniques. In Ginger: The Genus Zingiber, Ravindran and Babu (2005) summarized that cultured explant type did not have any noticeable differences in plant morphology in nurseries, which is consistent with our study that we did not observe noticeable changes in plant growth, except for Chinese White. Xing et al. (2022) and Antala et al. (2019) suggested that differences in ginger rhizome growth between cultivars is most likely due to genetic variability within the plants.
Composition analysis of ginger rhizomes
Gingerols and shogaols represent the predominant pungent constituents of ginger and are considered responsible for ginger’s medicinal properties. Mashhadi et al. (2013) reported that gingerols and shogoals serve as effective antioxidant and anti-inflammatory agents. It was reported that micropropagated ginger had high phytochemical contents (Min et al 2017). There is a correlation between antioxidant activity and 6-gingerol content comparing in vitro ginger cultures with conventionally grown ginger (Pawar et al 2015). We hypothesized that ginger cultivars when grown in vitro with different PGRs, would result in long-term effects on gingerols and shogoals produced in ginger rhizomes. Similar to Rani et al. (2022), who observed a genotype specific response to gingerol and shogaol production in vitro, we observed a cultivar specific response as well. Chinese White produced the least amount of all gingerols and shogoals in comparison to both Hawaii Yellow and Khing Yai, regardless of initial PGR treatment, but this could be due to the fact that Chinese White had the highest rhizome yield on average. Das et al. (2022) determined in a study comparing multiple ginger genotypes that there is an inverse reaction of measured crude fiber and essential oils in ginger rhizomes, and that as ginger rhizome increases in size the amount of gingerols and shogoals, found in the essential oil, will also decrease.
However, Hawaii Yellow produced more gingerols and shogoals than Khing Yai when grown with BA, while Khing Yai produced more gingerols and shogoals than Hawaii Yellow when grown with KT. Our data indicated that 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, 8-shogoal and 10-shogoal develop in all ginger rhizomes but that the amounts of these phytochemicals are cultivar specific. Other research into the effect of micropropagation protocols on gingerol and shogaol accumulation in ginger plants support that differing media conditions during micropropagation can lead to differences in gingerol and shogaol accumulation in ginger plants (Ma and Gang, 2006). To increase the amount of phytochemicals produced in specific cultivars, we can only project that Hawaii Yellow be micropropagated with BA and Khing Yai with KT. However, more research is needed to make a conclusive suggestion.
Materials and Methods
Explant materials
Ginger rhizome buds were collected from ginger grown at North Carolina A&T State University and put through a strict sterilization routine. Three ginger (Zingiber officinale Rosc.) cultivars tested in this study included Chinese White (CW), Hawaii Yellow (HY), and Khing Yai (KY). Axillary buds (growing points) from rhizomes (after harvesting) of 1-year old ginger (ginger grown for one season) were gently cut from rhizome and scrubbed with 1% bleach solution (0.94% NaOCl) using Kimwipes to remove the outer layer of skin, dirt and debris from bud (Figure 1A). Using a Grade 10 scalpel, the outer layers of the bud were cut and removed until no more layers could be removed, making sure to avoid cutting the tip of the growth point. Buds were then disinfected with 1% bleach solution (2 drops Tween 20 per 100 ml of solution) for 15 minutes, soaked in 70% ethanol solution for 5 seconds and a 15% bleach solution (0.94% NaOCl) for 10 minutes, then rinsed with sterile distilled deionized water.
Initiation of sterile culture
In vitro cultures were established (Figure 1B) in petri dishes with Murashige and Skoog (MS) Basal Salt Medium supplemented with 3% sucrose and technical grade agar at pH 5.8. Cultures were placed in plant tissue culture grade growth chambers with a completely randomized design (CRD) in complete darkness for one month at 23℃/18℃ at 16/8-h intervals. After one month, cultures were transferred to new MS media supplemented with TDZ at 3.0 mg∙L-1, 1-Naphthaleneacetic acid at 0.6 mg∙L-1, 3% sucrose and pH 5.8 and introduced to light (cool white fluorescent tubes) in growth chamber (23℃/18℃ at 16/8-h light photoperiod of 40 umol m-2s-1).
After 1 month in light, buds were transferred to final MS medium supplemented with either 6-Benzylaminopurine (BA) or kinetin (KT) plant growth regulators (PGRs) at 3.0 mg∙L-1 (plant growth regulator treatments), 1-Naphthaleneacetic acid at 0.6 mg∙L-1, 3% sucrose and agar at pH 5.8 and maintained in this media. At this time cultures were transferred into Magenta GA-7 culture box vessels, 25 culture vessels for each cultivar and plant growth regulator treatment, 20 buds/shoots per box, and media was changed each month to keep media fresh (Figure 1C).
After initiation of buds into final media, data was collected once a month for nine months. Total number of shoots and total number of buds were counted per culture vessel, then the number of shoots at different stages of growth were measured. Different stages of growth were recorded by shoot length (Figure 2) as Stage 1 (<0.5 cm < 1 cm), Stage 2 (<1.0 cm < 2.0 cm), Stage 3 (< 2.0 cm <3.0 cm) and Stage 4 (>3.0 cm).
Planting mature (Stage 4) ginger tissue culture seedlings in greenhouse
Mature Stage 4 (>3.0 cm shoot length) ginger tissue culture seedlings (Figure 1D and Supplementary Figure 1A) were planted in large yellow crates (Figure 1E and Supplementary Figure 1B) between March and June 2021 under 40% shade cloth for acclimatization, and then transplanted to individual pots on Nov. 11, 2021. No rooting stage was necessary, as Stage 4 seedlings consistently grew roots in culture for all cultivars tested. One seedling was planted per pot (2-gallon sized pots) (Figure 1F and Supplementary Figure 1C) and grown in 2:1 Metro-Mix:Compost soil. Seedlings were grown in Reid Greenhouse on one long bench, with a randomized complete block design (main plot = cultivar, sub plot = PGR) with 4 replications/ 18 plants per replication for a total of 72 pots. Plants were maintained by hand watering when needed and fertilized with slow-release fertilizer (15-15-15).
Due to transplanting in November, ginger plants went into a brief dormancy period during the winter months, and the top of the plants were removed when they began to brown. Plants reemerged in June 2022 and plant growth data was collected in September, roughly two months after re-emergence during peak growth of the plant. Plant growth data included stem diameter (mm), stem length (cm), and number of stems per initial micropropagated ginger seedling from mature ginger plants (Supplementary Figure 1D). Data was analyzed using Microsoft Excel and SAS OnDemand for Academics with PROC GLM analyzed at the 0.05 level of significance.
Harvest of ginger rhizome and yield data
Six months after reemergence of ginger plants (June 2022) ginger rhizomes (Figure 1G & Supplementary Figure 1E) were harvested by hand by removing them from pots, cutting away foliage and using a water hose to wash away all soil residue. Biological roots were removed and then the number of fingers (pieces) of the rhizome were counted and biological root weight, edible root weight (rhizome without biological root weight that would typically be sold to supermarkets for consumption), and total rhizome weight (edible and biological root weight) were measured in grams. Samples for composition analysis of 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 8-shogaol, and 10-shogaol were collected for edible rhizomes. Data was analyzed using Microsoft Excel and SAS OnDemand for Academics with PROC GLM analyzed at the 0.05 level of significance.
Phytochemical compositional analysis of edible rhizome samples
Sample preparation was done by pre-cutting and placing ginger rhizome pieces into petri dishes in the -80 freezer for 36 hours and then placed in the freeze dryer (Labconco, FreeZone 2.5 Liter -84C Benchtop Freeze Dryer) at -84℃ temperature and minimum chamber pressure control at -80℃, for 48 hours. Samples were then powdered and sent to the Drumetix Laboratories where they conducted the following protocol to measure 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 8-shogaol, and 10-shogaol amounts in sample. Sample extraction was conducted by weighing powdered samples (10-15 mg of ginger sample) into a 3-mL plastic vial and 200 µL of methanol were added to each vial and vortexed at room temperature for 18 hours. This was repeated six times for each sample so that six replicates were measured for each. Sample preparation in the 96-well plate was done by combining 20 µL of ginger sample extract, 100 µL of dimethyl sulfoxide (DMSO):Water (1:1) solution, 50 µL of IS solution (500 ng/mL diclofenac sodium and 200 ng/mL propranolol in methanol:water 1:1), and 200 µL of water before being compared to working standard solutions of 2.5, 7.5, 25, 75, 250, 750, 2500, and 7500 ng/mL 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 8-shogaol, and 10-shogaol in DMSO:Water 1:1 and 20000 ng/mL 6-gingerol in DMSO:Water 1:1. Wells were then capped and vortexed before being centrifuged at 3500 rpm for 10 minutes. Samples were analyzed using liquid chromatography (HPLC instrument Shimadzu LC-20AD Pumps and PE 200 Autosampler) with tandem mass spectrometry (AB Sciex API 5500 with Analyst 1.7.2 software).
Statistical analysis with ANOVA and Interaction Plots
Experimental design of In vitro cultures of ginger were placed in completely randomized design (CRD) inside plant growth chambers, in Magenta GA-7 culture box vessels; 25 culture vessels for each cultivar and plant growth regulator treatment, 20 buds/shoots per box (500 replicates per cultivar/PGR treatment) for a total population of 150 culture boxes and 3,000 replicates with data analyzed once a month. Experimental design of plants in the greenhouse was randomized complete block design (main plot= cultivar, sub plot = PGR) with 4 replications/ 18 plants per replication for a total population of 72 pots.
PROC GLM procedure was run at the 0.05 level of significance with SAS OnDemand for Academics to determine ANOVA and test plant growth response to different PGRs across all ginger cultivars, specific ginger cultivars across all PGRs tested, and ginger cultivars when micropropagated with specific PGRs.
If the P value was less than 0.05 then the code line “lsmeans PGR*cultivar / diff lines stderr” was run to generate an interaction plot. The interaction plot indicated whether specific ginger cultivars would have different plant growth responses when micropropagated with either BA or KT PGR. If no interaction occurred on the plot, then data was analyzed using the Type I SS Test. If significant interaction did occur, then data was analyzed using Type III SS. If interaction did occur, then plant growth response exhibited cultivar specific change to either KT or BA PGR.
Conclusion
Ginger micropropagation has been reported as the most effective method of preserving ginger genotypes and producing disease-free seed ginger material. However, more comprehensive field studies of micropropagated ginger plants are required on subsequent plant growth and rhizome yield performance following tissue culture (micropropagation). Our data indicated that tissue culture protocol had a significant impact on subsequent ginger plant growth, from initiation of culture to harvesting of rhizome, in addition to the observation of ginger cultivar-specific micropropagation.
Acknowledgments
This research was supported by North Carolina A&T State University’s Agricultural Research Program through USDA/NIFA/Evans-Allen project: NC.X-343-5-22-130-1. The authors wish to express their gratitude to Mrs. Lydian L. Bernhardt (Director of Agricultural Communications, CAES) for her review and edit on the manuscript.
References
Aleksandra R (2019) World Ginger Market Report 2019. https://romanenko-indexbox.medium.com/world-ginger-market-report-2019-6c82d31570f2.
Ali RA, G AA, Dai L, Weiner J, Estes SK, Yalavarthi S, Gockman K, Sun D, and Knight JS (2020) Antineutrophil properties of natural gingerols in models of lupus. JCI Insight, https://insight.jci.org/articles/view/138385.
Alqadasi AS, Al-madhagi I, Al-kershy A, Al-Samaei M (2021) Effect of Cytokinin Type and pH Level on Regeneration of Ginger in vitro. International Journal of Horticultural Science and Technology. 9(3): 265–274. https://doi.org/ 10.22059/IJHST.2021.321158.454
Antala V, Narayanan S, Radadiya N, Chaudhari H, Desai H, Patil G (2019) Assessment of genetic diversity by using RAPD and ISSR markers in ginger (Zingiber officinale Rosc.) genotypes. International Journal of Chemical Studies. 7(4): 363–372.
Archana CP, Pillai GS, Balachandran I (2013) In vitro mcirorhizome induction in three high yielding cultivars of Zingiber officinale Rosc. and their phytopathological analysis. International Journal of Advanced Biotechnology and Research. Vol 4(3): 296-300.
Das A, Behera DU, Sahoo RK, Barik DP, Subudhi E (2022) Phytochemical and morphological traits of ginger cultivars are modulated by agro-climatic conditions. Proc Natl Acad Sci India, Sect. B Biol Sci. https://doi.org/10.1007/s40011-022-01361-3
Datta S, Guha S, Sharangi AB (2015) Value addition of horticultural crops: Recent trends and future directions, In “Value Addition in Spice Crops” (eds. Sharangi AB and S Datta), DOI 1007/978-81-322-2262-0_4, Springer India, pp 59-82.
de Lange JH, Willers P, Nel MI (1987) Elimination of nematode from ginger by tissue culture. Journal of Horticultural Sciences. 62: 249-252.
de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata AMOF, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, and Melo-Cavalcante AAC (2018) Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother Res. 32(10):1885-1907. doi: 10.1002/ptr.6134.
Edison S, Ramana KV, Sasikumar B, Nirmal Babu K and Santhosh JE (1997) Biotechnology of spices, medicinal & aromatic plants. Indian Society for Spices, Calicut, Kerala, India. 1997.
Food and Agriculture Organization of the United Nations (2020) Banding together to re-root the Jamaican ginger industry, http://www.fao.org/fao-stories/article/en/c/1318751/.
Global Ginger Market (2019) U.S. imports increases robustly, turning the country into the most promising market. https://www.globaltrademag.com/global-ginger-market-2019-u-s-imports-increases-robustly-turning-the-country-into-the-most-promising-market/.
Global Ginger Market (2020) Industry Key Players, Trends, Sales, Supply, Demand, Analysis and Forecast 2027. http://www.digitaljournal.com/pr/4914556.
Grzanna R, Lindmark L, Frondoza CG (2005) Ginger: An herbal medicinal product with broad anti-inflammatory actions. Journal of Medicinal Food. 8(2): 125–132.
Grzanna R, Phan P, Polotsky A, Lindmark L, Frondoza CG (2005) Ginger extract inhibits β-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. The Journal of Alternative and Complementary Medicine. 10(6):1009-1013. https://doi.org/10.1089/acm.2004.10.1009.
Hawaii Agricultural Statistics Service, Hawaii Department of Agriculture, Hawaii Ginger Root, September 12, 2008, http://www.nass.usda.gov/hi/
Hicks A, Sharma PN (2001) Value-added food products processing for micro-income generation of rural communities in Myanmar. http://www.fao.org/docrep/006/AD379E/AD379E00.htm
Hossain A, Hassan L, Patwary AK, Sultan MM, Ahmad SD, Shah AH, Batool F (2010) Establishment of a suitable and reproducible protocol for in vitro regeneration of ginger. Pakistan Journal of Botany. 42(2): 1965-1074.
Karyanti T, Sukarnih Y, Rudiyana NF, Hanifah N, Sa'adah, Dasumiati (2020) Micropropagation of red ginger (Zingiber officinale Rosc. var. rubrum) using several types of cytokinins. Journal of Physics: Conference Series, 51751. https://doi.org/10.1088/1742-6596/1751/1/012051
Kone D, Kouadio OKS, Silue O, N’Guessan AR, Yeo N, Kouakou TH (2022) Optimization of bud disinfection technique and influence of growth regulators on micropropagation in ginger (Zingiber officinale Rosc.). International Journal of Biological and Chemical Sciences. 16(6): 2892–2904. https://doi.org/10.4314/ijbcs.v16i6.33
Lincy A, Sasikumar B (2010) Enhanced adventitious shoot regeneration from aerial stem explants of ginger using TDZ and its histological studies. Turk J Bot. 34: 21-29.
Ma X, Gang DR (2006) Metabolic profiling of in vitro micropropagated and conventionally greenhouse grown ginger (Zingiber officinale). Phytochemistry. 67(20): 2239–2255. https://doi.org/10.1016/j.phytochem.2006.07.012
Mashhadi NS, Ghiasvand R, Askari G, Hariri M, Darvishi L, Mofid MR (2013) Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med. Apr:4(Suppl 1):S36-42.
Meenu G, and Jebasingh T (2019) Diseases of ginger, ginger cultivation and its antimicrobial and pharmacological potentials, Haiping Wang, IntechOpen, DOI: 10.5772/intechopen.88839. https://www.intechopen.com/books/ginger-cultivation-and-its-antimicrobial-and-pharmacological-potentials/diseases-of-ginger.
Min BR, Marsh LE, Brathwaite K, Daramola AO (2017) Effects of tissue culture and mycorrhiza applications in organic farming on concentrations of phytochemicals and antioxidant capacities in ginger (Zingiber officinale Roscoe) rhizomes and leaves. J Food Sci. 82(4):873-881. doi: 10.1111/1750-3841.13661.
Miri SM (2019) Micropropagation, callus induction and regeneration of ginger (Zingiber officinale Rosc.). Open Agriculture. 5: 75-84. https://doi.org/10.1515/opag-2020-0008
Mosie T (2019) “A review on influence of growth regulator and culture condition on micro- propagation of Ginger (zingiber officinale).” International Journal of Food Science and Agriculture. 3(3): https://doi.org/10.26855/ijfsa.2019.09.009.
Murashige T (1966) Principles of in vitro culture. Proc Int Plant Prop Soc. 16: 80-88.
Murashige T (1974) Plant propagation through tissue culture. Ann Rev Plant Physiol. 25: 135-166.
Nair KP (2019) The Diseases of Ginger. In: Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.) - World's Invaluable Medicinal Spices. Springer, Cham. https://doi.org/10.1007/978-3-030-29189-1_21.
National Agricultural Research Institute, Technical Bulletin No. 23 Ginger Postharvest Care and Market Preparation 1–11 (2004). Bourda Georgetown.
Pawar N, Pai S, Nimbalkar M, and Dixit G (2015) RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from in vitro cultures of Zingiber officinale Roscoe. Plant Science Today. (2015) 2(1): 24-28 doi:10.14719/pst.2015.2.1.103.
Rani M, Shylaja MR, Mathew D, Girija D, Shankar MA, Sureshkumar P, Beena C (2022) Potential of microrhizomes for in vitro gingerol and shogaol synthesis in ginger (Zingiber officinale Rosc.). Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 92(1): 121–129. https://doi.org/10.1007/s40011-021-01314-2
Ravindran PN, Nirmal Babu K (2005) Ginger: the genus Zingiber. CRC Press.
Sathyagowri S and TH Seran (2011) In vitro plant regeneration of ginger (Zingiber officinale Rosc.) with emphasis on initial culture establishment. Int J Med Arom Plants. 1(3): 195-202.
Semwal RB, Semwal DK, Combrinck S, Viljoen AM (2015) Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 117:554-568. doi: 10.1016/j.phytochem.2015.07.012.
Shahrajabian MH, Sun W, Cheng Q (2018) Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agriculturae Scandinavica, Section B - Soil & Plant Science. https://doi.org/10.1080/09064710.2019.1606930
Solanki RU, MJ Parekh and SR Patel (2014) Regeneration of ginger (Zingiber officinale Rosc.) through shoot tip culture. Journal of Cell Tissue Research Vol. 14(2): 4409-4412.
Tamang S, Medda PS, Das S (2022) Response of single bud sprout technique on different ginger (Zingiber officinale Rosc.) cultivars under sub-himalayan plains of West Bengal. International Journal of Bio-Resource and Stress Management. 13(9): 899–905. https://doi.org/10.23910/1.2022.3139
Valenzuela H (2011) Farm and Forestry Production and Marketing Profile for Ginger (Zingiber officinale). Specialty Crops for Pacific Island Agroforestry. 1-13 (http://agroforestry.net/scps).
Xing H, Li Y, Ren Y, Zhao Y, Wu X, Li H-L (2022) Genome-wide investigation of microRNAs and expression profiles during rhizome development in ginger (Zingiber officinale Roscoe). BMC Genomics, 23(1). https://doi.org/10.1186/s12864-021-08273-y
Yang G, Lu Z, Gu S, Robinson JC (2019) In vitro performance evaluation of two ginger cultivars. HortScience. 54(9):S201.
Zahid NA, Jaafar HZE, Hakiman M (2021) Micropropagation of ginger (Zingiber officinale Rosc.) “Bentong” and evaluation of its secondary metabolites and antioxidant activities compared with the conventionally propagated plant. Plants (Basel). 26:10(4):630. doi: 10.3390/plants10040630. PMID: 33810290; PMCID: PMC8066238.
Zitek T, Leitgeb M, Golle A, Daris B, Knez Z, and Hrncic MK (2020) The influence of hemp extract in combination with ginger on the metabolic activity of metastatic cells and microorganisms. Molecules. 25(21): 4992; https://doi.org/10.3390/molecules25214992.
Figure 1. Ginger life cycle from micropropagation of original bud explant to harvest of ginger rhizome. A) Bud explant used to initiate ginger tissue culture. B) Buds that have been sterilized and initiated in media. C) Callus grown on ginger tissue culture bud. D) Stage 4 tissue culture seedling ready for planting in greenhouse. E) Ginger tissue culture seedlings planted in individual pots for acclimatization in greenhouse. F) Ginger plant mid growth in 2-gallon pot. G)Harvested ginger rhizome with biological roots removed.
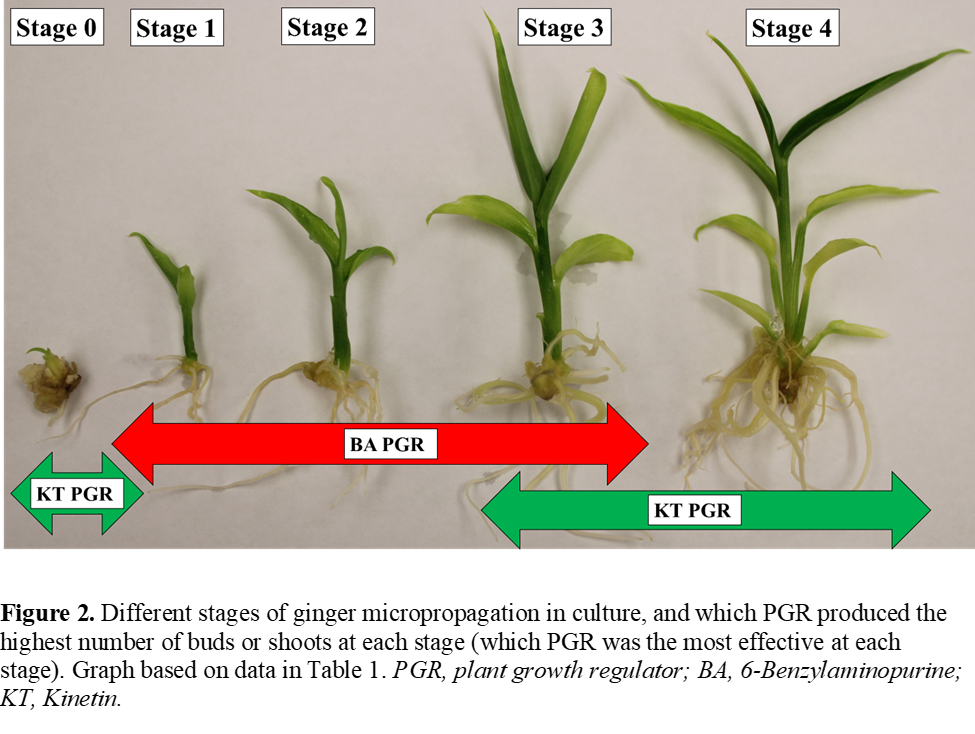
Figure 2. Different stages of ginger micropropagation in culture, and which PGR produced the highest number of buds or shoots at each stage (which PGR was the most effective at each stage). Graph based on data in Table 1. PGR, plant growth regulator; BA, 6-Benzylaminopurine; KT, Kinetin.
Table 1. Ginger tissue culture stages pf shoot growth and total number of buds & shoots grown in Magenta GA-7 vessels for three ginger cultivars and two plant growth regulator treatments.
| Stages of Shoot Length in Culture | |||||||
|---|---|---|---|---|---|---|---|
| Ginger Cultivar | PGR Treatment | Stage 0 (New Growth) |
Stage 1 (<0.5 < 1.0 cm) |
Stage 2 (<1.0 < 2.0 cm) |
Stage 3 (<2.0 < 3 cm) |
Stage 4 (>3.0 cm) |
Total Number of Shoots & Buds |
| Chinese White | BA | 14.3 (±2.4)a | 25.0 (±1.9)a | 36.0 (±1.3)a | 5.0 (±0.7)NS | 0.4 (±0.3)a | 80.7 (±3.7)a |
| KT | 20.9 (±1.0)b | 20.8 (±0.8)b | 18.4 (±0.6)b | 5.6 (±0.3)NS | 1.3 (±0.1)b | 66.9 (±1.6)b | |
| Hawaii Yellow | BA | 21.8 (±2.2)a | 26.0 (±1.8)a | 26.5 (±1.2)a | 5.5 (±0.6)NS | 1.1 (±0.3)a | 80.9 (±3.5)a |
| KT | 27.9 (±1.1)b | 25.1 (±0.8)a | 16.0 (±0.6)b | 4.9 (±0.3) NS | 1.8 (±0.2)a | 75.7 (±1.7)a | |
| Khing Yai | BA | 31.3 (±2.2)a | 27.2 (±1.2)a | 27.4 (±1.2)a | 4.8 (±0.6)NS | 0.5 (±0.3)a | 91.2 (±3.4)a |
| KT | 30.6 (±1.0)a | 11.1 (±0.5)a | 11.1 (±0.5)b | 3.7 (±0.3) NS | 1.1 (±0.1)b | 70.2 (±1.5)b | |
PGR, plant growth regulator; BA, 6-Benzylaminopurine; KT, Kinetin. ZSignificant comparisons were conducted within a column between cultivar treatments. Significance is determined by Fishers protected LSD at P≤0.05 level of significance, and data is LSMEANS ± SE (least square means + standard error). Data marked with NS are not significant.
Table 2. LSMEANS ± SE for stem diameter, stem length, and number of stems of three cultivars of ginger tissue culture seedlings transplanted into pots in a greenhouse micropropagated with two plant growth regulators.
| Ginger Cultivar | PGR Treatment | Stem Diameter (mm) | Stem Length (cm) | Number of Stems |
|---|---|---|---|---|
| Chinese White | BA | 7.8 (±0.2)a | 101.4 (±2.7)a | 8.9 (±1.3)a |
| KT | 6.8 (±0.2)b | 88.4 (±2.7)b | 10.3 (±1.4)a | |
| Hawaii Yellow | BA | 7.2 (±0.2)a | 90.5 (±2.5)a | 11.1 (±1.4)a |
| KT | 6.9 (±0.2)a | 90.4 (±2.1)a | 15.7 (±1.4)a | |
| Khing Yai | BA | 8.1 (±0.2)a | 92.8 (±2.7)a | 9.4 (±1.4)a |
| KT | 7.9 (±0.2)a | 93.6 (±2.5)a | 10.6 (±1.4)a |
PGR, plant growth regulator; BA, 6-Benzylaminopurine; KT, Kinetin; ZSignificant comparisons within a column, between specific cultivar treatments, with the same letter in common are not significantly different according to Fishers Protected LSD at P ≤ 0.05 level of significance, and data is LSMEANS ± SE (least square means + standard error). Data marked with NS are not significant.
Table 3. LSMEANS ± SE for stem diameter, stem length, and number of stems of three cultivars of ginger tissue culture seedlings transplanted into pots in a greenhouse micropropagated with two plant growth regulators.
| PGR Treatment | Ginger Cultivar |
Biological Root Weight (g) | Edible Root Weight (g) | Total Rhizome Weight (g) | Number of Fingers (Pieces) |
|---|---|---|---|---|---|
| BA | Chinese White | 102.8 (±26.3)a | 1003.3 (±67.7)a | 1107.7 (±71.8)a | 50.5 (±6.1)ab |
| Hawaii Yellow | 175.1 (±25.1)b | 708.1 (±64.8)b | 883.5 (±68.7)b | 59.0 (±5.8)a | |
| Khing Yai | 163.2(±26.3)ab | 917.7(±67.7)a | 1076.2(±71.8)ab | 35.2 (±6.1)b | |
| KT | Chinese White | 69.2 (±29.0)a | 1026.3 (±74.9)a | 1095.0 (±79.4)a | 54.0 (±6.7)ab |
| Hawaii Yellow | 244.1 (±25.1)b | 612.5 (±64.8)c | 856.3 (±68.7)b | 64.9 (±5.8)a | |
| Khing Yai | 188.1 (±26.3)b | 801.5(±67.7)b | 994.9(±71.8)ab | 45.0 (±6.1)b |
PGR, plant growth regulator; BA, 6-Benzylaminopurine; KT, Kinetin; ZSignificant comparisons within a column, between specific cultivars (within a single treatment), with the same letter in common are not significantly different according to Fishers Protected LSD at P ≤ 0.05 level of significance, and data is LSMEANS ± SE (least square means + standard error). Data marked with NS are not significant.
Table 4. LSMEANS ± SE for 6-gingerol, 8-gingerol, 10-gingerol, 6-shogoal, 8-shogoal, and 10-shogoal of three cultivars of micropropagated ginger rhizomes grown with two plant growth regulators.
| Average Concentration (ug/g) of Ginger Rhizome | |||||||
|---|---|---|---|---|---|---|---|
| Ginger Cultivar | PGR Treatment | 6-gingerol | 8-gingerol | 10-gingerol | 6-shogoal | 8-shogoal | 10-shogoal |
| Chinese White | BA | 7,393.3 (±394.1)b | 1,095.0 (±63.8)NS | 1,846.7 (±77.1)d | 69.9 (±7.4)b | 10.0 (±1.0)d | 21.3 (±2.5)NS |
| KT | 7,290.0 (±321.2)b | 1,213.3 (±58.5)NS | 2,125.0 (±114.7)c | 58.0 (±4.7)c | 10.1 (±1.3)d | 23.2 (±3.9)NS | |
| Hawaii Yellow | BA | 9,208.3 (±299.0)a | 1,793.3 (±48.0)NS | 3,230.0 (±39.5)b | 91.6 (±5.8)a | 14.2 (±1.3)ab | 35.6 (±3.8)NS |
| KT | 7,041.7 (±438.0)b | 1,975.0 (±123.1)NS | 3,988.3 (±217.0)a | 56.8 (±4.1)c | 12.0 (±1.0)c | 34.3 (±3.4)NS | |
| Khing Yai | BA | 5,635.0 (±303.2)c | 1,615.0 (±76.9)NS | 3,176.7 (±114.0)b | 58.9 (±6.7)c | 13.1 (±1.4)bc | 36.5 (±4.5)NS |
| KT | 8,778.3 (±438.0)a | 1,746.7 (±78.7)NS | 3,220.0 (±148.1)b | 95.3 (±7.6)a | 14.9 (±1.5)a | 36.9 (±4.2)NS | |
PGR, plant growth regulator; BA, 6-Benzylaminopurine; KT, Kinetin; ZSignificant comparisons within a column with the same letter in common are not significantly different according to Fishers Protected LSD at P ≤ 0.05 level of significance, and data is LSMEANS ± SE (least square means + standard error). Data marked with NS are not significant.